Abstract
Advancing age is associated with a progressive loss of skeletal muscle (SkM) mass and function. Given the worldwide aging demographics, this is a major contributor to morbidity, escalating socio-economic costs and ultimately mortality. Previously, it has been established that a decrease in regenerative capacity in addition to SkM loss with age coincides with suppression of insulin/insulin-like growth factor signalling pathways. However, genetic or pharmacological modulations of these highly conserved pathways have been observed to significantly enhance life and healthspan in various species, including mammals. This therefore provides a controversial paradigm in which reduced regenerative capacity of skeletal muscle tissue with age potentially promotes longevity of the organism. This paradox will be assessed and considered in the light of the following: (i) the genetic knockout, overexpression and pharmacological models that induce lifespan extension (e.g. IRS-1/s6K KO, mTOR inhibition) versus the important role of these signalling pathways in SkM growth and adaptation; (ii) the role of the sirtuins (SIRTs) in longevity versus their emerging role in SkM regeneration and survival under catabolic stress; (iii) the role of dietary restriction and its impact on longevity versus skeletal muscle mass regulation; (iv) the crosstalk between cellular energy metabolism (AMPK/TSC2/SIRT1) and survival (FOXO) versus growth and repair of SkM (e.g. AMPK vs. mTOR); and (v) the impact of protein feeding in combination with dietary restriction will be discussed as a potential intervention to maintain SkM mass while increasing longevity and enabling healthy aging.
Keywords: AKT, AMPK, cachexia, calorie restriction, FOXO, high-protein diets, IGF-I, IRS-1, lifespan, longevity, MAFBx, mTOR, MURF, regeneration, sarcopenia, satellite cells, SIRT, SkM, TSC
Sarcopenia: demographics and impact on quality of life in humans
Life expectancy is increasing rapidly in many countries. As a consequence, there are a greater proportion of older people making up our global population. In the UK, 10 million people are currently over 65 years of age, with the latest projections suggesting that this will increase to 19 million people by 2050 (Cracknell, 2013). Age is the primary risk factor for a multitude of pathological conditions, including Alzheimer’s disease, cardiovascular disease, type II diabetes and sarcopenia. Sarcopenia is the age-related loss of Skeletal Muscle (SkM) mass and function (Rosenberg, 1997). Muscle loss is evident in sedentary humans at 25 years of age, with a 10% loss in peak lean SkM mass at 40 years of age, which increases to 40% at 70 years of age (Porter et al., 1995). Indeed, from age 50, muscle mass is lost at a rate of 1–2% per year (Hughes et al., 2001). This loss impacts negatively on functional and metabolic performance, maximal strength and muscle quality (Renault et al., 2002; Morse et al., 2005a,b; Rossi et al., 2008). Importantly, loss of functional capacity in skeletal muscle with age is strongly correlated with decreased quality of life and increased frailty, morbidity and early mortality (Rantanen et al., 2003). Given that approximately 40–50% of the population over 80 years of age suffers from sarcopenia, this condition has been recognized as a major geriatric clinical disorder (Cruz-Jentoft et al., 2010). Thus, ameliorating age-related SkM wasting is of high clinical importance if we are to improve quality of life and ultimately reduce the socio-economic impact of sarcopenia.
Overview and Rationale
This review will focus on the cellular and molecular mechanisms that underpin age-related muscle loss and will debate the trade-off that may occur between skeletal muscle maintenance and survival into old age versus whole organism life/healthspan. This concept emerges from the body of research investigating the molecular modulators of aging. It focuses on genetic knockout (KO) of IRS-1 and p70S6K1 as well as transgenic models such as FOXO, SIRT1 and finally pharmacological modulation including mTOR inhibition and sirtuin activation. All of these models have been shown to extend both lifespan and healthspan. Importantly however, all of these pathways are also inextricably shared with those that modulate skeletal muscle mass maintenance. Therefore, this review will seek to discuss the hypertrophic, degradative and sirtuin pathways in relation to their modulatory regulation of lifespan, healthspan and muscle cell survival particularly in inflamed aged environments. Finally, the potential importance of optimizing dietary restriction and amino acid uptake to ameliorate the reduction in SkM mass while promoting healthy aging will be discussed.
