Abstract
Objective
To determine the safety and efficacy of a home-based functional exercise program in spinal and bulbar muscular atrophy (SBMA).
Methods
Subjects were randomly assigned to participate in 12 weeks of either functional exercises (intervention) or a stretching program (control) at the National Institutes of Health in Bethesda, MD. A total of 54 subjects enrolled, and 50 completed the study with 24 in the functional exercise group and 26 in the stretching control group. The primary outcome measure was the Adult Myopathy Assessment Tool (AMAT) total score, and secondary measures included total activity by accelerometry, muscle strength, balance, timed up and go, sit-to-stand test, health-related quality of life, creatine kinase, and insulin-like growth factor-1.
Results
Functional exercise was well tolerated but did not lead to significant group differences in the primary outcome measure or any of the secondary measures. The functional exercise did not produce significantly more adverse events than stretching, and was not perceived to be difficult. To determine whether a subset of the subjects may have benefited, we divided them into high and low functioning based on baseline AMAT scores and performed a post hoc subgroup analysis. Low-functioning individuals receiving the intervention increased AMAT functional subscale scores compared to the control group.
Interpretation
Although these trial results indicate that functional exercise had no significant effect on total AMAT scores or on mobility, strength, balance, and quality of life, post hoc findings indicate that low-functioning men with SBMA may respond better to functional exercises, and this warrants further investigation with appropriate exercise intensity.
Introduction
Spinal and bulbar muscular atrophy (SBMA) is an X-linked neuromuscular disorder caused by polyglutamine repeat expansion in the androgen receptor.1,2 SBMA causes progressive muscle weakness, cramps, and tremor, with degeneration of motor neurons and muscle.3,4 These changes often lead to limitations in mobility and in the capacity to perform functions such as sitting up and standing from a seated position.5,6 The loss of these abilities leads to greater dependence on caregivers, with associated psychosocial ramifications.
Exercise training has been shown to improve function in older adults7 and in disease populations.8,9 In humans, exercise has been shown to increase levels of insulin-like growth factor-1 (IGF-1),10,11 which alleviates the manifestations of SBMA in transgenic mice.12 These findings suggest that exercise may be beneficial in SBMA.
Clinical studies of the efficacy of exercise training to improve physical function in persons with neuromuscular diseases (NMD) have had mixed results. One review concluded that exercise is likely to be effective in improving function13, whereas others were more equivocal.14 Some discrepancy in these studies may be due to the heterogeneity of diagnoses and various degrees of disease severity included in the studies.15 Interestingly, improvements in physical function after participating in home-based resistance plus balance training have been observed independent of changes in muscle strength in both elderly populations16 and subjects with amyotrophic lateral sclerosis.17 A previous trial of aerobic exercise in SBMA conducted with eight subjects did not show a benefit in the primary outcome measure of maximum oxygen uptake or in activities of daily living, however, a significant increase in maximal work capacity was observed.18
To date most trials of exercise interventions in NMD have used either resistance or aerobic exercises, with little if any research examining the effects of functional exercise interventions. Functional exercises are designed to resemble activities typically performed in daily life and are potentially of greater benefit to patients with activity limitations. The purpose of this study was to examine the effects of a home-based functional exercise program on physical function in a cohort of subjects with SBMA and to evaluate the safety of this approach.
Methods
Overview
We conducted a 12-week, randomized, evaluator-blinded functional exercise trial at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD, from July 2011 to January 2014 (Data S1). Subjects were randomly assigned to either an intervention group that performed a functional exercise program or a control group that performed a stretching program. Subjects were required to participate in telephone and video monitoring to ensure compliance and intervention fidelity. The minimum compliance requirement to be maintained in the study was completion of 80% of the telephone contact forms and other communications and 85% of the assigned exercise sessions.
Subjects
All subjects were men over 18 years of age with genetically confirmed SBMA. In addition, subjects were required to have an Adult Myopathy Assessment Tool (AMAT)5 score from 14 to 41. Subject group randomization was achieved using stratified block randomization based on AMAT scores with a block size of four.
Exercise programs
Both the functional exercise and stretching programs were developed by NIH physical therapists familiar with SBMA (Data S2). They were taught by unblinded physical therapy staff. Functional exercises included trunk sit back, sit-to-stand (STS), standing squat with theraband row, standing lunge with theraband forward reach, double limb heel raise, and wall pushup. Maximal functional exercise capacity for each exercise was assessed by subjects accurately performing as many repetitions as possible in 60 sec. Participants in the functional exercise group began the first week with one set of exercises performed on two nonconsecutive days at 50% of the maximal number of repetitions achieved at baseline in order to assure exercise safety (Fig. S1). After the first week, the repetitions were increased to 70% of the maximal baseline performance for the study duration. At week three, the frequency was increased to 3 days a week. For weeks 4–6, the subjects performed two sets of each exercise at each session, and at week seven they began three sets per session. Subjects were instructed to keep exercise logs. They were asked to provide their OMNI rating scale of perceived exertion (RPE)19,20 and soreness using two different 0–10 point scales, with 10 indicating the highest level of exertion and soreness. Exercise subjects were instructed to use the following scale when calculating their weekly RPE: 0–2 = very easy; 3–4 = easy; 5–6 = starting to get hard; 7–8 = very hard; 9–10 = so hard I am going to stop. Ratings of soreness were collected with the following scale: 0 = no soreness; 1–3 = mild soreness; 4–6 = moderate soreness; 7–10 = severe soreness.
