Abstract
Background
Treatment of melasma is unsatisfactory most of the times. Hormonal role is shown to exist in pathogenesis of the melasma, and sex-hormone related drugs may have an effect on melasma.
Aim
To investigate efficacy of 1% flutamide cream versus 4% hydroquinone cream on melasma.
Methods
In a parallel randomized clinical trial, 74 women with melasma were allocated to receive a sunscreen along with 4% hydroquinone cream or 1% flutamide cream. Melasma Area and Severity Index (MASI), mexameter melanin assay, and patient satisfaction were investigated.
Results
Mean age of the participants was 33.8 years. Mean length of time suffering from Melasma was 96.3 months. The subjects reported in average 1.1 hours per day of exposure to sunlight. Mean standardized total patient satisfaction score was 28.8 (standard deviation [SD] 17.2) in flutamide group patients versus 18 (SD 15.5) in control group (P<0.01). Regardless of treatment group, the skin darkness assessed upon MASI scales was reduced over the treatment course (P<0.001). Using mixed effects, longitudinal modeling showed better treatment efficacy based on MASI scale for flutamide group compared to the hydroquinone group (P<0.05). However, longitudinal analysis of mexameter scores did not reveal any significant difference in melanin measurements between flutamide and hydroquinone.
Conclusion
Topical flutamide appeared as effective as topical hydroquinone in treating melasma using mexameter assessment but with a better MASI improvement trend and higher patient satisfaction in flutamide treatment versus topical hydroquinone. As the present study is possibly the first clinical experience on efficacy of topical flutamide on melasma, it would be quite unreasonable to recommend clinical use of it before future studies replicate the results on its efficacy and safety.
Keywords: Pigmentation disorders, topical, treatment, dermatology, anti-androgenic agents, acquired increased skin pigmentation, Melasma Area and Severity Index (MASI) Mexamete
Introduction
Melasma is reported to account for 4%–10% of new dermatology hospital referrals. It is shown to be more common in people of Hispanic origin and among Asians.1 Treatment of melasma is unsatisfactory most of the times, even by tolerating various side effects such as contact dermatitis, irritation, and scarring.1–3 According to the most recent Cochrane review, evidence shows insufficiency to provide robust guidance for practice, and more randomized clinical trials are needed in the field of melasma treatment.4
The range of treatments investigated for melasma covers all systemic, procedural, and topical modalities. Topical treatments have their own advantage over systemic therapies being among the most common interventions investigated and used for treating melasma.4 Hormonal role is shown to exist in pathogenesis of the melasma.5–8 Therefore, flutamide as an anti-androgenic agent may theoretically be considered to have an effect in treating melasma. A safe way to assess such an effect would be to use its topical form. Topical flutamide has been used earlier for treatment of hair-loss. However, no previous study was identified to investigate topical flutamide in treating melasma. The aim of this study was to investigate efficacy of 1% flutamide cream versus 4% hydroquinone cream on melasma.
Methods
A parallel randomized clinical trial design was conducted to compare the effect of topical hydroquinone, as the standard treatment of melasma, with topical flutamide as the new test treatment. The study participants comprised 74 women with melasma referred to a private dermatology clinic in Ardabil from 2010 to 2013. Melasma was diagnosed by an experienced dermatologist and Wood’s light examination was done to assess the depth. The following criteria were set for excluding the subjects from the study:
Pregnancy
Breastfeeding
Use of oral contraceptives, spironolactone, or phenytoin
Being treated with tretinoin through the last 3 weeks
Being treated with hydroquinone through the last 6 months
Hydroquinone hypersensitivity reactions
Drug or alcohol addiction
Hepatic disease.
Randomization and blinding
Both the patients and outcome assessors were blinded to the treatment type. To ensure the blinding, both the hydroquinone and flutamide cream tubes were produced in the same size and appearance. Randomly permuted blocks were generated using Ralloc module in Stata 11 statistical software package and patients were allocated to receive either 1% flutamide topical cream or 4% hydroquinone topical cream.9 After baseline assessments, patients in both the groups were treated for 4 months.
