Abstract
Objective
The objective of this study was to determine the prevalence and incidence of sleep disordered breathing (SDB) in pregnancy among high-risk women.
Study Design
This was a prospective, observational study. We recruited women with a body mass index (BMI) 30 ≥ kg/m2, chronic hypertension, pregestational diabetes, history of preeclampsia, and/or a twin gestation. Objective assessment of SDB was completed between 6 and 20 weeks and again in the third trimester. SDB was defined as an apnea–hypopnea index (AHI) ≥ 5, and further grouped into severity categories: mild (5–14.9), moderate (15–29.9) and severe (≥30). Subjects who had a normal AHI at the baseline (AHI < 5), but an abnormal study in the third trimester (AHI 5) were classified as having “new-onset” SDB.
Results
A total of 128 women were recruited. In early pregnancy 21, 6 and 3% had mild, moderate, or severe SDB, respectively. These frequencies increased to 35, 7, and 5% in the third trimester (p < 0.001). About 27% (n = 34) experienced a worsening of SDB during pregnancy; 26 were cases of new-onset SDB, while the other 8 had SDB in early pregnancy that worsened in severity. The incidence of new-onset SDB was 20%. The majority of these new-onset cases were mild.
Conclusions
SDB in early pregnancy is common in high-risk women and new-onset SDB occurs in 20% of these women.
Keywords: sleep disordered breathing, sleep apnea, pregnancy
Sleep disordered breathing (SDB) refers to a group of disorders characterized by abnormal respiratory patterns (e.g., apneas, hypopneas) or abnormal gas exchange (e.g., hypoxia) during sleep.1,2 Obstructive sleep apnea, the most common type of SDB, is characterized by airway narrowing during sleep that leads to respiratory disruption, hypoxia, and nocturnal arousals.
Pregnancy has been associated with several alterations in sleep and a high incidence of sleep disturbances.3 Many studies have demonstrated that SDB symptoms (snoring, excessive daytime sleepiness) are common in pregnancy and that the prevalence of SDB symptoms increases as pregnancy progresses.3–7 This progression is at least partly related to the weight gain, edema, and hyperemia of pregnancy that lead to upper airway narrowing and increased airway resistance. Women who start off pregnancy over-weight or obese, or who suffer from excessive fluid retention during pregnancy (e.g., preeclampsia) are particularly sensitive to these upper airway changes.
SDB has been linked to poor sleep and impaired daytime function, but also to other health outcomes, such as cardiovascular and metabolic disease, in nonpregnant populations.8–14 Recent data also have demonstrated an association between SDB and adverse pregnancy outcomes such as hypertensive disorders of pregnancy, preterm birth, and gestational diabetes.15–22 In a large retrospective cohort study, Bourjeily et al23 found that snoring, a common symptom of SDB, was associated with gestational hypertension/preeclampsia, even after adjusting for multiple factors, including body mass index (BMI) at delivery (adjusted odds ratio [AOR], 2.3; 95% confidence interval [CI], 1.4–4). In a large retrospective cohort of women with polysomnogram-con-firmed SDB (n = 791), Chen et al16 reported that SDB was associated with an increased risk of preeclampsia (AOR, 1.6; 95% CI, 2.16–11.26), gestational diabetes (AOR, 1.63; 95% CI, 1.07–2.48), and preterm birth (AOR, 2.31; 95% CI, 1.77–3.01). Such data underscore the potential importance of SDB to pregnancy outcomes, and the importance of gaining a better understanding of the epidemiology of this disorder in pregnancy. However, most of the research regarding the epidemiology of SDB in pregnancy is retrospective or cross-sectional, and the majority of studies have relied on self-reported symptom assessments. Few investigators have prospectively evaluated SDB across pregnancy using objective measures. The objective of this study was to evaluate the prevalence of and trends in SDB across pregnancy in a cohort of women at high risk for hypertensive disorders of pregnancy, preterm birth, and gestational diabetes mellitus.
