Abstract
Background
Deep brain stimulation (DBS) electrode impedance is a major determinant of current delivery to target tissues, but long-term variation in impedance has received little attention.
Objectives
To assess the relationship between electrode impedance and time in a large DBS patient population and characterize the relationship between contact activity and impedance.
Methods
We collected retrospective impedance and programming data from 128 electrodes in 84 patients with Parkinson's disease, essential tremor, or dystonia. Effects of time, contact activity, stimulation voltage, and other parameters on impedance were assessed. We also examined impedance changes following contact activation and deactivation.
Results
Impedance decreased by 73 Ω/year (P < .001), with 72% of contacts following a downward trend. Impedance was on average 163 Ω lower in active contacts (P < .001). Contact activation and inactivation were associated with a more (P < .001) and less (P = .016) rapid decline in impedance, respectively. Higher stimulation voltages were associated with lower impedance values (P < .001). Contact number and electrode model were also significant predictors of impedance.
Conclusions
Impedance decreases gradually in a stimulation-dependent manner. These trends have implications for long-term programming, the development of a closed-loop DBS device, and current understanding of the electrode-tissue interface.
Keywords: deep brain stimulation, electrical stimulation, movement disorders
Introduction
Deep brain stimulation (DBS) is used in the treatment of certain movement and psychiatric disorders, including Parkinson's disease (PD), essential tremor (ET), dystonia, and obsessive-compulsive disorder. DBS therapy entails surgical implantation of an electrode into a specific brain target. The electrode is then connected via a subcutaneous extension cable to an implantable pulse generator (IPG) that provides sustained stimulation. Stimulation parameters (voltage or current, frequency, pulse width, specific contact(s), and polarity of stimulation) are selected in order to optimize therapeutic benefit in the individual patient. Electrical current delivery to the intended tissues is opposed by impedance, which is the resistance to current flow in an alternating current circuit [1-4]. The value of impedance depends in turn on the stimulation parameters selected [5]. In constant-voltage IPGs, the voltage of pulses from the IPG is controlled, but the current delivered varies based on impedance. Since electrode lifetime can be a decade or longer [6], changes in impedance over time may have significant implications for long-term DBS programming and efficacy, as well as for closed-loop DBS devices being developed to mitigate the need for reprogramming [7-10]. Moreover, identification of the factors responsible for variation in impedance is crucial for understanding current transfer from electrode to tissue, which is a key step in the mechanism of DBS.
Two types of impedance can be measured in clinical DBS systems: therapy and electrode impedance. Therapy impedance represents the resistance to current flow for the specific stimulation parameters being used to treat the patient. Electrode impedance is measured at standardized stimulation parameters and is monitored clinically because abnormal values may indicate electrode dysfunction such as a short or open circuit [11]. Impedance varies significantly between patients and has a substantial effect on the extent of tissue activation, as it affects the amount of current being delivered to the brain. A major determinant of impedance is the extent of the foreign body reaction around the electrode contacts [3]. The long-term tissue response to electrode implantation is characterized by gliosis, formation of a glial fibrillary acidic protein (GFAP)-positive capsule, and emergence of giant cells [12-14]. A higher degree of encapsulation has been associated with larger impedance values [15,16].
Several studies have described impedance changes over hours to days. Impedance has been found to decrease reversibly in the hours following acute stimulation [2,17,18]. One study found that in patients with PD, impedance decreases over the two days following surgery, but returns to two-thirds of the initial value after one month [19,20]. A trial of DBS in non-human primates found that impedance increases in a dramatic, transient manner in the first week following implantation and stabilizes at an elevated value at approximately 100 days after surgery [17]. These observations have been attributed to electrical stimulation causing the separation of proteins and cells from the electrode, possibly via the formation of an oxidative film surrounding the DBS electrode [17,18].
Four studies to date have examined long-term electrode impedance changes in patients undergoing DBS. A study of 24 patients with dystonia showed a stimulation-dependent decrease in impedance over the course of one year following surgery [21]. Recent work by our group found that in 18 patients with PD, impedance decreased over a three-year period and was lower in active contacts [22]. Conversely, in 191 patients receiving subdural and deep brain stimulation for epilepsy, impedance fluctuated during the first six months and was constant from one to four years after electrode implantation [23]. Lastly, a study of 94 patients with various movement disorders followed for up to five years after surgery found that impedance decreased over time and with contact activity, and varied by target, contact number, and cerebral hemisphere [24].