Outcomes
Physical function and endurance were measured with the AMAT5,21 which served as the primary outcome measure for this study. Quantitative muscle assessment (QMA) was done by measuring the maximal voluntary isometric muscle contraction of seven muscles bilaterally, including shoulder abduction, elbow flexion, hand grip, hip extension, knee extension, ankle dorsi, and plantar flexion.21 Only the right side was analyzed. All muscle strength data were normalized by body mass index and compared to control values for calculation of the percent predicted strength.22–24 The progressive height STS test was modified and used as described by Schenkman et al.25 Mobility was also measured with the Timed Up and Go (TUG) test and the Actical accelerometer (Philips Respironics, Bend, OR). The Actical was worn during the first 10 days of the trial and during the last 10 days before the final evaluation.26–28 In order to account for the subjects who did not fully comply with the 10-day wear period, all total variable counts were weighted by dividing the average value by the total number of days recorded for each subject. Subjects having ≥6 days of pre- and post activity data were analyzed. The Computerized Dynamic Posturography SMART EquiTest system and a long force plate (NeuroCom International, Clackamas, OR) were used for a quantitative assessment of balance. The Medical Outcomes Study 36-item Short Form version 2 (SF36 v2; Quality Metric software, Lincoln, RI) was used to assess the health-related quality of life and the Beck Depression Inventory (BDI) II (Pearson, San Antonio, TX) was used to screen for depressive symptoms. Blood work was performed at baseline and follow-up and included assessments of creatine kinase (CK), IGF-1, and testosterone.
An adverse events questionnaire was administered to subjects electronically every week to monitor any negative effects related to exercise, including pain, muscle fatigue, cramping, and soreness. Additional measures included the bulbar rating scale (reported as 0–100%) and serological markers as reported previously by our group.29
Statistical analysis
The sample size needed was calculated based on an anticipated 10% change in the AMAT total score in the intervention group compared to control. This change in AMAT would be roughly equivalent in magnitude to the amount of disease progression over 2 years.29 In order to obtain 80% power with alpha at 0.05 for this study we kept recruitment open until 50 subjects completed the study. Groups’ baseline characteristics were compared with independent sample t-tests. Effects of the intervention were analyzed with a two-way repeated measures ANOVA on each dependent variable with group (intervention vs. control) as a between factor, and time (pretest vs. posttest) as a within factor. Baseline age, disease duration, bulbar rating scale, serum IGF-1, testosterone and CK levels, and functional classification were explored as potential covariates. Post hoc analyses were performed to determine the effects of our intervention in subjects classified as low or high function. Classification was based on baseline AMAT functional subscale scores (Low ≤ 15, High > 15). The cutoff of 15 was chosen because it was the median for both groups. Significance was established at 0.05, without adjustment for multiple comparisons.
Results
Patient characteristics
Of 61 subjects screened, seven were not enrolled due to AMAT total scores above the cutoff, leaving 54 participants entering the trial (Fig.1). Altogether a total of 50 subjects completed the study and were included in the analysis, with 24 subjects in the intervention group, and 26 in the stretching control group. Baseline characteristics are shown in Table1 and were similar between groups. All subjects had a high compliance rate of 88.8%. Some subjects experienced adverse events such as cramping and falls, which are common in the SBMA population21 (Table S1). There was no significant difference between the number of adverse events reported by the intervention and control groups. All but one subject (in the control group) returned all self-reported questionnaires (one SF36 missing).
Figure 1.