Patients and Methods
This was a prospective, observational study. We sought to recruit a pregnancy cohort at greater risk for the adverse pregnancy outcomes that have been associated with SDB (i.e., hypertensive disorders of pregnancy, iatrogenic preterm birth, and gestational diabetes).15–20 Therefore, we recruited women with a self-reported prepregnancy BMI ≥ 30 kg/m2, chronic hypertension, pregestational diabetes (type 1 or type 2), history of prior preeclampsia, and/or a twin gestation. The study subjects were recruited as a convenience sample from ambulatory care practices at two University centers serving women with both private and public insurance. Objective assessment of SDB was completed once between 6 and 20 weeks and then again in the third trimester (between 28 and 37 weeks). At each study visit, subjects were also asked to complete a sleep questionnaire that included an assessment of snoring symptoms.
After signing informed consent, women completed at home, overnight sleep evaluation with the Watch-PAT100 (Itamar medical Ltd., Israel, ▶Fib. 1) during early pregnancy (between 6 and 20 weeks gestation) and were asked to repeat the study in late pregnancy (between 28 and 37 weeks gestation). The Watch-PAT, which has a peripheral arterial tonometry (PAT) finger plethysmograph and a standard oxygen saturation (SpO2 probe), allows the recording of the PAT signal, heart rate, and oxyhemoglobin saturation. Sleep time is estimated using an inbuilt actigraph.24 Analysis of these signals allows for the determination of a respiratory disturbance index (RDI), an apnea–hypopnea index (AHI), and an oxygen desaturation index (ODI), all of which are measures of SDB.
The Watch-PAT proprietary software algorithm was used to analyze the PAT signal amplitude along with the heart rate and Spo2 to estimate the RDI, AHI, and ODI. Specifically, an RDI event was scored if one of the following three criteria were met: (1) PAT amplitude reduction occurred with acceleration in the pulse rate or increase in wrist activity; (2) PAT amplitude reduction occurred with ≥ 3% oxyhemoglobin desaturation; or (3) ≥ 4% oxyhemoglobin desaturation occurred.25 The AHI includes only the last two of these events while the ODI incorporates only the third. Studies in nonpregnant populations have shown that the respiratory indices, such as the AHI, derived from the Watch-PAT are strongly correlated (r = 0.90) with those obtained from in-laboratory polysomnography (PSG), and have also demonstrated that the Watch-PAT is an accurate and reliable ambulatory method for the detection of sleep apnea (sensitivity, 89%; specificity, 69%).24–28 It is well established that certain biological parameters measured by the Watch-PAT are altered in pregnancy. Specifically, resting heart rate is known to increase while systemic vascular resistance decreases. However, the Watch-PAT scoring algorithm takes into consideration that every individual, pregnant or not, has a different baseline heart rate and peripheral arterial tone and therefore, events are scored when there is a change in baseline (increase in heart rate, increase in peripheral arterial tone).28 Pulse oximetry and actigraphy measures, also used by the Watch-PAT, should not be different when measured in pregnancy. Therefore, we believe that there is no good biologic foundation to suggest that the objective measures of the Watch-PAT will correlate less well with PSG measures just because a woman is pregnant. Moreover, O'Brien et al recently presented data comparing Watch-PAT to full PSG in third trimester pregnant subjects. Their results indicate that among pregnant women, the Watch-PAT AHI correlated very well with PSG AHI (r = 0.76, p < 0.0001) and that the Watch-PAT demonstrated excellent sensitivity (88%) and specificity (86%) for identification of SDB in pregnancy.29
SDB was defined as an AHI ≤ 5, and further subgrouped into severity categories: no SDB (0–4.9), mild SDB (5–14.9), moderate SDB (15–29.9), and severe SDB (≤30).2,30,31 SDB severity classification (none, mild, moderate, and severe) in early versus late pregnancy was compared and subjects were classified as having “no change in,” “improved,” or “worsening” SDB. Subjects who had a normal AHI at the baseline (AHI < 5), but an abnormal study in the third trimester (AHI ≤ 5) were classified as having “new-onset” SDB.