The active treatment group received 4% hydroquinone cream (Sobhan Daru Drug Industries, Rasht, Iran) at nights after cleaning the face. Through the day, after cleaning the face, a sunscreen with sun protection factor (SPF) 30 (Ardene, Pars Haiat Industries, Tehran, Iran) was used three times, 1 g each time at 9, 12, and 15 o’clock. This treatment was continued for 4 months and melasma status was assessed at the end of each month. The patients in test trial group received 1% flutamide cream (produced by Tabriz Faculty of Pharmacy, Iran) at nights after cleaning the face. Use of sunscreen and outcome assessments were similarly done for this group. Pigmentation reduction as the primary outcome of the study was assessed through various methods analyzed and reported as follows:
Melasma Area and Severity Index (MASI): This method is a relevant method of assessing improvement of melasma after treatment.10
Mexameter melanin assay: Mexameter was used to assess pigmentation in five facial locations: cheeks (left and right), forehead, upper lip, and nasal area. At the baseline, assessment measurement was done on highest darkness area in each of the five locations. These points were marked on a facial mapping sheet in patient records in order to repeat the measurements on the same points through the follow-up assessments. The mexameter scores recorded for each location was then averaged to form a total score. Mexameter examination was done in a semidark room and after cleaning the face with water and soap.
Patient satisfaction: The patient satisfaction was assessed through five Likert-type scale questions: 1 – improvement of melasma patches; 2 – satisfaction with drug and potential side effects; 3 – skin succulence improvement; 4 – skin darkness improvement; and 5 – overall satisfaction with treatment.
One patient in the intervention group did not participate after randomization list was generated. Per-protocol analysis was run and data were analyzed using Stata version 11 statistical software package (StataCorp LP, College Station, TX, USA). To study the trend of MASI and mexameter outcome scales, measured repeatedly over time, mixed-effects models were selected to analyze the longitudinal data. Nonparametric tests of comparing numeric scales were applied to compare the effect of topical flutamide and hydroquinone on patient satisfaction. A P-value <0.05 was considered to be statistically significant.
The study protocol was approved by regional committee of ethics in Ardabil University of Medical Sciences. Informed consent was obtained from all the participants. The study was registered in Iranian Registry of Clinical Trials clinical registration center approved by the World Health Organization under the number IRCT138905114310N4.
Results
Mean age of the participants was 33.8 years with a standard deviation (SD) of 6.7 years (range: 20–52 years). Mean (SD) of age was 33.2 (6) years in flutamide group versus 34.4 (7.4) years in hydroquinone group. Housewives comprised 83% of the participants. This figure was 82.35% for flutamide group and 83.8% for the hydroquinone group patients. Above 90% of subjects had history of at least one child birth. The percentage of child birth history was 92.7% for flutamide group compared with 88.5% in hydroquinone group. Mean length of time suffering from melasma was 96.3 months (SD 69.5) and this time did not differ significantly between the trial groups. Table 1 compares the past medical history of the patients in trial groups.
Table 1.