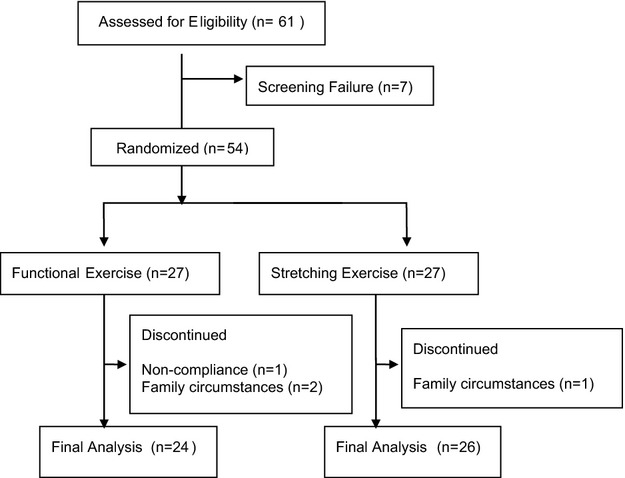
Flowchart of the trial. One subject in the functional exercise group dropped out due to noncompliance with uncompleted compliance surveys, videos, and weekly exercise forms. Two other subjects in the functional group and one in the stretching group dropped out because of family circumstances.
Table 1.
Baseline characteristics comparing intervention versus control.
| Characteristics | Intervention (n = 24) | Control (n = 26) | P value | Reference range |
|---|---|---|---|---|
| Age (years) | 53.8 (10.0) | 56.5 (8.1) | 0.28 | |
| CAG repeat length | 47.3 (4.9) | 46.9 (2.7) | 0.68 | <39 |
| CK (U/L) | 1038 (616) | 1232 (1171) | 0.47 | 52–386 |
| IGF1 (ng/mL) | 137.8 (51.1) | 155.3 (39.5) | 0.18 | 87–283 |
| Testosterone, total (ng/dL) | 385.2 (109.6) | 382.4 (153.9) | 0.94 | 181–758 |
| Disease duration (years) | 15.1 (7.5) | 16.0 (10.7) | 0.11 | |
| Bulbar Rating Scale | 92.3 (4.1) | 92.3 (4.7) | 0.99 | |
| Body mass index | 28.2 (5.1) | 28.3 (10.7) | 0.61 | |
| QMA total strength (% predicted) | 41.0 (16.9) | 39.3 (20.8) | 0.68 | |
| QMA UE strength | 40.0 (16.0) | 36.0 (20.0) | 0.97 | |
| QMA LE strength | 41.7 (17.6) | 41.9 (21.3) | 0.41 |
Data are given as mean (SD). CAG, cytosine adenine guanine; CK, creatine kinase; IGF-1, insulin-like growth factor 1; QMA, quantitative muscle assessment; UE, upper extremity; LE, lower extremity.
Outcome measures
The comparisons between intervention versus control groups at pretest versus posttest are shown in Table2. No significant improvements in the primary outcome measure, the AMAT total score, or in the AMAT functional and endurance subscales were observed for either group. Similarly, no significant changes were observed for any other measures of mobility, or other outcome categories. Our analysis showed elevation of CK levels in the intervention group by 61 U/L and the control group decreasing by 79. RPE reported in the exercise logs were relatively low for the entire cohort, with a mean score of 3.2 ± 1.5 (week 1), to 3.5 ± 1.5 (week 6), and 3.3 ± 1.6 (weeks 9 and 12). Ratings of soreness after performing the exercise routine were also low for the entire cohort, ranging from mean 2.31 (week 1), to 2.14 (week 6), and 2.28 (weeks 9 and 12). Functional variables measured in the Neurocom force plate including forward lunge, STS, step up and over, and step quick turn tests were not reported in the analysis because a great number of data were missing due to test floor effect as many patients were unable to safely perform the tasks.
Table 2.
Comparisons between intervention versus control groups at pretest versus posttest.
| Intervention group | Control group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Change | N | Pretest | Posttest | Change | N | P-value | |
| Mobility | |||||||||
| AMAT total1 (0–45) | 29.3 (6.8) | 29.9 (6.6) | 0.6 | 24 | 28.9 (6.7) | 29.0 (7.7) | 0.2 | 26 | 0.60 |
| AMAT functional1 (0–21) | 14.6 (4.0) | 15.3 (3.7) | 0.6 | 24 | 15.4 (3.4) | 15.1 (4.0) | −0.3 | 26 | 0.08 |
| AMAT endurance1 (0–24) | 14.7 (3.3) | 14.7 (3.6) | 0.0 | 24 | 13.5 (3.7) | 13.9 (4.0) | 0.4 | 26 | 0.29 |
| STS scale1 (% of knee height) | 103.3 (22) | 103.8 (24.3) | 0.4 | 24 | 102.3 (22.5) | 103.1 (23.5) | 0.8 | 26 | 0.86 |
| TUG (sec)1 | 10.8 (6.5) | 11.0 (6.5) | 0.2 | 24 | 9.5 (3.2) | 9.6 (3.7) | 0.1 | 26 | 0.93 |
| Actical total activity (average count per day)1, 2 | 53,949 (42,610) | 61,797 (48,383) | 7848 | 20 | 69,326 (51,539) | 70,498 (50,508) | 1171 | 23 | 0.19 |
| Molecular | |||||||||
| CK (U/L) | 1038 (616) | 1098 (671) | 61 | 24 | 1232 (1174) | 1153 (1183) | −79 | 26 | 0.06 |