Differences between the baseline and third trimester SDB indices were compared using the paired t-test for continuous data or McNemar test for categorical data. Associations were explored between SDB patterns and patient characteristics through the use of the t-test for continuous variables and the chi-square and Fischer exact tests for categorical variables. All tests were two-tailed and p < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS 19.0 statistical software (SPSS Inc., Chicago, IL).
Accounting for the paired design and assuming the prevalence of SDB among this high-risk cohort to be 15% at baseline,32 we calculated that 105 subjects would be needed to detect a 10% increase in the SDB prevalence across pregnancy, with an α of 0.05 and a power of at least 80%. On the assumption of 30% study failure rate and 30% rate of loss to follow-up, we established that a sample size of 180 women would be required to complete the baseline study. This study was approved by the Institutional Review Board of Northwestern University and NorthShore University Hospital System.
Results
A total of 233 women consented to participate. Of the 233 women, 188 women had a valid early pregnancy sleep study, and 128 had a valid repeat study in the third trimester. The reasons for incomplete sleep data are presented in ▶Fig. 2. Our overall sleep study failure rate was 8.4%. This is consistent with data from numerous other studies that have used ambulatory devices for SDB diagnosis.26,33 The mean gestational age (±standard deviation) was 16.7 ± 3.5 and 32.6 ± 2.4 weeks at the first and second sleep study, respectively. Demographic characteristics of the study population are provided in ▶Table 1. About 62% of subjects were obese, 30% had chronic hypertension, 57% had pregestational diabetes, 16% had a prior history of preeclampsia, 6% had a twin gestation. About 54% of women had more than one qualifying risk factor. For analytical purposes, we chose to use self-reported prepregnancy BMI rather than BMI at first prenatal visit (mean, 32.8 vs. 33.2, respectively), given the very small difference between these two values, and the fact that all participants did not present for their first prenatal visit at the same gestational age.
Fig. 2.
Study recruitment. * 24% of study failures (7/29) were due to parcipant errors/non-compliance
Table 1.
Characteristics of study participants (N = 188)
| Age (y), mean ± SD | 33.0 ± 5.9 |
| Ethno-racial status | % |
| White | 39.24 |
| Black | 25.5 |
| Hispanic | 20.2 |
| Other | 14.9 |
| Prepregnancy BMI (kg/m2), mean ± SD | 32.8 ± 8.7 |
| Maternal history | % |
| Chronic hypertension | 29.8 |
| Pregestational diabetes | 57.4 |
| Twins | 5.9 |
| Nulliparous | 29.3 |
| Prior preeclampsia | 16.5 |
Abbreviations: BMI, body mass index; SD, standard deviation.
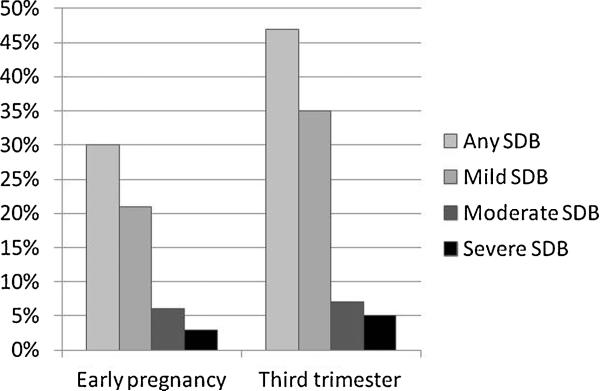
Mean RDI, AHI, and ODI values at baseline and in the third trimester are shown in ▶Table 2. All indices were significantly higher in the third trimester. The prevalence and severity of SDB also increased in the third trimester (▶Fig. 3). In early pregnancy 21, 6, and 3% of women had mild, moderate, or severe SBD, respectively. These frequencies increased to 35, 7, and 5% in the third trimester (p < 0.001). About 70% (95% CI, 62–78%) of women had no change in their SDB severity (none, mild, moderate, or severe) across pregnancy, 3% (95% CI, 0.001–6%) improved, and 27% (95% CI, 19–34%) worsened. Of the 34 women who worsened, 26 were cases of new-onset SDB, while the other 8 had SDB in early pregnancy that worsened in severity the third trimester (e.g., mild SDB that worsened to moderate SDB). Thus, the incidence of new-onset SDB in pregnancy was 20% (95% CI, 13–27%). The majority of these new-onset cases were mild SDB (24/ 26), although one woman developed moderate SDB and one woman developed severe SDB.