In the present study, we quantified the relationship between electrode impedance and time in a large DBS movement disorder population, including patients with PD, ET, and dystonia. In addition, we characterized the relationship between contact activity and impedance. Given the findings of our recent smaller-scale study [22], we hypothesized that impedance would decrease over time, with lower impedances in active versus inactive contacts. Based on the evidence that electrical stimulation decreases impedance by causing tissue to separate from active contacts, we further hypothesized that impedance would decrease more rapidly in contacts following activation and less rapidly following deactivation, and that higher stimulation voltages would be associated with lower impedances.
Methods
Study design and population
This study was approved by the University of Minnesota Institutional Review Board. Data were collected retrospectively from the electronic medical record (EMR) system, and were derived from those patients with PD, ET, or dystonia who underwent DBS electrode implantation by the author (AA), from 2006-2011. Electrodes implanted in separate hemispheres of the same patient were analyzed independently.
Inclusion criteria for DBS surgery at the University of Minnesota Medical Center have been published previously [9,25]. Subjects were included in this analysis if they possessed one or more DBS electrodes connected to a single-channel Medtronic Soletra IPG (model #7426; Medtronic Corporation; Fridley, MN), and had at least one DBS programming visit during which impedance values were recorded in the EMR. We excluded electrodes in the event of substantial post-operative complications, specifically electrode infection (N = 4), hydrocephalus (N = 1), or subdural hematoma (N = 1). Electrodes were also excluded if their location was ever surgically revised due to suboptimal placement, hardware malfunction, or lack of benefit with stimulation (N = 11). In the case of IPG replacement with an IPG other than the Soletra model, we recorded data only during the lifetime of the Soletra IPG, so as to eliminate the potential variable of including impedance data collected using a different IPG model.
Data collection
Electrode impedance values were measured during DBS device programming visits with a programming wand (N'Vision model #8840) at 30 Hz and 1.5 V. Demographic data obtained from the EMR included indication for DBS (diagnosis), DBS anatomical target, target laterality (left or right), and electrode model. For each programming visit, we recorded the date, each contact's status (active or inactive), each contact's impedance, and voltage, all measured prior to any programming changes made during that particular visit. Study data were collected and managed using REDCap electronic data capture tools, hosted by the Clinical and Translational Science Institute (CTSI) of the University of Minnesota [26].
Statistical analysis
Baseline impedance differences between contacts selected to be active or inactive in the initial programming visit were compared using an unpaired, two-tailed t-test. A Mann-Whitney-Wilcoxon test was also carried out due to minor skew in the initial impedances. Finally, to address the possibility that the impedances within electrodes (across contacts) might not be independent, we examined the effect of initial contact activity status on impedance using a mixed linear regression model. In this test, contact status (active or inactive) was analyzed as a fixed effect, and a random effect for electrode was introduced to account for variation between electrodes.
We examined the effects of time and contact activity on electrode impedance using a mixed linear regression model. Time since implantation and contact status were analyzed as fixed effects, and a random effect for electrode was again included. Data were analyzed for an interaction effect between time and contact activity. Similar parallel analyses were carried out for diagnosis, anatomical target, electrode model, target laterality, and contact number, each included with time as a fixed effect. Lastly, we performed a Pearson's chi-squared goodness-of-fit test comparing the number of visits in which each contact number was active to the expected number of such visits.
To demonstrate the variability in the time course of impedance, linear regression for impedance versus time was performed separately for each contact within each electrode. Because the individual regressions were often based upon a small number of measurements (2-5), several extreme outliers were found in the slopes of these regression lines. These outliers were discarded using the conservative 3×IQR approach, in which slopes that lay more than three multiples of the inter-quartile range (IQR) below the first quartile or above the third quartile are identified as extreme outliers. Mean and standard deviation were subsequently calculated for the regression slopes.
To further explore effects of contact activity on impedance, we took the impedance value of each contact from a given visit and subtracted that contact's impedance value from the previous visit; these differences were then classified by relative contact activity in the given visit versus the previous visit (i.e., active to active, inactive to inactive, active to inactive, or inactive to active).
A mixed linear regression model was again used, this time with the dependent variable as impedance change, the fixed effects as time between visits and relative contact activity, and a random effect for electrodes. Visit pairs more than two years apart were not included so as to avoid giving undue statistical influence to a small number of distant visit pairs.
The influence of the degree of contact electrical activity on impedance was assessed with a mixed linear regression model comparing impedance (at a given visit) to programming voltage (recorded at the given visit, but set at the prior visit). Voltage was considered a fixed effect, and a random effect for electrodes was again introduced. All statistical analyses were performed with SAS version 9.2 (SAS Institute Inc.; Cary, NC).
