Abstract
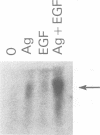
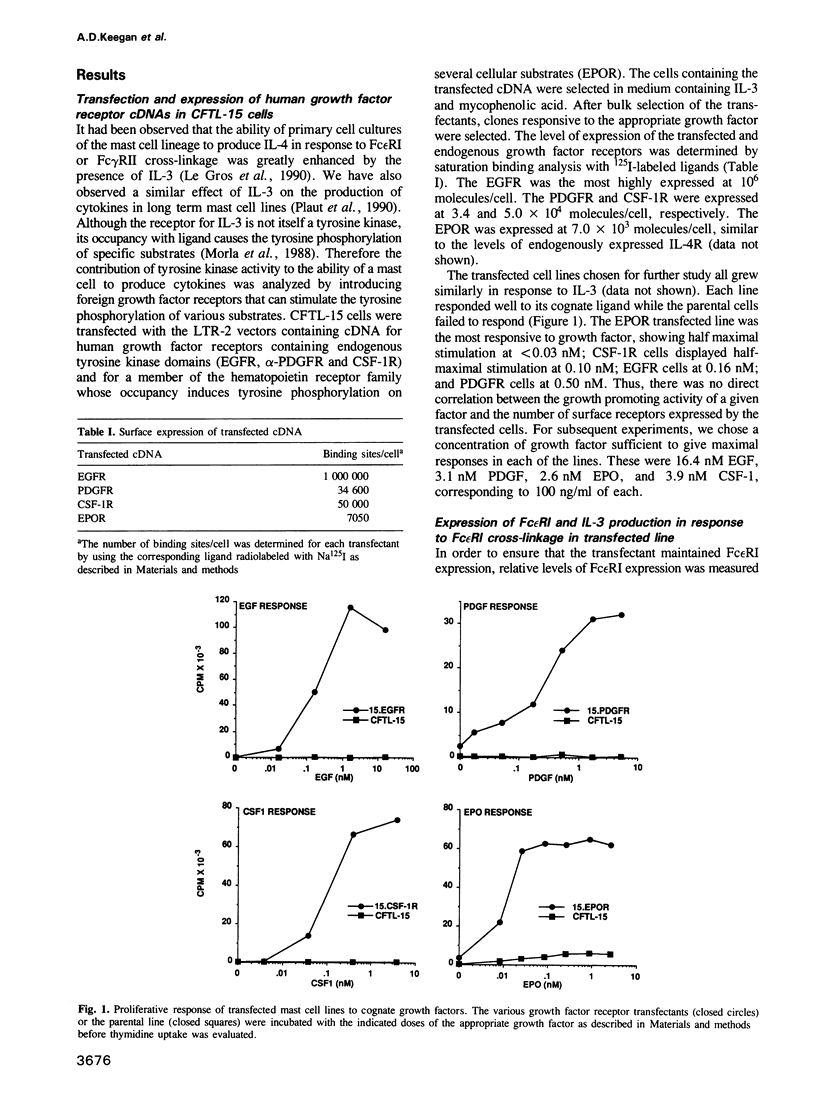
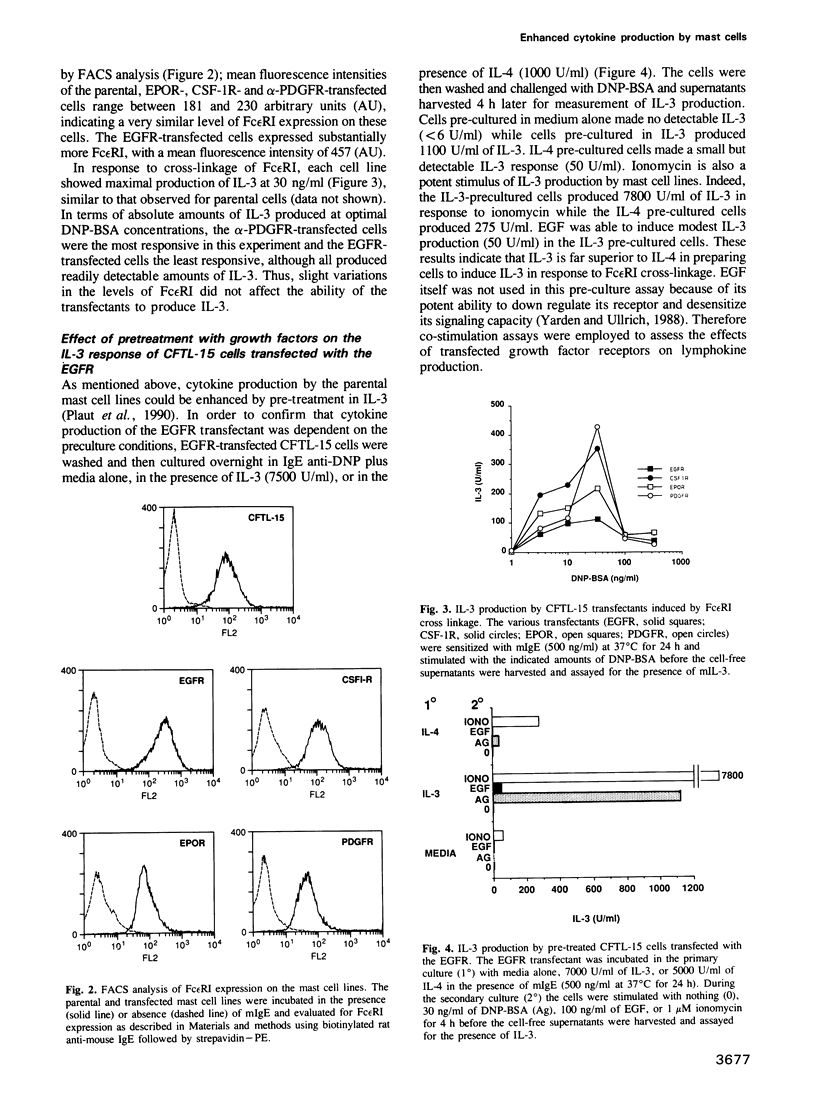
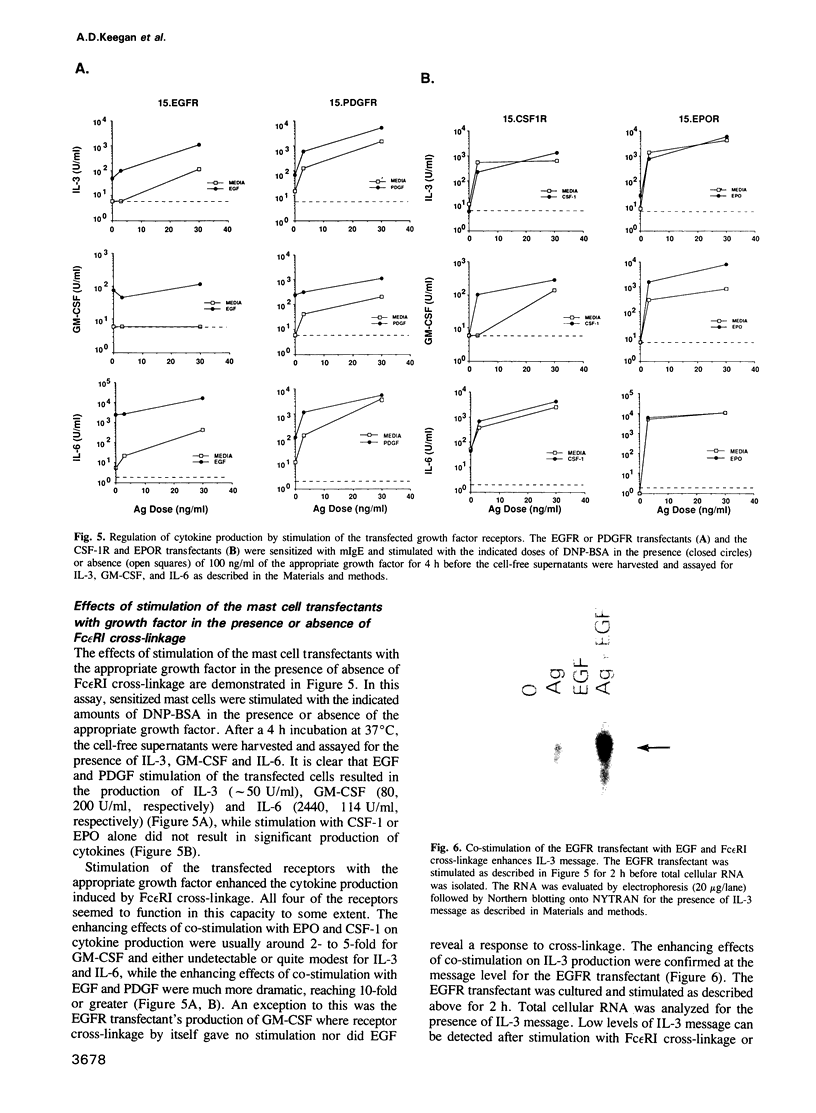
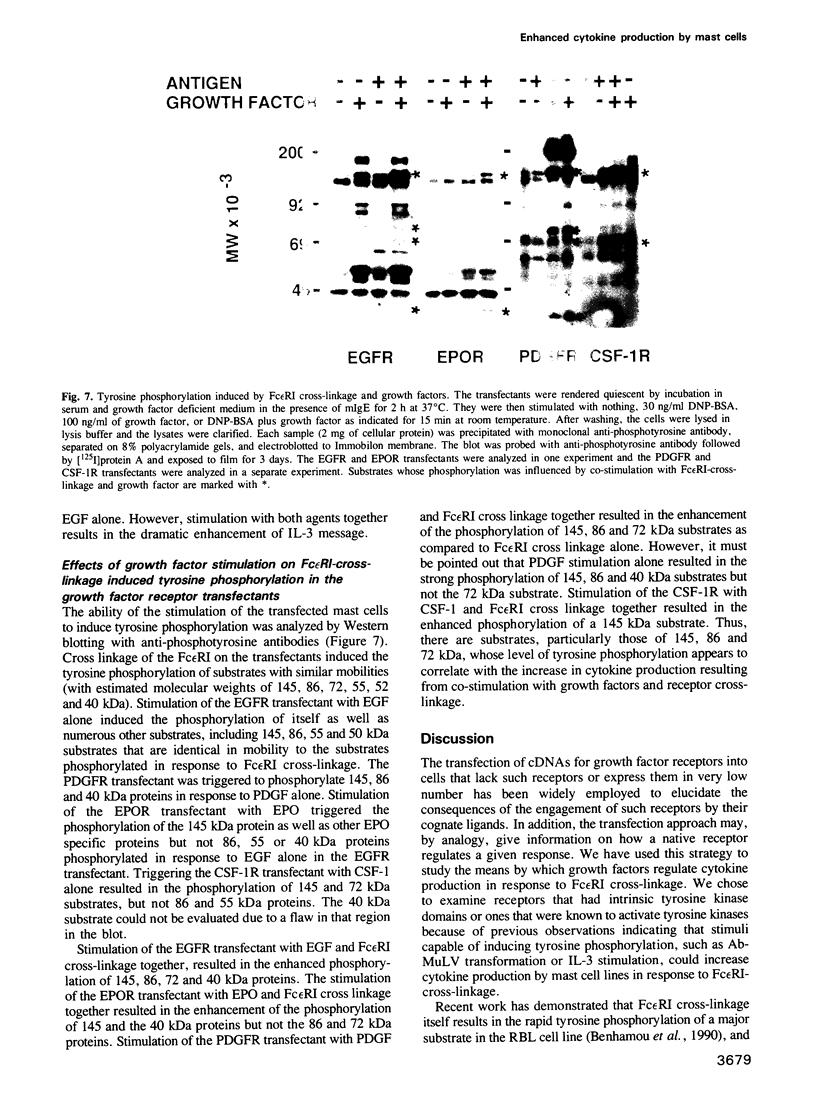
IL-3 dependent mast cell lines produce cytokines in response to Fc receptor cross-linkage or to ionomycin. In this study we have observed that cells pre-cultured in IL-3 produce 10-100 times more cytokine after receptor cross-linkage in comparison with IL-4 pre-cultured cells. Although several hematopoietin receptors, including those for IL-3, IL-4 and EPO, do not contain tyrosine kinase domains, their occupancy with ligand causes tyrosine phosphorylation of specific cellular substrates. Therefore, the contribution of tyrosine kinase activation to the ability of an IL-3 dependent mast cell line, CFTL-15, to produce cytokines was analyzed. The CFTL-15 cells were transfected with growth factor receptors containing ligand-inducible tyrosine kinase domains (EGFR and PDGFR, and CSF-IR) or with the EPOR. All of the transfectants were able to proliferate in response to IL-3 or to their respective growth factor and to produce IL-3 in response to IgE receptor cross-linkage. Stimulation of the EGFR and PDGFR transfectants with their respective ligands resulted in the production of IL-3, IL-6, and GM-CSF. Stimulation of the CSF-1R or EPOR transfectants with growth factor alone failed to induce cytokine