Past medical history of the participants in two groups of the clinical trial comparing effect of flutamide and hydroquinone on melasma
| Clinical history | Trial group | Current suffering frequency (%) | Previous history frequency (%) | Not suffered frequency (%) |
|---|---|---|---|---|
| Menstrual irregularities | Flutamide | 13 (37.1) | 3 (8.6) | 19 (54.3) |
| Hydroquinone | 9 (24.3) | 4 (10.8) | 24 (64.9) | |
| Total | 22 (30.6) | 7 (9.7) | 19 (54.3) | |
| Ovarian cysts | Flutamide | 2 (6.1) | 8 (24.3) | 23 (69.7) |
| Hydroquinone | 3 (8.3) | 8 (22.2) | 25 (69.4) | |
| Total | 5 (7.25) | 16 (23.2) | 48 (69.6) | |
| Infertility | Flutamide | 1 (3.45) | 1 (3.45) | 27 (93.1) |
| Hydroquinone | 3 (12) | 0 (0) | 22 (88) | |
| Total | 4 (7.4) | 1 (1.85) | 49 (90.7) | |
| Hair loss | Flutamide | 14 (41.2) | 1 (2.9) | 19 (55.9) |
| Hydroquinone | 11 (31.4) | 0 (0) | 24 (68.6) | |
| Total | 25 (36.2) | 1 (1.45) | 43 (62.3) | |
| History of Addison’s disease, Cushing’s syndrome, or hyperthyroidism | Only one patient in the flutamide group had history of hyperthyroidism | |||
| Drug history | ||||
| History of OCP usage | Flutamide | Yes: 20 (55.6) | No: 16 (44.4) | |
| Hydroquinone | Yes: 12 (32.4) | No: 25 (67.6) | ||
| Total | Yes: 32 (43.8) | No: 41 (56.2) | ||
| History of spironolactone usage | Flutamide | Yes: 1 (2.8) | No: 35 (97.2) | |
| Hydroquinone | Yes: 0 (0) | No: 37 (100) | ||
| Total | Yes: 1 (1.4) | No: 72 (98.6) | ||
| History of phenytoin usage | No one reported use of phenytoin among participants | |||
| Family history | ||||
| Hirsutism in mother | Flutamide | Yes: 0 (0) | No: 35 (100) | |
| Hydroquinone | Yes: 3 (8.1) | No: 34 (91.9) | ||
| Total | Yes: 3 (4.2) | No: 69 (95.8) | ||
| Melasma in mother | Flutamide | Yes: 4 (11.1) | No: 32 (88.9) | |
| Hydroquinone | Yes: 8 (21.6) | No: 29 (78.4) | ||
| Total | Yes: 12 (16.4) | No: 61 (83.6) | ||
| Hirsutism in sister | Flutamide | Yes: 9 (25.7) | No: 26 (74.3) | |
| Hydroquinone | Yes: 6 (16.2) | No: 31 (83.8) | ||
| Total | Yes: 15 (20.8) | No: 57 (79.2) | ||
| Melasma in sister | Flutamide | Yes: 20 (55.6) | No: 16 (44.4) | |
| Hydroquinone | Yes: 26 (70.3) | No: 11 (29.7) | ||
| Total | Yes: 46 (63) | No: 27 (37) | ||
| Hirsutism in either one of mother, sister, or aunts | Flutamide | Yes: 11 (31.4) | No: 24 (68.6) | |
| Hydroquinone | Yes: 9 (24.3) | No: 28 (75.7) | ||
| Total | Yes: 20 (27.8) | No: 52 (68.6) | ||
| Melasma in either one of mother, sister, or aunts | Flutamide | Yes: 24 (66.7) | No: 12 (33.3) | |
| Hydroquinone | Yes: 31 (83.8) | No: 6 (16.2) | ||
| Total | Yes: 55 (75.3) | No: 18 (24.7) | ||
Abbreviation: OCP, oral contraceptive pill.
The subjects reported an average 1.1 hours per day of exposure to sunlight (SD 1). The mean exposure time for the past 24 hours prior to participation in the study was 1.4 hours (SD 1.3). The sun exposure length of time did not significantly differ between the trial groups.
Mean standardized total patient satisfaction score was 28.8 (SD 17.2) in flutamide group patients versus 18 (SD 15.5) in control group (P<0.01). When compared with the control group, median satisfaction rank was significantly higher in flutamide group patients in all the four satisfaction items: 1 – improvement of melasma patches (P<0.01); 2 – satisfaction with drug and side effects (P<0.01); 3 – skin succulence improvement (P<0.05); and 4 – skin darkness improvement (P<0.01). Total dissatisfaction was observed in 35% of hydroquinone group patients versus 22% in flutamide group, nevertheless, the observed difference in this measure was not statistically significant (intervention risk ratio [RR]: 0.63, 95% confidence interval [CI]: 0.3–1.3).
MASI
Regardless of the treatment group, the skin darkness assessed upon MASI scales was reduced over the treatment course (P<0.001). However, skin homogeneity was not found to be different over treatment time. Similarly, the total percentage of the face area involving melasma did not also change significantly over the time period of investigation.