Results
Demographics
Data from 128 DBS electrodes in 84 patients met criteria for inclusion in this study. Subject follow-up ranged from 34 days to 6.5 years, with a median of 2.7 years. Demographic statistics are provided in Table 1. The patient population included PD (N = 64), ET (N = 14), dystonia (N = 1), and an additional patient with mixed PD and ET features. In PD patients, 93 of 98 electrodes were located in the subthalamic nucleus (STN), while the remaining 5 targeted the globus pallidus pars interna (GPi). All electrodes in ET patients were implanted in the ventral intermediate nucleus of the thalamus (VIM), and all electrodes in dystonia patients were situated in GPi. Medtronic model #3389 electrodes were used exclusively in PD patients, while Medtronic model #3387 electrodes were used in ET and dystonia patients as well as a small number of PD patients (4 STN and 5 GPi electrodes).
Table 1. Patient demographics and electrode information.
| Electrodes (patients) | |
|---|---|
| Diagnosis | |
| Parkinson's disease (PD) | 98 (64) |
| Essential tremor (ET) | 20 (14) |
| Mixed PD and ET features | 1 (1) |
| Dystonia | 9 (5) |
| Total | 128 (84) |
|
| |
| Target | |
| STN | 94 |
| GPi | 14 |
| VIM | 20 |
|
| |
| Electrode | |
| Medtronic #3387 | 38 |
| Medtronic #3389 | 90 |
|
| |
| Hemisphere | |
| Left | 75 |
| Right | 53 |
Time and contact activity
A total of 2022 individual contact impedance measurements were recorded. Figure 1 shows these impedance values plotted as a function of time and contact activity. Impedances recorded in the initial programming visit, before any stimulation had occurred, were compared between contacts selected to be active or inactive. T-testing, nonparametric Mann-Whitney-Wilcoxon testing, and mixed linear regression model analysis all demonstrated no significant difference in baseline impedance (P = .51, P = .54, and P = .40, respectively). The mixed linear regression analysis yielded a significant effect for both time (P < .001) and contact activity (P < .001) on impedance (Table 2). Impedance decreased by 73 Ω/year, and active contacts had lower impedances than inactive contacts by 163 Ω. No significant interaction was found between time and contact activity (P = .53).
Figure 1.
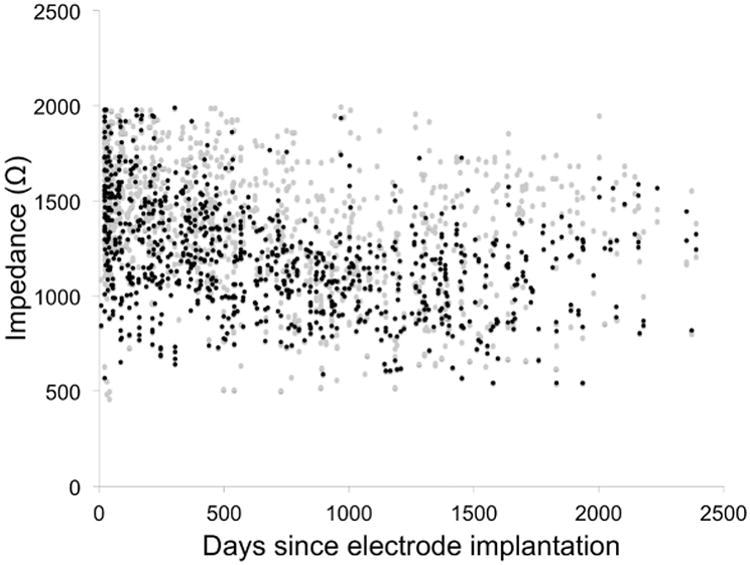
Impedance versus time since electrode implantation. Black points represent active contacts and gray points represent inactive contacts.
Table 2. Mixed linear regression findings for fixed effects paired with time since electrode implantation.
| Impedance | Effect (Ω) | P | |||
|---|---|---|---|---|---|
| Time | -73/year | < .001 | |||
| Contact activity | Inactive | > | Active | 163 | < .001 |
|
| |||||
| Diagnosis | PD | > | ET | 171 | < .001 |
| PD | > | Dystonia | 310 | < .001 | |
| ET | ≈ | Dystonia | - | .08 | |
|
| |||||
| Anatomical target | STN | > | GPi | 246 | < .001 |
| STN | > | VIM | 173 | < .001 | |
| GPi | ≈ | VIM | - | .30 | |
|
| |||||
| Electrode | #3389 | > | #3387 | 181 | < .001 |
|
| |||||
| Hemisphere | Left | ≈ | Right | - | .18 |
In order to assess variability in the temporal course of impedance, we carried out simple regression analysis for each contact. The mean slope was -80 Ω/year with a standard deviation of 183 Ω/year, and 72% of the slopes were negative. The distribution of these individual impedance time courses is illustrated in Figure 2.