Table 2.
SDB indices in early and later pregnancy
| Early pregnancy, n = 60 with no valid 2nd study | Early pregnancy, n = 128 with 2 valid studies | Third trimester, n = 128 with 2 valid studies | p valuea | |
|---|---|---|---|---|
| RDI, mean ± SD | 7.9 ± 7.2 | 8.9 ± 10.7 | 11.9 ± 12.7 | 0.001 |
| AHI, mean ± SD | 4.7 ± 7.1 | 5.6 ± 10.8 | 8.8 ± 12.9 | < 0.001 |
| ODI, mean ± SD | 2.0 ± 3.2 | 2.7 ± 7.6 | 4.2 ± 8.6 | 0.005 |
Abbreviations: AHI, apnea hypopnea index; ODI, oxygen desaturation index; RDI, respiratory disturbance index; SD, standard deviation; SDB, sleep disordered breathing.
Comparing early vs. third trimester in 128 subjects with 2 valid studies.
Fig. 3.
Trends in SDB across pregnancy. SDB, sleep disordered breathing.
The characteristics of women who developed new-onset SDB (n = 26) were compared with those who tested negative for SDB at both time points (n = 63) (▶Table 3). The two groups did not differ with respect to age, race/ethnicity, or prepregnancy BMI. Compared with women who never developed SDB, women who developed new-onset SDB were more likely to be carrying a twin gestation (19.2 vs. 1.6%, p = 0.008). Mean pregnancy weight gain in women with new-onset SDB was 4 pounds greater, but this difference was not statistically significant. Similarly, the proportion of women gaining greater than 30 pounds, or gaining weight in excess to the BMI-based, Institute of Medicine criteria,34 during pregnancy did not differ between the two groups. Only 41 of the 88 women included in this analysis completed sleep questionnaires at both time points. The percentage of women who reported new-onset frequent snoring (≤3 times /week in the third trimester, but not in early pregnancy) was much higher in women with new-onset SDB (31 vs. 7%) however, the difference did not reach statistical significance given our sample size limitations.
Table 3.
Characteristics of women who developed new-onset SDB compared with women who tested negative for SDB at both time points
| No SDB (early and 3rd trimester), n = 63 | New-onset SDB, n = 26 | p value | |
|---|---|---|---|
| GA at second sleep study, mean ± SD | 32.6 ± 2.4 | 32.2 ± 1.7 | |
| Age (y), mean ± SD | 32.3 ± 6.1 | 33.1 ± 6.5 | 0.6 |
| BMI | |||
| Mean ± SD | 30.1 ± 8.6 | 31.1 ± 8.0 | 0.7 |
| < 25, % | 39.7 | 26.9 | |
| 25–29, % | 15.9 | 15.4 | |
| 30–34, % | 14.3 | 34.6 | 0.3 |
| 35–39, % | 12.7 | 7.7 | |
| ≥40, % | 17.5 | 15.4 | |
| Ethno-racial status | |||
| White, % | 39.7 | 60 | 0.2 |
| Black, % | 25.4 | 7.7 | |
| Hispanic, % | 22.2 | 15.4 | |
| Other, % | 12.7 | 16.9 | |
| Maternal characteristics | |||
| Chronic hypertension, % | 27.0 | 11.5 | 0.1 |
| Pregestational diabetes, % | 61.9 | 80.8 | 0.1 |
| Twins, % | 1.6 | 19.2 | 0.008 |
| Nullip, % | 27.0 | 34.6 | 0.5 |
| Prior preeclampsia, % | 19.2 | 17.5 | 0.9 |
| New-onset of self-reported frequent snoring (≥ 3 times/week), % | 7.1 (2/28) | 30.8 (4/13) | 0.07 |
| Maternal weight gain | |||
| Mean ± SD | 25.7 ± 14.5 | 29.6 ± 14.5 | 0.3 |
| > 30 Lbs, % | 36.5 | 34.6 | 0.9 |
| Weight gain in excess of Institute of Medicine recommendations, % | 41.3 | 46.2 | 0.7 |
Abbreviations: BMI, body mass index; SD, standard deviation; SDB, sleep disordered breathing.