The trend of change in skin darkness compared for flutamide and hydroquinone is shown in Figure 1A. The trend of variations in skin homogeneity measurements compared for flutamide and hydroquinone is shown in Figure 1B. The MASI score had a decreasing trend over the treatment course for both the groups (Figure 2). Descriptive results of melanin measurement compared for flutamide and hydroquinone are given in Table 2. Using mixed effects, longitudinal modeling showed better treatment efficacy based on MASI scale for flutamide group compared to the hydroquinone group (P<0.05). However, longitudinal analysis of mexameter scores did not reveal any significant difference in melanin measurements between flutamide and hydroquinone.
Figure 1.
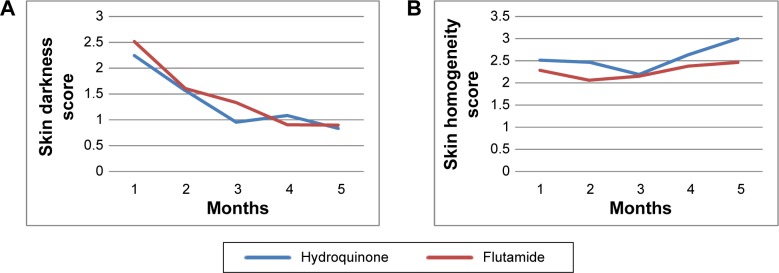
The trend of change in skin darkness and homogeneity scores compared for flutamide and hydroquinone randomized clinical trial groups.
Notes: (A) Skin darkness and (B) skin homogeneity trend. Measurement time points from baseline (1) to final measurement (5), 4 months after treatment. Skin darkness and homogeneity scores assessed by the physician (possible range: 0–4).
Figure 2.
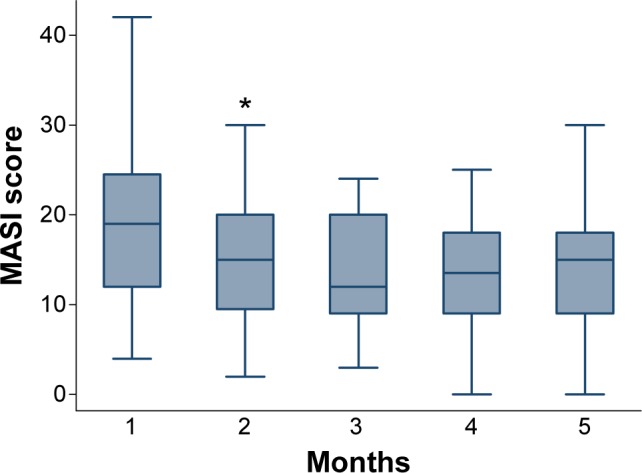
Box plot presenting the changes in MASI score over the time among patients treated either by topical flutamide or hydroquinone. *Indicates an outlier.
Abbreviation: MASI, Melasma Area and Severity Index.
Table 2.