Figure 2.
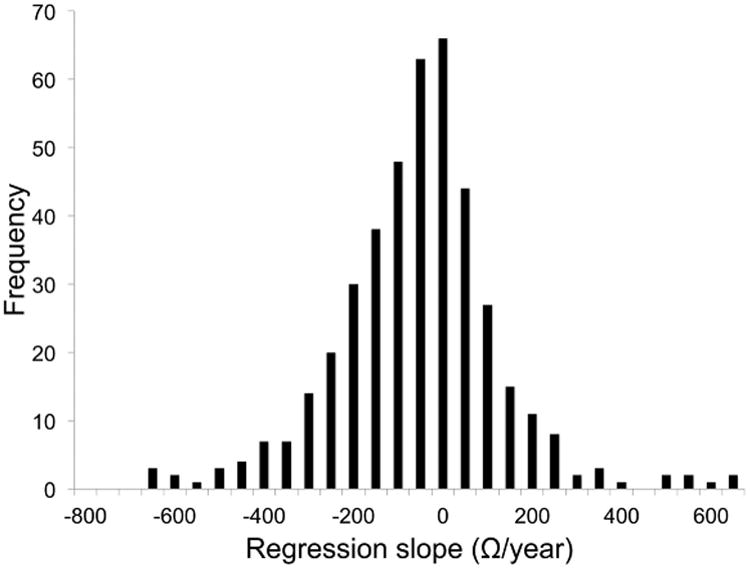
Variation in impedance trends by contact. Figure illustrates distribution of slopes from individual simple linear regression analysis of impedances calculated for each contact. Mean = -80 Ω/year, standard deviation = 183 Ω/year. 72% of the slopes were negative. Compare mean slope to the mixed linear regression effect size of -73 Ω/year.
Diagnosis, target, electrode model, and target laterality
We also evaluated diagnosis, anatomical target, electrode model, and target laterality (right vs. left cerebral hemisphere) as independent predictors of impedance after controlling for time following electrode implantation (Table 2). The impedance of electrodes implanted in PD patients was higher than electrodes implanted in ET patients (by 171 Ω, P < .001) and dystonia patients (by 310 Ω, P < .001); there was a marginally significant difference between electrode impedances in ET and dystonia patients (P = .08). Electrodes located in STN were characterized by higher impedances than those in GPi (by 246 Ω, P < .001) and VIM (by 173 Ω, P < .001); there was not a significant difference between impedances of electrodes implanted in GPi versus VIM. Impedances were 181 Ω higher for Medtronic model #3389 electrodes than for model #3387 electrodes (P < .001). Finally, no significant difference was identified between impedances recorded from left-hemispheric versus right-hemispheric electrodes (P = .18).
Contact number
Contact activity and impedance also varied by contact number (Table 3). The likelihood of a given contact being active in any given programming visit varied significantly (P < .001) between contacts, with usage rates lower in the outer contacts (29% and 44% in contacts 3 and 0, respectively) and higher in the middle contacts (49% and 51% in contacts 1 and 2, respectively). Mean impedance followed a similar trend, with higher impedances in the outer contacts (1347 and 1309 Ω in contacts 0 and 3, respectively) and lower impedances in the middle contacts (1265 and 1230 Ω in contacts 1 and 2, respectively). Mixed linear regression identified significant differences between all contact numbers (P = .015 for contacts 0 vs. 3, P = .013 for contacts 1 vs. 2, and P < .001 for all other comparisons).
Table 3. Impedance differences between contacts.
Usage refers to the relative frequency of a contact being active; this likelihood varied significantly between contacts (P < .001).
Impedances with the same symbol (* or †) differed with P < .05 while impedances with different symbols differed with P < .001.
Contact activation/deactivation and voltage
Impedance also depended on contact activation or deactivation, as well as voltage of stimulation. We found significant effects from activating or deactivating contacts during the programming visit following the change. Relative to a contact left off in both visits, activation of a contact was associated with a more rapid decline in impedance (121 Ω lower at the follow-up visit, P < .001), while deactivation of a contact was associated with a less rapid decline in impedance (81 Ω higher, P = .016). Contacts left active were similar to those left inactive (P = 0.12). Impedance decreased significantly (P < .001) with increasing voltage, at a rate of -150 Ω /V.
Discussion
Changes in impedance over time may affect the long-term stability of both electrical current delivery and detection of local field potentials [22,27]. Consequently, these temporal patterns have implications for long-term DBS device programming and for the design of a closed-loop DBS system. Additionally, elucidation of the factors that underlie variation in DBS electrode impedance is essential for understanding the electrode-tissue interface. This retrospective study was designed to assess longitudinal trends in electrode impedance in a large patient population, as well as to elucidate the effects of contact activity on impedance. In addition to time and contact activity, we also examined variation in impedance by indication for DBS surgery, anatomical target, electrode model, and contact number.