Conclusions
Our data demonstrate that SDB is prevalent in high-risk women (defined in our cohort as obese women, women with chronic hypertension, pregestational diabetes, a history of preeclampsia and/or a twin gestation). Specifically, nearly 30% of the study population demonstrated SDB in early pregnancy with the majority of the cases being mild. In addition, new-onset SDB occurred in 20% of our population, with again most cases of incident SDB in pregnancy being mild. Thus, by the end of pregnancy, approximately 50% of this high-risk cohort demonstrated sleep indices consistent with SDB.
Previously, in an analysis of how best to screen for SDB in early pregnancy, we described the characteristics associated with early pregnancy SDB and reported that maternal age, prepregnancy BMI, the diagnosis of chronic hypertension, and self-reported frequent snoring were all associated with a higher likelihood of testing positive for SDB in early pregnancy.35 In this analysis describing incident SDB in the third trimester, the only factor significantly associated with new-onset SDB was twin gestation. However, given our sample size (11 twins) we are unable to provide a precise risk estimate. Subjective new-onset of frequent snoring may be a good predictor as well, though subject noncompliance with questionnaires left us underpowered to detect a statistically significant difference. In our cohort, gestational weight gain did not differ significantly between those who developed new-onset SDB and those who did not. However, new-onset SDB was more common in twin gestations. In our cohort, twin pregnancies, as expected, had greater maternal weight gain. This suggests that maternal weight, weight distribution, and body composition may play a role in incident SDB during pregnancy but that assessing this by just measuring BMI and pounds gained in pregnancy is inadequate. Other measures, such as changes in neck circumference, body composition, and the trajectory of weight gain warrant further study as risk factors for incident SDB in pregnancy. Overall, given our study size and findings, we cannot soundly derive a predictive model for new-onset SDB in pregnancy. Larger, prospective studies with serial SDB measures are needed.
This study was designed to address the epidemiology of SDB in pregnancy. We believe these data on the prevalence and trajectory of SDB will help inform the study design of future trials examining the impact of SDB on clinically meaningful obstetrical outcomes. Although our study is one of the largest prospective and objective evaluations of SDB across pregnancy, the study population all had high-risk conditions and the findings cannot be generalized to all obstetrical populations.
Most SDB detected, whether at baseline or in the third trimester, was mild. This finding is not surprising. Our population, while high risk, was relatively young and it is well-established that SDB prevalence and severity increases with age.36 In addition, women are less likely than men to suffer from severe SDB.37,38 The implications of suffering from mild SDB during some or all of pregnancy are yet to be determined. In fact, in pregnancy we have yet to determine what degree of SDB is clinically relevant and what indices are of greatest concern. In nonpregnant populations, an AHI of ≤5 is considered abnormal, but higher AHI levels have the greatest correlation with adverse outcomes. In certain populations, conversely, lower values of indices are considered abnormal; in children, an AHI ≤2 is considered abnormal.39 Given that pregnancy is a relatively short period (40 weeks) in a woman's life, it may require exposure to a very high AHI to adversely affect maternal and neonatal outcomes. On the contrary, pregnancy may turn out to be a particularly vulnerable state for the mother and fetus and very low AHI levels may be clinically significant. Although data that are accumulating suggest an association between SDB in pregnancy and adverse pregnancy outcomes, the majority of data is derived from cross-sectional or retrospective analyses, and involved subjective SDB assessment. Also, these studies typically have not adequately adjusted for confounding factors such as BMI or clearly defined a temporal relationship between SDB and the subsequent development of pregnancy complications.