Mexameter melanin repeated measurements on various face spots compared for topical flutamide and hydroquinone over the treatment course
| Trial group | Location | Measurement time points and mexameter measurement score
|
||||
|---|---|---|---|---|---|---|
| Baseline | Month 1 | Month 2 | Month 3 | Month 4 | ||
| Hydroquinone | Forehead | 22.6 | 20.7 | 19.7 | 17.7 | 15.8 |
| Right malar | 20.7 | 18.2 | 18.1 | 16.2 | 16.3 | |
| Left malar | 20.1 | 18.2 | 17 | 18.5 | 15.5 | |
| Lip | 30.7 | 23.9 | 23.8 | 23 | 20.4 | |
| Nose | 26.5 | 25.5 | 26.6 | 24.7 | 21.7 | |
| Topical flutamide | Forehead | 25.8 | 19.1 | 18.5 | 17.1 | 15.4 |
| Right malar | 24.2 | 18.2 | 16.2 | 16.7 | 15.7 | |
| Left malar | 23.2 | 17.9 | 15.4 | 14.9 | 15.3 | |
| Lip | 27.4 | 20.8 | 21 | 20.2 | 19 | |
| Nose | 27.5 | 23.4 | 21.6 | 20.1 | 21.5 | |
Discussion
The results of present study confirmed that both the hydroquinone and flutamide creams were effective in treating the melasma. However, flutamide appeared to have higher efficacy than hydroquinone with respect to the patient satisfaction score as well as the MASI scale score. Hydroquinone is considered to be the most widely investigated topical treatment for melasma. Several randomized clinical trials have been conducted in recent years, to compare hydroquinone with other alternatives. Most studies revealed that hydroquinone cream had higher efficacy compared to new treatments (Table 3). Nonrandomized clinical trials have also been conducted, the results of which should be interpreted cautiously. In a Mexican 12-week study, the patients who received 1% dioic acid cream were compared with those receiving 2% hydroquinone cream according to MASI score. No significant difference between treatments were observed and the side effects were similar; however, pruritus was more common in hydroquinone group.11 Another nonrandomized study had compared the clinical efficacy and safety of the association of Belides, Emblica, and Licorice 7%, with hydroquinone 2% in the treatment of melasma. Similarly with the Mexican study, this study also did not reveal the superiority of the new alternative even to the 2% hydroquinone treatment.12 Contrary to most melasma studies, these two studies used 2% hydroquinone twice daily. Use of recommended 4% hydroquinone might have changed the results of testing the hypothesis of different efficacy. Some studies have investigated the efficacy of hydroquinone with other agents mostly reporting higher efficacy of combined treatments; however, the cost-effectiveness of these treatment needs to be investigated in more detail.13–15
Table 3.
Randomized clinical trials conducted to compare hydroquinone as the standard treatment with other topical alternative treatments
| Comparison groups | Outcome(s) | Author conclusions | References |
|---|---|---|---|
| 1 – Hydroquinone 4% cream 2 – 1% flutamide cream |
1 – Patient satisfaction 2 – MASI 3 – Mexameter melanin assay |
Comparable efficacy in objective assessments but higher patient satisfaction for 1% flutamide cream | Present study |
| 1 – Hydroquinone 4% cream 2 – 3% Rubus occidentalis cream |
Patient satisfaction | Occidentalis 3% cream is a safe and effective skin-lightening agent for melasma and is comparable in efficacy with 4% hydroquinone cream | 25 |
| 1 – Hydroquinone 4% cream 2 – Kojic acid cream (0.75% kojic acid and 2.5% vitamin C) |
MASI | Kojic acid cream found to be less effective than hydroquinone 4% cream | 26 |
| 1 – Hydroquinone 4% cream 2 – Zinc sulfate 10% solution |
MASI | Zinc sulfate 10% solution found to be less effective than hydroquinone 4% cream | 27 |
| 1 – Hydroquinone 4% cream 2 – Azelaic acid cream |
MASI | 20% Azelaic acid cream applied twice daily may be more effective than hydroquinone 4% in reducing mild melasma | 28 |
| 1 – Hydroquinone 4% on one side of the face 2 – Niacinamide cream on other side of the face |
1 – Colorimetric measures 2 – Histological sections |
Generally, similar efficacy but proportion of good to excellent improvement higher for hydroquinone | 29 |
| 1 – Hydroquinone 2% cream as priming agent 2 weeks before peeling with glycolic acid 2 – Tretinoin and 0.025% cream as priming agent 2 weeks before peeling with glycolic acid |
MASI | Results are better with hydroquinone as priming agent compared to tretinoin in enhancing the results with glycolic acid peels in melasma and in decreasing postpeel postinflammatory hyperpigmentation | 30 |
| 1 – Hydroquinone 4% cream 2 – A triple combination (TC) cream |
Static global severity assessment of melasma | Was more effective than the hydroquinone cream for the treatment of moderate-to-severe facial melasma | 31 |
| 1 – Hydroquinone 5% cream on one side of the face 2 – Ascorbic acid cream on other side of the face |
1 – Colorimetry 2 – Digital photography and regular color slides |
Hydroquinone showed a better response than ascorbic acid | 32 |
Abbreviation: MASI, Melasma Area and Severity Index.