Effects of time and stimulation on impedance
Changes in impedance over time have received little attention in the DBS literature but have important implications for device programming, current delivery to target brain structures, and DBS efficacy [1-3,5]. Trials in human patients and animal models have documented fluctuations in impedance during the first 30-100 days following implantation [17,19,20]; beyond this period there have been four previous reports published. Consistent with these prior studies of long-term changes in human DBS populations [21-24], we found that on average, impedance decreased slowly over time, at a rate of 73 Ω/year. We did not observe any evidence of the previously described stabilization in impedance after one year [23]. Over the study period, impedance decreased in 72% of individual contacts (Figure 2). Thus, while impedance values varied significantly between electrodes, a large majority of electrode impedances followed a similar downward trend. Although a decrease in impedance by 73 Ω/year is unlikely to have major effects on therapeutic efficacy, those patients in whom a more rapid decline in impedance was noted may have experienced significant changes in symptom control and adverse effects. Future research examining the relationship between impedance variability and clinical outcome would serve to clarify the practical implications of these changes.
We found that electrically active contacts had lower impedances than inactive contacts, also consistent with past work [22,24]. In order to explore this relationship further, we examined the consequences of contact activation and deactivation at the subsequent follow-up programming visit, as well as the effects of stimulation voltage on impedance. Relative to contacts left in the off state, activated contacts decreased more in impedance while deactivated contacts decreased less. Higher voltages were associated with lower impedances. This description of contact activity and stimulation magnitude constitutes a novel characterization of the effects of stimulation on impedance and provides evidence for a causal relationship in which electrical activity reduces impedance.
Histological encapsulation of the electrode due to the foreign body response has a significant effect on impedance [3,15]. More extensive gliosis and a thicker capsule are associated with higher impedance values [12,15]. A possible explanation for gradually decreasing impedance over time is accumulation of cerebrospinal fluid (CSF) around the electrode, such as might be observed in cerebral atrophy of the sort seen in PD [28] or with normal aging [29]. Consistent with this hypothesis, the blood-brain barrier has been found to be more permeable in the vicinity of implanted electrodes [30]. While histological studies do not mention peri-electrode CSF accumulation [12], these fluid spaces may not be readily apparent in postmortem tissue specimens. In the 28% of contacts associated with increasing impedance over time, ongoing electrode encapsulation may have outweighed CSF accumulation.
Previous authors have proposed that contact stimulation decreases tissue adhesion to the DBS electrode by oxidation at the electrode-tissue interface, and in fact, acute stimulation has been used in a research context to reduce impedance [2,17,18]. We have shown that impedance is dependent on contact activity status as well as the degree of activity (i.e., voltage). The diminished decay in impedance observed following electrode deactivation may reflect re-adhesion of tissue structures to the electrode, while the accelerated decrease in impedance with activation may be due to acute separation of the capsule elements from the electrode. We found that impedance decreases over time in both active and inactive contacts, which suggests that our explanations for decreasing impedance over time and with contact activity are compatible. Future biochemical and histological work—for example, quantitative evaluation of peri-electrode fluid—may elucidate the specific tissue processes that underlie these impedance trends.
Electrode model and impedance
We found significant effects of indication for DBS surgery, target nucleus, and electrode model on impedance. Higher impedances were found in patients with PD, electrodes implanted in STN, and Medtronic model #3389 electrodes. Patients with ET or dystonia and Medtronic model #3387 electrodes implanted in VIM or GPi were associated with lower impedance values. Past research has similarly found higher impedance in electrodes implanted in STN relative to those implanted in GPi [24], but the relationship between impedance, surgical indication, and electrode model has not previously been examined. Given that all ET and dystonia patients were implanted with model #3387 electrodes, the absence of significant impedance differences between ET-VIM and dystonia-GPi stimulation suggests that electrode model accounts for most—if not all—of the variability in impedance among the potential variables assessed. A difference is not surprising due to the geometric configurations of the two electrode models studies here, which vary only in the spacing between contacts (1.5 mm and 0.5 mm in the model #3387 and #3389 electrodes, respectively). A computer modeling study found the opposite result from the present study— with higher impedances associated with model #3387 rather than model #3389 electrodes— although that study analyzed bipolar rather than monopolar stimulation [3]. The higher average impedance observed in the more closely spaced contacts, as well as the presence of this difference during monopolar stimulation (in which adjacent contacts are inactive), is somewhat surprising and does not immediately suggest an explanatory mechanism. However, the presence of electric fields around inactive contacts [31] may be relevant.
