Abstract
A heterotrimeric G-protein in vertebrate photoreceptor cells is called transducin (T alpha beta gamma), whose gamma-subunit is a mixture of two components, T gamma-1 and T gamma-2. T gamma-2 is S-farnesylated and partly carboxyl methylated at the C-terminal cysteine residue, whereas T gamma-1 lacks the modified cysteine residue. To elucidate the physiological significance of the double modifications in T gamma, we established a simple chromatographic procedure to isolate T gamma-1, methylated T gamma-2 and non-methylated T gamma-2 on a reversed phase column. Taking advantage of the high and reproducible yield of T gamma from the column, we analyzed the composition of T gamma subspecies in the T alpha-T beta gamma complex which did not bind with transducin-depleted rod outer segment membranes containing metarhodopsin II. The binding of T alpha-T beta gamma with the membranes was shown to require the S-farnesylated cysteine residue of T gamma, whose methylation further enhanced the binding. This synergistic effect was not evident when T alpha was either absent or converted to the GTP-bound form which is known to dissociate from T beta gamma. Thus we concluded that a formation of the ternary complex, T alpha-T beta gamma-metarhodopsin II, is enhanced by the farnesylation and methylation of T gamma. This suggests that the double modifications provide most efficient signal transduction in photoreceptor cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
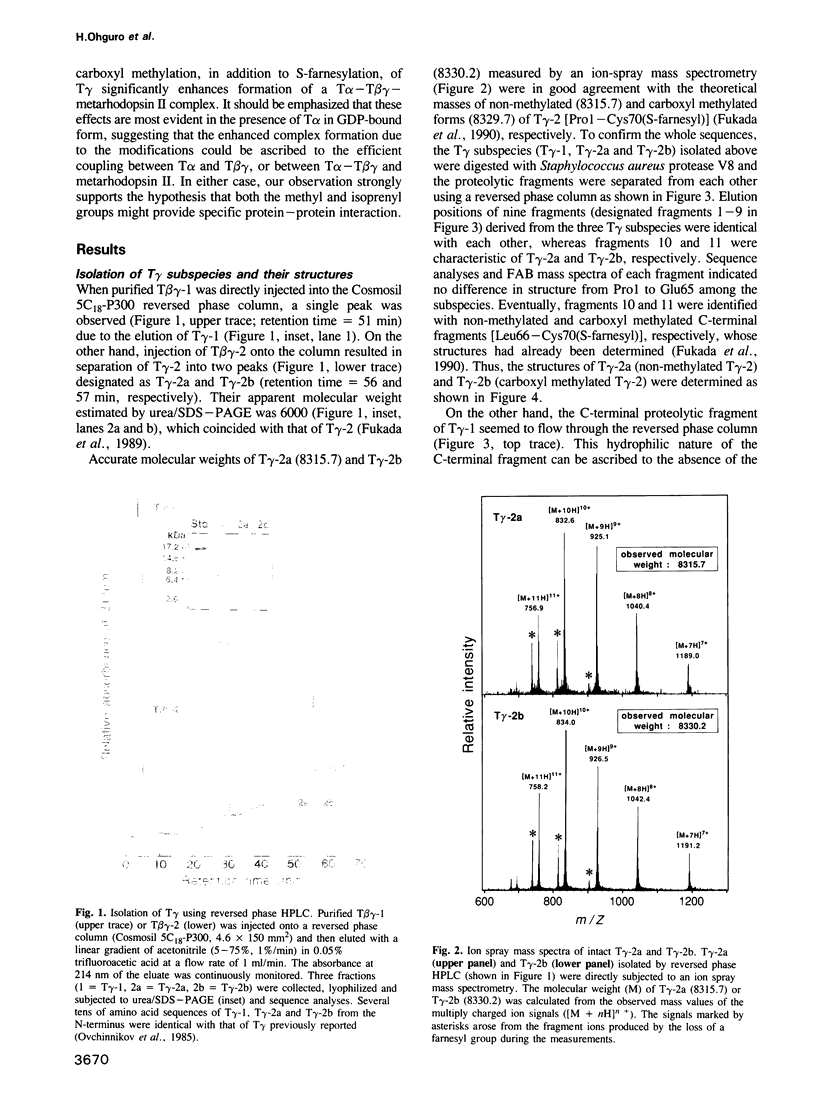
- Fukada Y., Ohguro H., Saito T., Yoshizawa T., Akino T. Beta gamma-subunit of bovine transducin composed of two components with distinctive gamma-subunits. J Biol Chem. 1989 Apr 5;264(10):5937–5943. [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Fukada Y., Yoshizawa T. Activation of phosphodiesterase in frog rod outer segment by an intermediate of rhodopsin photolysis. II. Biochim Biophys Acta. 1981 Jul;675(2):195–200. doi: 10.1016/0304-4165(81)90226-9. [DOI] [PubMed] [Google Scholar]
- Fung B. K. Characterization of transducin from bovine retinal rod outer segments. I. Separation and reconstitution of the subunits. J Biol Chem. 1983 Sep 10;258(17):10495–10502. [PubMed] [Google Scholar]
- Fung B. K., Yamane H. K., Ota I. M., Clarke S. The gamma subunit of brain G-proteins is methyl esterified at a C-terminal cysteine. FEBS Lett. 1990 Jan 29;260(2):313–317. doi: 10.1016/0014-5793(90)80132-3. [DOI] [PubMed] [Google Scholar]
- Gautam N., Baetscher M., Aebersold R., Simon M. I. A G protein gamma subunit shares homology with ras proteins. Science. 1989 May 26;244(4907):971–974. doi: 10.1126/science.2499046. [DOI] [PubMed] [Google Scholar]
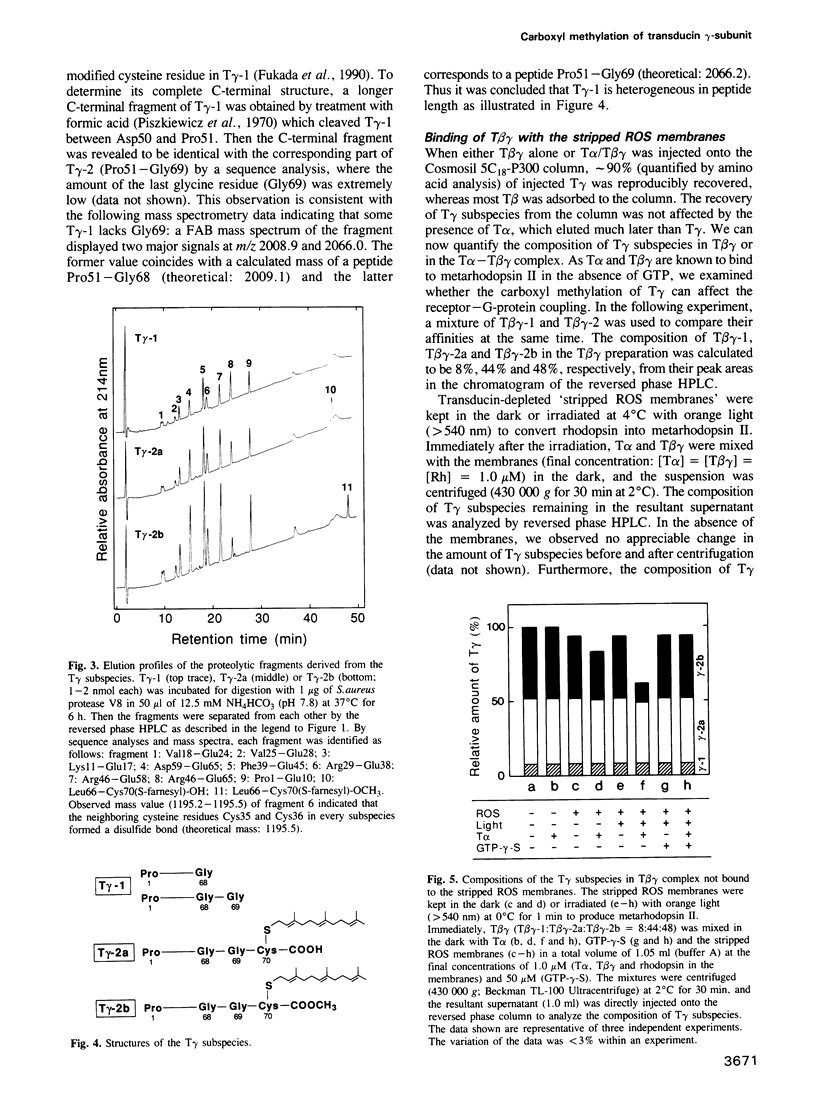