In the present study, topical flutamide was shown to be relatively safe when compared with hydroquinone. Although the present study seems to be the first study published on the efficacy of topical flutamide in treating melasma, flutamide in its topical or oral forms has been used earlier for treatment of acne, hirsutism, and hair loss with promising results in human and animal studies.16–18 The preliminary results of an ongoing case–control study by the authors are also in line with the role of androgenic disorders in melasma. Considering some ambiguity in etiologic mechanisms of melasma, it is also very hard to understand the exact mechanism of action observed for the effect of flutamide on melasma. The initiative behind this trial was the available clinical experience of improved melasma coexisting with acne when the patients were treated with flutamide. There is theoretical evidence of potential association between melasma and other androgenic-related conditions such as acne, polycystic ovarian disease, and hirsutism. The mechanism behind the effect may lie in modifications on alpha-melanocyte-stimulating hormone or cyclic adenosine monophosphate-elevating agents affecting the melanin synthesis; however, both future animal and clinical studies are recommended to investigate the mechanism in detail.19,20 A case report has also been published on possible effects of topical flutamide in pigmentation problems.21 The use of topical drugs are much more attractive for dermatologists because there is the potential for relatively mild systemic adverse effects during topical use of drugs. Topical flutamide would be more safe compared to its oral form taking into account potentials for severe side effects when oral flutamide has been used.22 As the present study is possibly the first clinical experience on efficacy of topical flutamide on melasma, it would be quite unreasonable to recommend the product for clinical use before future studies replicate the results on its efficacy and safety.
All three types of outcomes used in present study to assess the treatment efficacy have been shown to be relevant outcomes for such purpose.1 While patient satisfaction is a subjective assessment, MASI is a semiquantitative objective scale and mexameter assessment is a full quantitative and objective measure. MASI has been the most common endpoint for evaluation of the treatment effect in randomized clinical trials on melasma. Soft subjective endpoints such as patient satisfaction, although criticized in earlier decades to be used in clinical trials, have gained more attention in recent years. Moreover, the more the patients are satisfied with the treatment, the more likely they are to comply with medical treatment ensuring to have a better outcome.23,24 It seems that as beauty turns to be a major demand in the field of dermatology, and especially with respect to pigmentation disorders, it would be quite critical to include patient satisfaction among the evaluation outcomes of melasma improvement preferably along with the objective measures such as MASI. The present study investigated patient satisfaction as a subjective soft endpoint, MASI as a semiquantitative objective measure as well as full-quantitative objective measure of skin melanin by mexameter. However, the study also suffers limitations. In present research, the length of study was decided to be 120 days; however, future longer studies may yield more information to build the evidence. Moreover, as it was the first time flutamide was being used for melasma treatment, a study with much larger sample size was not planned, but considering the preliminary promising results of the present study, larger clinical trials could now be planned in order to better depict the potential applicability of topical flutamide. Combined treatments including flutamide may also be the focus of future research.
Conclusion
Topical flutamide appeared as effective as topical hydroquinone in treating melasma with higher patient satisfaction. As the present study is possibly the first clinical experience on efficacy of topical flutamide on melasma, it would be quite unreasonable to recommend the product for clinical use before future studies replicate its results on efficacy and safety.