| IGF-1 (ng/mL) | 137.8 (51.0) | 137.6 (54.6) | −0.3 | 24 | 155.3 (39.5) | 154.0 (41.3) | −1.3 | 26 | 0.88 |
| Testosterone, total (ng/dL) | 385.2 (109.6) | 396.2 (124.3) | 10.9 | 24 | 382.4 (153.9) | 406.4 (172.5) | 24 | 26 | 0.57 |
| Overall health | |||||||||
| BDI (0–21) | 8.4 (5.6) | 8.8 (5.7) | 0.3 | 24 | 10.6 (8.1) | 8.7 (6.7) | −1.9 | 26 | 0.07 |
| SF36v2 PCS1 | 32.9 (7.0) | 33.0 (7.3) | 0.4 | 23 | 33.1 (6.9) | 34.1 (7.4) | 1.0 | 26 | 0.74 |
| SF36v2 MCS1 | 54.6 (9.4) | 53.3 (10.0) | −1.3 | 23 | 53.1 (12.0) | 54.4 (10.3) | 1.3 | 26 | 0.26 |
| SF36v2 VT | 45.4 (20.6) | 46.7 (20.5) | 1.4 | 23 | 44.5 (20.3) | 48.6 (19.5) | 4.1 | 26 | 0.48 |
| Strength | |||||||||
| QMA total (scaled to BMI)1 | 9.26 (2.51) | 9.08 (2.59) | −0.18 | 24 | 8.77 (3.65) | 8.93 (3.87) | 0.16 | 24 | 0.08 |
| QMA upper extremity | 2.71 (1.17) | 2.74 (1.15) | 0.03 | 24 | 2.62 (1.14) | 2.62 (1.19) | 0.00 | 25 | 0.75 |
| QMA lower extremity | 6.43 (2.42) | 6.54 (2.68) | 0.11 | 24 | 6.13 (1.98) | 5.98 (1.96) | −0.14 | 25 | 0.13 |
| Balance | |||||||||
| mCTSIB composite (COG sway velocity in deg/sec)1 | 1.5 (0.8) | 1.5 (0.7) | −0.2 | 20 | 1.3 (0.5) | 1.3 (0.5) | 0.0 | 23 | 0.73 |
| MCT composite (latency in msec) | 154.0 (15.2) | 154.3 (15.5) | 0.3 | 21 | 146.4 (10.4) | 149.2 (10.9) | 2.8 | 22 | 0.23 |
STS scale utilized seven levels from 80% to 140% of knee height, which was measured from tibial crest to the floor. The SF36v2 is reported as a norm-based number (0–100 with 50 as the mean) calculated with Quality Metric software (Lincoln, RI). Data are given as mean (SD). AMAT, Adult Myopathy Assessment Tool; CK, creatine kinase; IGF-1, insulin-like growth factor 1; BDI, Beck Depression Inventory; SF36v2, short form quality of life survey; PCS, physical component summary; MCS, mental component summary; VT, vitality component; STS, progressive height sit-to-stand test; TUG, time up and go test; QMA, quantitative muscle assessment; BMI, body mass index; COG, center of gravity; mCTSIB, modified clinical test of sensory interaction on balance; MCT, motor control test.
Classification (low or high function) is a significant covariate.
Age is a significant covariate.
Post hoc analysis
We found that the AMAT functional subscale baseline scores (low vs. high function) were a significant covariate (Table2), and we therefore carried out post hoc analyses to determine the influence of functional level on our cohort’s response to the intervention.
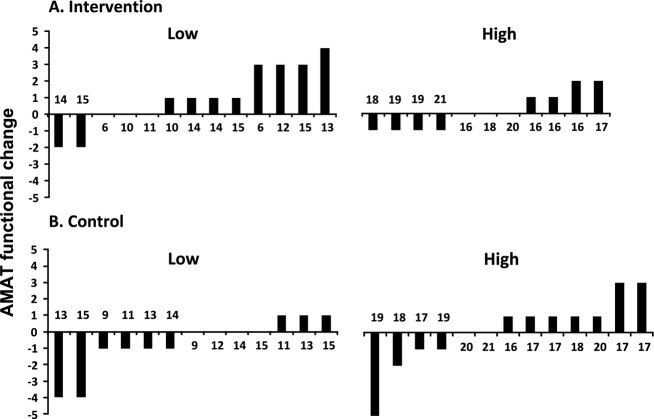
When groups were separated according to baseline AMAT functional subscale scores (Fig.2), we found that individuals with lower AMAT scores who received the intervention improved physical function as measured by the AMAT functional subscale, whereas individuals with similarly low AMAT baseline scores in the stretching control group declined. Figure2 shows that eight subjects with low function in the functional exercise intervention group (top left) improved compared to only three subjects with low function in the control group (bottom left). Individuals with higher AMAT baseline functional subscale scores who received the intervention became more active, showing an increase in total activity count per day by accelerometry when compared to the control group, accounting for age differences (Fig. S2). These changes in total activity do not appear to be an indication of the exercise stimulus itself, as the time the intervention was performed occupied only a small fraction of the total activity time analyzed (Fig. S3). An independent t-test on change scores confirmed this finding (P = 0.03).