production. However, in co-stimulation assays each of the growth factors enhanced the amount of cytokine produced in response to Fc epsilon RI cross-linkage. The ability of these stimuli to induce tyrosine phosphorylation in the transfectants was analyzed. Fc epsilon RI cross-linkage in the transfectants routinely induced the tyrosine phosphorylation of 145, 86 and 72 kDa proteins, with occasional phosphorylation of 55, 52, and 40 kDa proteins.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Sasson S. Z., Le Gros G., Conrad D. H., Finkelman F. D., Paul W. E. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1421–1425. doi: 10.1073/pnas.87.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou M., Gutkind J. S., Robbins K. C., Siraganian R. P. Tyrosine phosphorylation coupled to IgE receptor-mediated signal transduction and histamine release. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5327–5330. doi: 10.1073/pnas.87.14.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Rogers H. W., Gordon J. R., Martin C. A., Jayaraman S., Wilson S. D., Dvorak A. M., Galli S. J., Dorf M. E. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989 Jul 1;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Kraus M. H., Segatto O., King C. R., Aaronson S. A. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987 Jul 10;237(4811):178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- Heidaran M. A., Pierce J. H., Lombardi D., Ruggiero M., Gutkind J. S., Matsui T., Aaronson S. A. Deletion or substitution within the alpha platelet-derived growth factor receptor kinase insert domain: effects on functional coupling with intracellular signaling pathways. Mol Cell Biol. 1991 Jan;11(1):134–142. doi: 10.1128/mcb.11.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Abraham S., Krystal G., Lansdorp P., Lemoine F., Eaves C. J. Activation of multiple hemopoietic growth factor genes in Abelson virus-transformed myeloid cells. Exp Hematol. 1988 Oct;16(9):774–781. [PubMed] [Google Scholar]
- Isfort R., Huhn R. D., Frackelton A. R., Jr, Ihle J. N. Stimulation of factor-dependent myeloid cell lines with interleukin 3 induces tyrosine phosphorylation of several cellular substrates. J Biol Chem. 1988 Dec 15;263(35):19203–19209. [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Le Gros G., Ben-Sasson S. Z., Conrad D. H., Clark-Lewis I., Finkelman F. D., Plaut M., Paul W. E. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol. 1990 Oct 15;145(8):2500–2506. [PubMed] [Google Scholar]
- Lee W. T., Conrad D. H. Murine B cell hybridomas bearing ligand-inducible Fc receptors for IgE. J Immunol. 1986 Jun 15;136(12):4573–4580. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- Matsui T., Heidaran M., Miki T., Popescu N., La Rochelle W., Kraus M., Pierce J., Aaronson S. Isolation of a novel receptor cDNA establishes the existence of two PDGF receptor genes. Science. 1989 Feb 10;243(4892):800–804. doi: 10.1126/science.2536956. [DOI] [PubMed] [Google Scholar]