Acknowledgments
The authors are thankful to Dr. Hadi Valizadeh for his kind help in preparation of the medication we had planned to use in present study.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Salim A, Rengifo-Pardo M, Vincent S, Cuervo-Amore LG. Melasma. In: Williams H, editor. Evidence-based Dermatology. 2nd ed. Malden: Blackwell Publishing; 2008. pp. 497–510. [Google Scholar]
- 2.Sardesai VR, Kolte JN, Srinivas BN. A clinical study of melasma and a comparison of the therapeutic effect of certain currently available topical modalities for its treatment. Indian J Dermatol. 2013;58(3):239. doi: 10.4103/0019-5154.110842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivas S, Pandya AG. Treatment of melasma with topical agents, peels and lasers: an evidence-based review. Am J Clin Dermatol. 2013;14(5):359–376. doi: 10.1007/s40257-013-0038-4. [DOI] [PubMed] [Google Scholar]
- 4.Jutley GS, Rajaratnam R, Halpern J, Salim A, Emmett C. Systematic review of randomized controlled trials on interventions for melasma: an abridged Cochrane review. J Am Acad Dermatol. 2014;70(2):369–373. doi: 10.1016/j.jaad.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Passeron T. Melasma pathogenesis and influencing factors – an overview of the latest research. J Eur Acad Dermatol Venereol. 2013;27(1):5–6. doi: 10.1111/jdv.12049. [DOI] [PubMed] [Google Scholar]
- 6.Sialy R, Hassan I, Kaur I, Dash RJ. Melasma in men: a hormonal profile. J Dermatol. 2000;27(1):64–65. doi: 10.1111/j.1346-8138.2000.tb02122.x. [DOI] [PubMed] [Google Scholar]
- 7.Ortonne JP, Arellano I, Berneburg M, et al. A global survey of the role of ultraviolet radiation and hormonal influences in the development of melasma. J Eur Acad Dermatol Venereol. 2009;23(11):1254–1262. doi: 10.1111/j.1468-3083.2009.03295.x. [DOI] [PubMed] [Google Scholar]
- 8.Hassan I, Kaur I, Sialy R, Dash RJ. Hormonal milieu in the maintenance of melasma in fertile women. J Dermatol. 1998;25(8):510–512. doi: 10.1111/j.1346-8138.1998.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 9.Asghari-Jafarabadi M, Sadeghi-Bazargani H. Randomization: techniques and software-aided implementation in medical studies. J Clin Res Gov. 2014;3(2) doi: 10.13183/jcrg.v3i2.105.. [DOI] [Google Scholar]
- 10.Pandya AG, Hynan LS, Bhore R, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol. 2011;64(1):78–83. doi: 10.1016/j.jaad.2009.10.051. [DOI] [PubMed] [Google Scholar]
- 11.Tirado-Sanchez A, Santamaria-Roman A, Ponce-Olivera RM. Efficacy of dioic acid compared with hydroquinone in the treatment of melasma. Int J Dermatol. 2009;48(8):893–895. doi: 10.1111/j.1365-4632.2009.04105.x. [DOI] [PubMed] [Google Scholar]
- 12.Costa A, Moises TA, Cordero T, Alves CR, Marmirori J. Association of emblica, licorice and belides as an alternative to hydroquinone in the clinical treatment of melasma. An Bras Dermatol. 2010;85(5):613–620. doi: 10.1590/s0365-05962010000500003. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh PW, Aljuffali IA, Fang CL, Chang SH, Fang JY. Hydroquinone-salicylic acid conjugates as novel anti-melasma actives show superior skin targeting compared to the parent drugs. J Dermatol Sci. 2014;76(2):120–131. doi: 10.1016/j.jdermsci.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Deo KS, Dash KN, Sharma YK, Virmani NC, Oberai C. Kojic acid visa-vis its combinations with hydroquinone and betamethasone valerate in melasma: a randomized, single blind, comparative study of efficacy and safety. Indian J Dermatol. 2013;58(4):281–285. doi: 10.4103/0019-5154.113940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alikhan A, Daly M, Wu J, Balkrishnan R, Feldman SR. Cost-effectiveness of a hydroquinone/tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21(5):276–281. doi: 10.3109/09546630903200612. [DOI] [PubMed] [Google Scholar]
- 16.Scheinfeld N. A review of hormonal therapy for female pattern (androgenic) alopecia. Dermatol Online J. 2008;14(3):1. [PubMed] [Google Scholar]