Figure 2.
Change in the functional Adult Myopathy Assessment Tool (AMAT) score following intervention (A) and stretching (B) by individual. The terms low and high are used to separate the groups of individuals with an initial functional AMAT score below and including 15, classified as the low function group or above 15, classified as the high function group. Bars above zero indicate an improvement on functional AMAT at posttest compared to pretest, whereas bars below zero indicate deterioration. No bars indicate that the Functional AMAT score was the same at posttest compared to pretest. The numbers displayed above or below each bar indicate individual initial functional AMAT score. Within each group, individuals are ordered by difference in score.
Additionally, high-function individuals who received the intervention reported increased depressive symptoms as measured with the BDI when compared to those in the control group (group by time interaction F(1,22) = 4.88, P = 0.04). An independent t-test on change scores confirmed this finding (P = 0.04). It is important to note that the high-functioning individuals in the control group had increased depressive symptoms at baseline (Table S2). There was a borderline difference in average CK change between the intervention and control groups (P = 0.06). Six subjects in the intervention group had an increase in CK by over 200 U/L compared to one subject in the control group. None of the other variables were significantly different between the groups when individuals were classified as low or high function.
Discussion
Functional exercise is safe and well tolerated
All subjects achieved a minimum completion rate of 30/34 exercise sessions. Our analysis showed a modest elevation of CK in the intervention group compared to the control group, which could reflect more muscle break down. However, this degree of CK elevation is smaller than reported in other studies assessing aerobic exercise30 and strength training.31 In addition, RPE during the exercises and soreness after performing the exercise were relatively low for the entire cohort, suggesting that the exercises were perceived as low intensity. These data coupled with the observation that muscle strength did not appreciably decline during the study period lead us to conclude that there was little if any muscle damage.
Functional exercise did not improve the primary outcome measure
Functional exercise had no significant effect on the total AMAT, which includes both functional performance and endurance. Post hoc analyses offered some insights into why the total AMAT did not capture functional task performance changes in the intervention group, as virtually all of this groups’ change was seen in the AMAT functional subscale. The lack of change in serum IGF-1 levels may be related to the time point after exercise that was tested or the relatively low intensity of exercise. Previous reports of increased IGF-1 with exercise showed effects soon after high-intensity cycle ergometry.10,11
Functional exercise may improve functional task performance in individuals with low function
While the trial results showed no effect on the total AMAT, post hoc analysis showed that our cohort had a wide range of functional levels, which may have influenced intervention response. Individuals with low baseline function in the intervention group did better than those with low baseline function in the control group. The improvement seen in those with lower function was unaccompanied by significant changes in total AMAT, the endurance subscore, other measures of mobility, strength, balance, and quality of life, or biochemical markers. We do not think this improvement in the low function intervention group resulted from a practice effect since the high function group did not show a similar improvement.
Several factors could have influenced why those with high function did not improve functional task performance. Although we attempted to individualize the functional exercise dose, it is possible that those with higher initial function were underworked. The RPE ratings suggest the entire cohort felt the intensity of functional exercise and stretching was light rather than the desired moderate intensity. Finally, those with high function may have had a ceiling effect, since those with higher AMAT functional subscale baseline scores (e.g., 19, 20, or 21), may have had less room to increase their score, having only one or two tasks that could be improved upon, given the maximum possible score of 21. For example, our use of the AMAT total score with study exclusion criteria above 41, as the primary outcome measure, did not allow us to appreciate that nine subjects with functional subscale scores of 19, 20, and 21 were enrolled. Only one of these nine subjects improved on the AMAT functional subscale.
In a previous study from our group, the mean total AMAT score decreased by 9.1% over 2 years.29 The AMAT functional subscale accounted for over 80% of that decline, indicating that the functional subscale outperformed the endurance subscale in detecting change in SBMA (Fernandez-Rhodes et al., unpublished). Additionally, our exercise intervention was designed to improve upright functional tasks with less emphasis on improving endurance. Given these considerations, we felt it was appropriate to explore the analysis of the AMAT functional subscale alone.