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohguro H., Fukada Y., Yoshizawa T., Saito T., Akino T. A specific beta gamma-subunit of transducin stimulates ADP-ribosylation of the alpha-subunit by pertussis toxin. Biochem Biophys Res Commun. 1990 Mar 30;167(3):1235–1241. doi: 10.1016/0006-291x(90)90656-8. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Shuvaeva T. M., Bogachuk A. P., Shemyakin V. V. Complete amino acid sequence of gamma-subunit of the GTP-binding protein from cattle retina. FEBS Lett. 1985 Jan 1;179(1):107–110. doi: 10.1016/0014-5793(85)80201-5. [DOI] [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- Pérez-Sala D., Tan E. W., Cañada F. J., Rando R. R. Methylation and demethylation reactions of guanine nucleotide-binding proteins of retinal rod outer segments. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3043–3046. doi: 10.1073/pnas.88.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss Y., Goldstein J. L., Seabra M. C., Casey P. J., Brown M. S. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990 Jul 13;62(1):81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- Resh M. D., Ling H. P. Identification of a 32K plasma membrane protein that binds to the myristylated amino-terminal sequence of p60v-src. Nature. 1990 Jul 5;346(6279):84–86. doi: 10.1038/346084a0. [DOI] [PubMed] [Google Scholar]
- Robishaw J. D., Kalman V. K., Moomaw C. R., Slaughter C. A. Existence of two gamma subunits of the G proteins in brain. J Biol Chem. 1989 Sep 25;264(27):15758–15761. [PubMed] [Google Scholar]
- Schafer W. R., Kim R., Sterne R., Thorner J., Kim S. H., Rine J. Genetic and pharmacological suppression of oncogenic mutations in ras genes of yeast and humans. Science. 1989 Jul 28;245(4916):379–385. doi: 10.1126/science.2569235. [DOI] [PubMed] [Google Scholar]
- Simonds W. F., Butrynski J. E., Gautam N., Unson C. G., Spiegel A. M. G-protein beta gamma dimers. Membrane targeting requires subunit coexpression and intact gamma C-A-A-X domain. J Biol Chem. 1991 Mar 25;266(9):5363–5366. [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteway M., Hougan L., Dignard D., Thomas D. Y., Bell L., Saari G. C., Grant F. J., O'Hara P., MacKay V. L. The STE4 and STE18 genes of yeast encode potential beta and gamma subunits of the mating factor receptor-coupled G protein. Cell. 1989 Feb 10;56(3):467–477. doi: 10.1016/0092-8674(89)90249-3. [DOI] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami K., Pandya B. V., Oprian D. D., Khorana H. G. cDNA-derived amino acid sequence of the gamma subunit of GTPase from bovine rod outer segments. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1936–1940. doi: 10.1073/pnas.82.7.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]