It should be noted that the impedance difference between ET and dystonia did not reach statistical significance but suggested the presence of a trend (P = 0.08). Impedance may be affected by other target-specific factors not assessed in this study, such as degree of myelination within grey matter, as well as contact location relative to the intended target. However, the simplest explanation for impedance differences observed in these different disorders and anatomic targets is variation in the electrodes used based on indication and target.
Contact location and impedance
We found that both contact usage and impedance varied by contact number. Consistent with past research [24,32], the middle electrode contacts (1 and 2) were used more frequently than the outer contacts (0 and 3) and were associated with lower impedance values. Lower impedance in the middle contacts may be related to stimulation through optimally located contacts, as follows from the evidence above that electrical stimulation decreases impedance. Additionally, because contact number may be a proxy for anatomic location, middle contacts may have lower impedance by virtue of being more likely than outer contacts to reside in grey matter, which itself is characterized lower impedance than white matter [33,34]. This latter possibility is opposed by our observation that baseline impedance—measured during the initial postoperative programming visit, prior to the delivery of any stimulation—was similar in contacts selected to be active compared to contacts selected to be inactive. There remains some controversy regarding whether maximal benefit arises from stimulation of the grey matter of STN itself, or from stimulation of the white matter of the zona incerta [35,36]. However, if it is the case that contacts located in grey matter are selected more often than contacts in white matter as a result of symptomatic benefit during programming, impedance may reflect the anatomic position of a given contact.
Study limitations
The retrospective design of this study presents some limitations to data interpretation. First, not all programming visits were recorded in the medical record. This is due to a combination of recent institutional EMR changes as well as the occurrence of some patient programming visits outside of our hospital system. Many patients had large gaps between recorded visits, across which temporal trends were extrapolated. Furthermore, these visits fell between some visits that we considered to be consecutive for the purposes of the contact activation-deactivation analysis.
Second, our findings pertain to electrode impedance (measured at uniform stimulation parameters) rather than therapy impedance (measured at patient-specific stimulation parameters). The use of electrode impedance instead of therapy impedance was necessary to ensure valid comparison between impedances recorded from different patients and at different time points. The measurement voltage for impedance testing (1.5 V) is consistent with typical therapeutic stimulation parameters, and while the measurement frequency (30 Hz) is lower than typical therapeutic stimulation frequency, impedance measurements at lower frequency reflect the electrode-tissue interface impedance more specifically than impedance measurements at higher frequency [37]. Additionally, while therapy impedance constitutes the barrier to therapeutic current flow in a specific patient [4], monopolar electrode impedance reflects the microenvironment of each contact [3]. Therefore, the aggregate electrode impedance for all active contacts should be closely related to therapy impedance.
Third, while the manufacturer of the Soletra IPG has estimated the accuracy of the device's impedance measurements to be ± 50-100 Ω, the precise accuracy is difficult to report due to a number of variables involved (e.g. stimulation parameters and daily impedance changes). Additionally, the accuracy of these measurements has been reported to decline with decreasing IPG charge [38]. Nonetheless, the effect sizes in the present study all exceed 100 Ω.
Fourth, the programming wand used to check impedance values in this study had a maximum measurement threshold of 2000 Ω. The impedance testing voltage is often increased in order to drive impedance below 2000 Ω and rule out the presence of an open circuit, but for consistency we did not include impedances recorded at test voltages other than 1.5 V. Impedances above 2000 Ω were subjectively observed to occur more frequently in early postoperative programming visits, and excluding these impedance recordings may have resulted in an underestimation of the effect of time and other variables on impedance.
Finally, this study was restricted to electrodes associated with an older voltage-controlled IPG model in order to ensure a large sample size. Current-controlled IPGs have recently been developed to minimize the effect of impedance changes on voltage distribution [2]. Repeating this study using the newer, current-controlled IPGs might yield different results in electrode impedance trends and relationships.
Conclusions
In this retrospective study, we examined trends in electrode impedance in a large population of patients with movement disorders receiving stimulation through implanted, constant-voltage, DBS devices. We present evidence for a downward trend in impedance over time, as well as a potentially causal effect in which electrical stimulation led to lower impedance values, and contact deactivation slowed the rate of decline in impedance over time. Our results suggest that impedance variation between different disorders and targets is predominantly due to the use of different electrode models. Finally, we found that impedance was lower in the middle electrode contacts (1 and 2), due to stimulation at those contacts and/or to anatomical location within grey matter.