Our results also show that high function individuals performing exercise increased their activity during the last 10 days of the study period compared with the first 10 days, in comparison with those who performed stretching. We believe this improvement was not related to activity measurement during the intervention itself since the intervention sessions represented a small segment of each 10-day measurement period, and no significant increase in activity was observed during the intervention sessions themselves. Also, the activity increase was not seen in those with low function who exercised. It is possible that those with higher functional capacity can increase their activity level through exercise without measurable effects on their function or endurance testing.
Surprisingly, depressive symptoms increased in those with high function at baseline who received the intervention. BDI scores indicate that these individuals were not depressed initially, but ended the trial with higher scores indicating mild depressive symptoms. This finding may be related to the high BDI scores in the control group at baseline and subsequent improvement in scores within this group. It is also possible that the intervention may have contributed to increased depression by requiring men with relatively high, and yet abnormal function and strength, to perform challenging activities that remind them of their functional limitations, while those in the control group performed stretching which may have improved their overall well-being.
Additional considerations for future studies
In retrospect, a future study could include closer monitoring to achieve a higher intensity exercise for a longer duration. One may consider powering a study based primarily on functional testing. Advantages of the AMAT functional subscale include the aforementioned improved ability to detect functional change in SBMA with the functional subscale compared with the endurance subscale. Other functional measures are also available, including the newly developed SBMA-FRS.32 On the other hand, studies examining aerobic exercise, may consider powering based primarily on endurance scales. Nevertheless, careful consideration should be taken when choosing a primary outcome measure so that the study population fits within the dynamic range of the measure.
In this study and in previous work by our group, we detected that core strength could be diminished in SBMA as evidenced by difficulty in performing the sit-up task.5 The exercise regimen in this trial included careful instruction to focus on contracting trunk muscles in an attempt to maintain a stable core during all functional exercises, but we did not include a direct measure of core strength.
Limitations
This study did not provide direct exercise supervision or an objective measure of the work performed during exercise. The functional exercises were designed to achieve moderate intensity, at 70% of baseline performance. However, the level of RPE after adjustments in exercise intensity and frequency indicates that the exercise was considered light rather than moderate intensity by the participants. Finally, although we found an improvement on the AMAT functional subscale in those entering with low function on post hoc analysis, the minimal clinically important change in scores has not yet been determined for the AMAT or its subscales in subjects with SBMA.
Conclusion
This study showed that a 12-week course of light functional exercise has no significant effects on muscle function, strength, balance, or quality of life when compared to stretching in a wide functional range of subjects with SBMA. However, functional exercise had a favorable safety profile, and post hoc analysis indicates that functional exercise may improve task performance in those with low baseline function. Subjects with high baseline function had an increase in overall activity, but also an increase in depressive symptoms. Further studies should consider longer trial duration, higher intensity exercise, use of function-based scales as appropriate, and a more targeted study population to reduce group variability when assessing outcomes in interventions designed to improve functional task performance.
Acknowledgments
The authors thank Nicholas Maragakis, MD (Professor of Neurology, Johns Hopkins University) for his role as the independent safety monitor during this study. We also thank John Collins, PhD (Statistician, NIH clinical center and Professor, George Mason University) for providing important additional statistical support for this study. We thank Annamarie Shrader, BA (Florida State University) for creating the weekly exercise log sheets and the functional exercise instructional video. Lastly, the authors thank Elizabeth Hartnett, BA (NINDS, National Institutes of Health) for her help in scheduling subjects during the study, the clinical staff of the outpatient NIH neurology clinic for their assistance during the subjects’ visits, and the research subjects who made this study possible. This work was supported by intramural research funds from the National Institute of Neurological Disorders and Stroke. The authors report no disclosures relevant to this manuscript.
Author Contributions
J. S., C. Z., I. K., and B. E. D. wrote the initial draft and edited the manuscript, analysis/interpretation of data. J. S., E. L., G. O. J, A. K., M. R. S., B. E. D., J. G. W., C. Z., W. C., and C. G. contributed to study concept/design, acquisition of data. K. H. F., C. G. contributed to study concept/design, writing and editing of manuscript, analysis/interpretation of data. M. R. S., W. C., and E. L. were involved in instruction of the study exercises. A. K. was the study coordinator. C. Z. and S. A. performed the statistical analysis, L. G., D. F. performed the acquisition of data. A. B. S was involved in study concept/design.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Twelve weeks of exercise intervention.
Figure S2. Post hoc comparison of total activity level between intervention and control group. Change in accelerometer total activity count following intervention (A) and stretching (B) by individual. The terms low and high are used to separate the groups of individuals with an initial functional AMAT score below and including 15, classified as the low function group or above 15, classified as the high function group. Bars above zero indicate an improvement in total activity at posttest compared to pretest, whereas bars below zero indicate deterioration. The numbers displayed above or below each bar indicate the total activity count. Within each group, individuals are ordered by difference in activity.