- 17.Cusan L, Dupont A, Gomez JL, Tremblay RR, Labrie F. Comparison of flutamide and spironolactone in the treatment of hirsutism: a randomized controlled trial. Fertil Steril. 1994;61(2):281–287. doi: 10.1016/s0015-0282(16)56518-2. [DOI] [PubMed] [Google Scholar]
- 18.Adalatkhah H, Pourfarzi F, Sadeghi-Bazargani H. Flutamide versus a cyproterone acetate-ethinyl estradiol combination in moderate acne: a pilot randomized clinical trial. Clin Cosmet Investig Dermatol. 2011;4:117–121. doi: 10.2147/CCID.S20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi Y, Hearing VJ. Physiological factors that regulate skin pigmentation. Biofactors. 2009;35(2):193–199. doi: 10.1002/biof.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi Y, Morita A, Maeda A, Hearing VJ. Regulation of skin pigmentation and thickness by Dickkopf 1 (DKK1) J Investig Dermatol Symp Proc. 2009;14(1):73–75. doi: 10.1038/jidsymp.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taheri A, Mansoori P, Sandoval LF, Feldman SR. Treatment of Becker nevus with topical flutamide. J Am Acad Dermatol. 2013;69(3):e147–e148. doi: 10.1016/j.jaad.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Kackar RR, Desai HG. Hepatic failure with flutamide. Indian J Gastroenterol. 2003;22(4):149–150. [PubMed] [Google Scholar]
- 23.Guldvog B. Can patient satisfaction improve health among patients with angina pectoris? Int J Qual Health Care. 1999;11:233–240. doi: 10.1093/intqhc/11.3.233. [DOI] [PubMed] [Google Scholar]
- 24.Asadi-Lari M, Tamburini M, Gray D. Patients’ needs, satisfaction, and health related quality of life: towards a comprehensive model. Health Qual Life Outcomes. 2004;2:32. doi: 10.1186/1477-7525-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendoza CG, Singzon IA, Handog EB. A randomized, double-blind, placebo-controlled clinical trial on the efficacy and safety of 3% Rumex occidentalis cream versus 4% hydroquinone cream in the treatment of melasma among Filipinos. Int J Dermatol. 2014;53(11):1412–1416. doi: 10.1111/ijd.12690. [DOI] [PubMed] [Google Scholar]
- 26.Monteiro RC, Kishore BN, Bhat RM, Sukumar D, Martis J, Ganesh HK. A comparative study of the efficacy of 4% hydroquinone vs 0.75% kojic acid cream in the treatment of facial melasma. Indian J Dermatol. 2013;58(2):157. doi: 10.4103/0019-5154.108070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iraji F, Tagmirriahi N, Gavidnia K. Comparison between the efficacy of 10% zinc sulfate solution with 4% hydroquinone cream on improvement of melasma. Adv Biomed Res. 2012;1:39. doi: 10.4103/2277-9175.100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farshi S. Comparative study of therapeutic effects of 20% azelaic acid and hydroquinone 4% cream in the treatment of melasma. J Cosmet Dermatol. 2011;10(4):282–287. doi: 10.1111/j.1473-2165.2011.00580.x. [DOI] [PubMed] [Google Scholar]
- 29.Navarrete-Solis J, Castanedo-Cazares JP, Torres-Alvarez B, et al. A double-blind, randomized clinical trial of niacinamide 4% versus hydroquinone 4% in the treatment of melasma. Dermatol Res Pract. 2011;2011:379173. doi: 10.1155/2011/379173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg VK, Sarkar R, Agarwal R. Comparative evaluation of beneficiary effects of priming agents (2% hydroquinone and 0.025% retinoic acid) in the treatment of melasma with glycolic acid peels. Dermatol Surg. 2008;34(8):1032–1039. doi: 10.1111/j.1524-4725.2008.34202.x. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira CT, Hassun K, Sittart A, De Lourdes Viegas M. A comparison of triple combination cream and hydroquinone 4% cream for the treatment of moderate to severe facial melasma. J Cosmet Dermatol. 2007;6(1):36–39. doi: 10.1111/j.1473-2165.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 32.Espinal-Perez LE, Moncada B, Castanedo-Cazares JP. A double-blind randomized trial of 5% ascorbic acid vs 4% hydroquinone in melasma. Int J Dermatol. 2004;43(8):604–607. doi: 10.1111/j.1365-4632.2004.02134.x. [DOI] [PubMed] [Google Scholar]