The relationships between time, contact activity, and impedance found in this study may be due to increased volume of a CSF-filled channel around the implanted electrode—associated with progressive cerebral atrophy—and current-induced separation of glial capsule components from the electrode. The trends reported here have implications for DBS programming over the years following surgery. In addition, these effects need to be considered in the development of novel closed-loop DBS devices that rely on feedback—of both impedances and brain signals—rather than external device programming by clinical personnel. Lastly, the dependence of impedance upon time, stimulation, and electrode model provides insight into the nature of the electrode-tissue interface and the current transfer that constitutes a critical step in the mechanism of DBS.
Acknowledgments
Financial support for REDCap and the Clinical and Translational Science Institute comes from grant UL1TR000114 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
References
- 1.Miocinovic S, Lempka SF, Russo GS, Maks CB, Butson CR, Sakaie KE, et al. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Exp Neurol. 2009;216:166–176. doi: 10.1016/j.expneurol.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lempka SF, Johnson MD, Miocinovic S, Vitek JL, McIntyre CC. Current-controlled deep brain stimulation reduces in vivo voltage fluctuations observed during voltage-controlled stimulation. Clin Neurophysiol. 2010;121:2128–2133. doi: 10.1016/j.clinph.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butson CR, Maks CB, McIntyre CC. Sources and effects of electrode impedance during deep brain stimulation. Clin Neurophysiol. 2006;117:447–454. doi: 10.1016/j.clinph.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery EB. Deep Brain Stimulation Programming: Principles and Practice. Birmingham, AL: Oxford University Press; 2010. [Google Scholar]
- 5.Wei XF, Grill WM. Impedance characteristics of deep brain stimulation electrodes in vitro and in vivo. J Neural Eng. 2009;6 doi: 10.1088/1741-2560/6/4/046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Oroz MC, Moro E, Krack P. Long-term outcomes of surgical therapies for Parkinson's disease. Mov Disord. 2012;27:1718–1728. doi: 10.1002/mds.25214. [DOI] [PubMed] [Google Scholar]
- 7.Kringelbach ML, Jenkinson N, Owen SL, Aziz TZ. Translational principles of deep brain stimulation. Nat Rev Neurosci. 2007;8:623–635. doi: 10.1038/nrn2196. [DOI] [PubMed] [Google Scholar]
- 8.Pedoto G, Santaniello S, Montgomery EB, Gale JT, Fiengo G, Glielmo L, et al. System identification of local field potentials under deep brain stimulation in a healthy primate. Conf Proc IEEE Eng Med Biol Soc. 2010:4144–4147. doi: 10.1109/IEMBS.2010.5627356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ince NF, Gupte A, Wichmann T, Ashe J, Henry T, Bebler M, et al. Selection of optimal programming contacts based on local field potential recordings from subthalamic nucleus in patients with Parkinson's disease. Neurosurgery. 2010;67:390–397. doi: 10.1227/01.NEU.0000372091.64824.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinberger M, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson's disease patients. J Neurophysiol. 2009;101:789–802. doi: 10.1152/jn.90837.2008. [DOI] [PubMed] [Google Scholar]
- 11.Allert N, Markou M, Miskiewicz AA, Nolden L, Karbe H. Electrode dysfunctions in patients with deep brain stimulation: a clinical retrospective study. Acta Neurochir (Wien) 2011;153:2343–2349. doi: 10.1007/s00701-011-1187-y. [DOI] [PubMed] [Google Scholar]
- 12.Haberler C, Alesch F, Mazal PR, Pilz P, Jellinger K, Pinter MM, et al. No tissue damage by chronic deep brain stimulation in Parkinson's disease. Ann Neurol. 2000;48:372–376. [PubMed] [Google Scholar]
- 13.Yousif N, Bayford R, Bain PG, Liu X. The peri-electrode space is a significant element of the electrode-brain interface in deep brain stimulation: a computational study. Brain Res Bull. 2007;74:361–368. doi: 10.1016/j.brainresbull.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun DA, Yu H, Spooner J, Tatsas AD, Davis T, Abel TW, et al. Postmortem analysis following 71 months of deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2008;109:325–329. doi: 10.3171/JNS/2008/109/8/0325. [DOI] [PubMed] [Google Scholar]
- 15.Williams JC, Hippensteel JA, Dilgen J, Shain W, Kipke DR. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007;4 doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- 16.Grill WM, Mortimer JT. Electrical properties of implant encapsulation tissue. Ann Biomed Eng. 1994;22:23–33. doi: 10.1007/BF02368219. [DOI] [PubMed] [Google Scholar]