Figure S3. Representative accelerometer activity counts during the beginning and end of intervention. Data show the activity counts from a representative individual during the initial and final days of study intervention. All subjects were instructed to record the time of the intervention by depressing a button on the accelerometer device. Arrows indicate the 1 h time intervals during which the intervention was performed. Activity data during this hour are indicated in black.
Table S1. Adverse events reported by more than 10% of subjects in either group. Data are the number of subjects reporting each adverse event, with the number of events shown in parentheses. P values are for comparison of the number of subjects reporting adverse events with stretching or functional exercises, based on chi-square analysis.
Table S2. Baseline characteristics comparing low function versus high function individuals under each group (intervention and control). CK, creatine kinase; IGF1, insulin-like growth factor 1; QMA, quantitative muscle assessment. *P < 0.05.
Data S1. Clinical protocol. Effect of functional exercise in patients with spinal bulbar muscular atrophy.
Data S2. Description of stretching and functional exercises.
References
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–680. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- La Spada AR, Wilson EM, Lubahn DB, et al. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Orr CR, Montie HL, Liu Y, et al. An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy. J Biol Chem. 2010;285:35567–35577. doi: 10.1074/jbc.M110.146845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AP, Yu Z, Murray S, et al. Peripheral androgen receptor gene suppression rescues disease in mouse models of spinal and bulbar muscular atrophy. Cell Rep. 2014;7:774–784. doi: 10.1016/j.celrep.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Love MO, Fernandez-Rhodes L, Joe G, et al. Assessing function and endurance in adults with spinal and bulbar muscular atrophy: validity of the adult myopathy assessment tool. Rehabil Res Pract. 2014;2014:873872. doi: 10.1155/2014/873872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Katsuno M, Banno H, et al. Walking capacity evaluated by the 6-minute walk test in spinal and bulbar muscular atrophy. Muscle Nerve. 2008;8:964–971. doi: 10.1002/mus.21077. [DOI] [PubMed] [Google Scholar]
- Thiebaud RS, Funk MD, Abe T. Home-based resistance training for older adults: a systematic review. Geriatr Gerontol Int. 2014;14:750–757. doi: 10.1111/ggi.12326. [DOI] [PubMed] [Google Scholar]
- Aitkens SG, McCrory MA, Kilmer DD, Bernauer EM. Moderate resistance exercise program: its effect in slowly progressive neuromuscular disease. Arch Phys Med Rehabil. 1993;74:711–715. doi: 10.1016/0003-9993(93)90031-5. [DOI] [PubMed] [Google Scholar]
- Krivickas L. Exercise in neuromuscular disease. J Clin Neuromuscul Dis. 2003;5:29–39. doi: 10.1097/00131402-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Bang P, Brandt J, Degerblad M, et al. Exercise-induced changes in insulin-like growth factors and their low molecular weight binding protein in healthy subjects and patients with growth hormone deficiency. Eur J Clin Invest. 1990;20:285–292. doi: 10.1111/j.1365-2362.1990.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Brasel JA, Hintz RL, et al. Acute effect of brief low- and high-intensity exercise on circulating insulin-like growth factor (IGF) I, II, and IGF-binding protein-3 and its proteolysis in young healthy men. J Clin Endocrinol Metab. 1996;81:3492–3497. doi: 10.1210/jcem.81.10.8855791. [DOI] [PubMed] [Google Scholar]
- Palazzolo I, Stack C, Kong L, et al. Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron. 2009;63:316–328. doi: 10.1016/j.neuron.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cup EH, Pieterse AJ, Broek-Pastoor T, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil. 2007;88:1452–1464. doi: 10.1016/j.apmr.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Voet NB, van der Kooi EL, Riphagen II, et al. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2010;1:1–40. doi: 10.1002/14651858.CD003907.pub3. [DOI] [PubMed] [Google Scholar]
- Abresch RT, Carter GT, Han JJ, McDonald CM. Exercise in neuromuscular diseases. Phys Med Rehabil Clin N Am. 2012;23:653–673. doi: 10.1016/j.pmr.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Layne JE, Bernstein MJ, et al. The effects of multidimensional home-based exercise on functional performance in elderly people. J Gerontol A Biol Sci Med Sci. 2004;59:154–160. doi: 10.1093/gerona/59.2.m154. [DOI] [PubMed] [Google Scholar]
- Bello-Haas VD, Florence JM, Kloos AD, et al. A randomized controlled trial of resistance exercise in individuals with ALS. Neurology. 2007;68:2003–2007. doi: 10.1212/01.wnl.0000264418.92308.a4. [DOI] [PubMed] [Google Scholar]
- Preisler N, Andersen G, Thøgersen F, et al. Effect of aerobic training in patients with spinal and bulbar muscular atrophy (Kennedy disease) Neurology. 2009;72:317–323. doi: 10.1212/01.wnl.0000341274.61236.02. [DOI] [PubMed] [Google Scholar]