In summary, SDB in early pregnancy is common in high-risk women and new-onset SDB occurs in 20% of these women. New-onset SDB during pregnancy is more common in twin pregnancies and the development of frequent snoring in the third trimester may be a useful predictor of new-onset SDB. In pregnancy, most cases of SDB are mild. Future studies are needed to determine the implications of SDB on pregnancy, and to evaluate the impact of treatment of this sleep disorder during pregnancy on maternal and fetal health.
Fig. 1.
Watch-PAT 100.
Acknowledgment
This study was supported with the fund from NIH/NICHD 1K12HD050121, Preeclampsia Foundation Vision Grant, Northwestern Memorial Foundation Dixon Translational Research Initiative.
Footnotes
Disclosure
The authors report no conflict of interest.
References
- 1.Berry RB, Budhiraja R, Gottlieb DJ, et al. American Academy of Sleep. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan S. The American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 3.Pien GW, Schwab RJ. Sleep disorders during pregnancy. Sleep. 2004;27(7):1405–1417. doi: 10.1093/sleep/27.7.1405. [DOI] [PubMed] [Google Scholar]
- 4.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28(10):1299–1305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 5.Facco FL, Kramer J, Ho KH, Zee PC, Grobman WA. Sleep disturbances in pregnancy. Obstet Gynecol. 2010;115(1):77–83. doi: 10.1097/AOG.0b013e3181c4f8ec. [DOI] [PubMed] [Google Scholar]
- 6.Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003;9(6):477–483. doi: 10.1097/00063198-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001;134(5):396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 8.Marshall NS, Wong KK, Phillips CL, Liu PY, Knuiman MW, Grunstein RR. Is sleep apnea an independent risk factor for prevalent and incident diabetes in the Busselton Health Study? J Clin Sleep Med. 2009;5(1):15–20. [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB, Nieto FJ, Guidry U, et al. Sleep Heart Health Study Research Group. Relation of sleep-disordered breathing to cardiovascular disease risk factors: the Sleep Heart Health Study. Am J Epidemiol. 2001;154(1):50–59. doi: 10.1093/aje/154.1.50. [DOI] [PubMed] [Google Scholar]
- 10.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 11.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 12.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young T, Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(Suppl 4):S122–S126. [PubMed] [Google Scholar]
- 14.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 15.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 16.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203(2):e1–e5. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-disordered breathing: a risk factor for adverse pregnancy outcomes? Am J Perinatol. 2012;29(4):277–282. doi: 10.1055/s-0031-1295658. [DOI] [PubMed] [Google Scholar]
- 19.Louis J, Auckley D, Miladinovic B, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120(5):1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202(3):e1–e5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 21.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC Womens Health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Brien LM, Bullough AS, Owusu JT, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):e1–e9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 24.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27(8):1560–1566. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 25.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004;27(5):923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29(3):367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 27.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 28.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003;4(5):435–442. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 29.O'Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry RB, Budhiraja R, Gottlieb DJ, American Academy of Sleep Medicine et al. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JG, Ramar K, Olson EJ. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clin Proc. 2011;86(6):549–554. doi: 10.4065/mcp.2010.0810. quiz 554–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Flemons WW, Littner MR, Rowley JA, et al. Home diagnosis of sleep apnea: a systematic review of the literature. An evidence review cosponsored by the American Academy of Sleep Medicine, the American College of Chest Physicians, and the American Thoracic Society. Chest. 2003;124(4):1543–1579. doi: 10.1378/chest.124.4.1543. [DOI] [PubMed] [Google Scholar]
- 34.IOM (Institute of Medicine) and NRC. (National Research Council . Weight Gain During Pregnancy: Rexamining the Guidelines. The National Academies Press; Washington, DC: 2009. [Google Scholar]
- 35.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8(4):389–394. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 37.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167(9):1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 38.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 39.American Thoracic Society. Standards and indications for cardio-pulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153(2):866–878. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]