- 17.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6 doi: 10.1088/1741-2560/6/4/046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans Neural Syst Rehabil Eng. 2005;13:160–165. doi: 10.1109/TNSRE.2005.847373. [DOI] [PubMed] [Google Scholar]
- 19.Rosa M, Marceglia S, Servello D, Foffani G, Rossi L, Sassi M, et al. Time dependent subthalamic local field potential changes after DBS surgery in Parkinson's disease. Exp Neurol. 2010;222:184–190. doi: 10.1016/j.expneurol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Rosa M, Giannicola G, Servello D, Marceglia S, Pacchetti C, Porta M, et al. Subthalamic local field beta oscillations during ongoing deep brain stimulation in Parkinson's disease in hyperacute and chronic phases. Neurosignals. 2011;19:151–162. doi: 10.1159/000328508. [DOI] [PubMed] [Google Scholar]
- 21.Hemm S, Vayssiere N, Mennessier G, Cif L, Zanca M, Ravel P, et al. Evolution of brain impedance in dystonic patients treated by GPi electrical stimulation. Neuromodulation. 2004;7:67–75. doi: 10.1111/j.1094-7159.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 22.Abosch A, Lanctin D, Onaran I, Eberly L, Spaniol M, Ince NF. Long-term recordings of local field potentials from implanted deep brain stimulation electrodes. Neurosurgery. 2012;71:804–814. doi: 10.1227/NEU.0b013e3182676b91. [DOI] [PubMed] [Google Scholar]
- 23.Sillay KA, Rutecki P, Cicora K, Worrell G, Drazkowski J, Shih JJ, et al. Long-term measurement of impedance in chronically implanted depth and subdural electrodes during responsive neurostimulation in humans. Brain Stimul. 2013;6:718–726. doi: 10.1016/j.brs.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Cheung T, Nuno M, Hoffman M, Katz M, Kilbane C, Alterman R, et al. Longitudinal impedance variability in patients with chronically implanted DBS devices. Brain Stimul. 2013;6:746–751. doi: 10.1016/j.brs.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 25.Keane M, Deyo S, Abosch A, Bajwa JA, Johnson MD. Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor. J Neural Eng. 2012;9 doi: 10.1088/1741-2560/9/4/046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stypulkowski PH, Stanslaski SR, Denison TJ, Giftakis JE. Chronic evaluation of a clinical system for deep brain stimulation and recording of neural network activity. Stereotact Funct Neurosurg. 2013;91:220–232. doi: 10.1159/000345493. [DOI] [PubMed] [Google Scholar]
- 28.Burton EJ, McKeith IG, Burn DJ, Williams ED, O'Brien JT. Cerebral atrophy in Parkinson's disease with and without dementia: a comparison with Alzheimer's disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 29.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 30.Winslow BD, Tresco PA. Quantitative analysis of the tissue response to chronically implanted microwire electrodes in rat cortex. Biomaterials. 2010;31:1558–1567. doi: 10.1016/j.biomaterials.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 31.Hemm S, Mennessier G, Vayssiere N, Cif L, El Fertit H, Coubes P. Deep brain stimulation in movement disorders: stereotactic coregistration of two-dimensional electrical field modeling and magnetic resonance imaging. J Neurosurg. 2005;103:949–955. doi: 10.3171/jns.2005.103.6.0949. [DOI] [PubMed] [Google Scholar]
- 32.Connolly PJ, Halpern CH, Baltuch GH, Danish SF, Jaggi JL. Implications for programming strategy of the location of the active contact in subthalamic nucleus deep brain stimulation. J Clin Neurosci. 2012;19:1029–1031. doi: 10.1016/j.jocn.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Haueisen J, Ramon C, Eiselt M, Brauer H, Nowak H. Influence of tissue resistivities on neuromagnetic fields and electric potentials studied with a finite element model of the head. IEEE Trans Biomed Eng. 1997;44:727–735. doi: 10.1109/10.605429. [DOI] [PubMed] [Google Scholar]
- 34.Gabriel C, Peyman A, Grant EH. Electrical conductivity of tissue at frequencies below 1 MHz. Phys Med Biol. 2009;54:4863–4878. doi: 10.1088/0031-9155/54/16/002. [DOI] [PubMed] [Google Scholar]
- 35.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115:2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129:1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 37.Ragheb T, Riegle S, Geddes LA, Amin V. The impedance of a spherical monopolar electrode. Ann Biomed Eng. 1992;20:617–627. doi: 10.1007/BF02368609. [DOI] [PubMed] [Google Scholar]
- 38.Patel NV, Oza CS, Wong S, Danish SF, Hargreaves EL. Impedance accuracy and reliability of Medtronic Soletra and Activa PC neuromodulation models as a function of battery charge. Society for Neuroscience; New Orleans, LA: 2012. [Google Scholar]