- Robertson RJ, Goss FL, Rutkowski J, et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc. 2003;35:333–341. doi: 10.1249/01.MSS.0000048831.15016.2A. [DOI] [PubMed] [Google Scholar]
- Robertson RJ, Goss FL, Dube J, et al. Validation of the adult OMNI scale of perceived exertion for cycle ergometer exercise. Med Sci Sports Exerc. 2004;36:102–108. doi: 10.1249/01.MSS.0000106169.35222.8B. [DOI] [PubMed] [Google Scholar]
- Rhodes LE, Freeman BK, Auh S, et al. Clinical features of spinal and bulbar muscular atrophy. Brain. 2009;132:3242–3251. doi: 10.1093/brain/awp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Congress of Rehabilitation Medicine and the American Academy of Physical and Medicine and Rehabilitation. Muscular weakness assessment: use of normal isometric strength data. Arch Phys Med Rehabil. 1996;77:1251–1255. doi: 10.1016/s0003-9993(96)90188-4. [DOI] [PubMed] [Google Scholar]
- Stoll T, Huber E, Seifert B, et al. Maximal isometric muscle strength: normative values and gender-specific relation to age. Clin Rheumatol. 2000;19:105–113. doi: 10.1007/s100670050026. [DOI] [PubMed] [Google Scholar]
- Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- Schenkman M, Riley PO, Pieper C. Sit to stand from progressively lower seat heights – alterations in angular velocity. Clin Biomech. 1996;11:153–158. doi: 10.1016/0268-0033(95)00060-7. [DOI] [PubMed] [Google Scholar]
- Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77:64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- Esliger DW, Probert A, Connor Gorber S, et al. Validity of the Actical accelerometer step-count function. Med Sci Sports Exerc. 2007;39:1200–1204. doi: 10.1249/mss.0b013e3804ec4e9. [DOI] [PubMed] [Google Scholar]
- Kayes NM, Schluter PJ, McPherson KM, et al. Exploring Actical accelerometers as an objective measure of physical activity in people with multiple sclerosis. Arch Phys Med Rehabil. 2009;90:594–601. doi: 10.1016/j.apmr.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Fernández-Rhodes LE, Kokkinis AD, White MJ, et al. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol. 2011;10:140–147. doi: 10.1016/S1474-4422(10)70321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney K, Landau ME, Gonzalez RS, et al. Serum creatine kinase after exercise: drawing the line between physiological response and exertional rhabdomyolysis. Muscle Nerve. 2012;45:356–362. doi: 10.1002/mus.22317. [DOI] [PubMed] [Google Scholar]
- Paschalis V, Koutedakis Y, Jamurtas AZ, et al. Equal volumes of high and low intensity of eccentric exercise in relation to muscle damage and performance. J Strength Cond Res. 2005;19:184–188. doi: 10.1519/R-14763.1. [DOI] [PubMed] [Google Scholar]
- Hashizume A, Katsuno M, Banno H, et al. A functional scale for spinal and bulbar muscular atrophy: cross-sectional and longitudinal study. Neuromuscul Disord. 2015 doi: 10.1016/j.nmd.2015.03.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Twelve weeks of exercise intervention.
Figure S2. Post hoc comparison of total activity level between intervention and control group. Change in accelerometer total activity count following intervention (A) and stretching (B) by individual. The terms low and high are used to separate the groups of individuals with an initial functional AMAT score below and including 15, classified as the low function group or above 15, classified as the high function group. Bars above zero indicate an improvement in total activity at posttest compared to pretest, whereas bars below zero indicate deterioration. The numbers displayed above or below each bar indicate the total activity count. Within each group, individuals are ordered by difference in activity.
Figure S3. Representative accelerometer activity counts during the beginning and end of intervention. Data show the activity counts from a representative individual during the initial and final days of study intervention. All subjects were instructed to record the time of the intervention by depressing a button on the accelerometer device. Arrows indicate the 1 h time intervals during which the intervention was performed. Activity data during this hour are indicated in black.
Table S1. Adverse events reported by more than 10% of subjects in either group. Data are the number of subjects reporting each adverse event, with the number of events shown in parentheses. P values are for comparison of the number of subjects reporting adverse events with stretching or functional exercises, based on chi-square analysis.
Table S2. Baseline characteristics comparing low function versus high function individuals under each group (intervention and control). CK, creatine kinase; IGF1, insulin-like growth factor 1; QMA, quantitative muscle assessment. *P < 0.05.
Data S1. Clinical protocol. Effect of functional exercise in patients with spinal bulbar muscular atrophy.
Data S2. Description of stretching and functional exercises.