- Metzger H. Molecular aspects of receptors and binding factors for IgE. Adv Immunol. 1988;43:277–312. doi: 10.1016/s0065-2776(08)60368-5. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Coligan J. E., Zoon K., Maloy W. L., Paul W. E. High-efficiency purification and chemical characterization of B cell stimulatory factor-1/interleukin 4. J Immunol. 1987 Aug 15;139(4):1127–1134. [PubMed] [Google Scholar]
- Pierce J. H., Di Marco E., Cox G. W., Lombardi D., Ruggiero M., Varesio L., Wang L. M., Choudhury G. G., Sakaguchi A. Y., Di Fiore P. P. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5613–5617. doi: 10.1073/pnas.87.15.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Collins M., Arai K., Miyajima A. EGF induces differentiation of an IL-3-dependent cell line expressing the EGF receptor. EMBO J. 1989 Dec 1;8(12):3677–3684. doi: 10.1002/j.1460-2075.1989.tb08542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Downes C. P. The haemopoietic growth factors interleukin 3 and colony stimulating factor-1 stimulate proliferation but do not induce inositol lipid breakdown in murine bone-marrow-derived macrophages. EMBO J. 1986 Dec 1;5(12):3281–3286. doi: 10.1002/j.1460-2075.1986.tb04640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]