Insulin-like growth factors (IGFs) and skeletal muscle
Overview of IGF’s and their role in skeletal muscle mass regulation
The insulin-like growth factor (IGF) family consists of the ligands, IGF-I and IGF-II, the type I and type II IGF cell surface receptors, six specific high-affinity binding proteins (IGFBP-1 to IGFBP-6), IGFBP proteases and other IGFBP-interacting molecules (Holly et al., 2000). They have a wide range of biological functions including embryonic, foetal and adult SkM development (reviewed in Stewart & Rotwein, 1996a). In vivo rodent studies have shown that KO of IGF-I, IGF-II or the IGF-I receptor (IGF-IR) results in animals that are phenotypically small for their gestational age with significant decreases in SkM mass and neonatal lethality (Nabeshima et al., 1993; Lau et al., 1994; Stewart & Rotwein, 1996a,b). Alternatively, increasing circulating IGF-I expression in transgenic mice results in SkM hypertrophy (Matthews et al., 1988). Furthermore, KO of IGF-IIR also results in SkM overgrowth; as IIR acts as a clearance receptor for IGF-II, thus its removal leads to an increase in circulating IGF-II and subsequent hypertrophy (Lau et al., 1994). Our group has extensively characterized the multifaceted roles of the IGF system where they are fundamental in the proliferation, survival, differentiation and hypertrophy of primary human and mouse SkM cells (Stewart et al., 1993; James et al., 1996; Stewart et al., 1996; Stewart & Rotwein, 1996b; Stewart et al., 1999a,b; Foulstone et al., 2001, 2003a,b, 2004; Grohmann et al., 2005; Saini et al., 2008; Stewart & Pell, 2010; Al-Shanti & Stewart, 2011; Saini et al., 2012; Sharples et al., 2013; Player et al., 2014) (Reviewed in Scime & Rudnicki, 2006). Skeletal muscle-derived IGF-I is also important in adult muscle hypertrophy, as demonstrated using liver IGF-I-deficient (LID) mice (Matheny et al., 2009). In this study, despite an 80% reduction in total circulating levels of IGF-I in LID versus control (L/L) mice, following 16 weeks of hypertrophy inducing resistance exercise there was no difference in locally produced IGF-I mRNA or IGF-IR activation between groups (Matheny et al., 2009). Despite these compelling data, the importance of IGF-I in mechanical load-induced hypertrophy following resistance exercise and the development of animal models of nonphysiological hypertrophy have been recently debated. This controversy is reviewed by our group elsewhere, and it not the focus of this current review (Stewart & Pell, 2010; Sharples & Stewart, 2011).
Reductions in IGF-I and associated signalling in aging skeletal muscle
With sarcopenia, a 33% reduction in circulating IGF-I (Benbassat et al., 1997) and a 45% decline in SkM-derived IGF-I mRNA are observed in older (70 ± 0.3 years) vs. younger (20 ± 0.3 years) human males (Leger et al., 2008). A corresponding attenuation in downstream intracellular signalling targets involved in protein synthesis with age has also been described. These include reductions in the activity of PI3K, Akt, mTOR, p70S6K1, 4E-BP1 and EIF2B in older vs. younger counterparts (Terada et al., 1994; Welsh et al., 1997; Pallafacchina et al., 2002; Cuthbertson et al., 2005; Leger et al., 2008). With impairments of these signalling pathways also observed with age following muscle contraction (Fry et al., 2011), a recent study using mouse models attempted to recapitulate declining human serum IGF-I concentrations with age. It should be noted that in rodents, serum IGF-I levels are consistently high and do not decrease until very old age when sarcopenia is observed, whereas in humans, serum IGF-I is highest during adolescence and declines earlier in the life course, starting in middle age and paralleling the onset of sarcopenia. This study suggested that mice with reduced serum IGF-I at 1 year of age had significantly deteriorated healthspans. They exhibited increased liver weight and inflammation and increased incidence of hepatic tumours. Importantly, in SkM tissue, increased oxidation of proteins was observed, indicative of increased oxidative stress (Gong et al., 2014), overall suggesting an important role for IGF-I in reducing some, but not all (see below), age-associated pathologies.
We have recently developed and begun to characterize the roles of the IGFs, their receptors and modulatory binding proteins in an in vitro murine cell model of SkM aging via the following: (i) comparisons of parental (older) vs. daughter (younger) cell populations and (ii) multiple population doublings as a way of artificially aging cells (Sharples et al., 2010, 2011, 2012, 2013). These studies demonstrated that IGF binding protein levels are increased in cells that display aging phenotypes via mechanisms that ultimately reduce the activity of Akt (Sharples et al., 2011, 2013). These observations correspond with impaired differentiation and hypertrophy of myotubes (Sharples et al., 2010, 2011, 2012; Deane et al., 2013). These phenotypes are also observed in primary human SkM cells isolated from aged vs. young donors (Collins et al., 2007; Bigot et al., 2008; Pietrangelo et al., 2009; Beccafico et al., 2010). These effects correspond with a loss of myogenicity (Hidestrand et al., 2008) in the face of unchanged telomere length and telomerase activity (O’Connor et al., 2009). Together, the majority of evidence (both in vitro and in vivo) therefore points towards the need for IGF-I and activation of its downstream signalling pathways to maintain skeletal muscle mass across the lifespan.
Reduced Insulin/Insulin-like-Growth Factor Signalling (IIS): enhanced longevity vs. reduced muscle mass in aging skeletal muscle
IGF and Insulin Receptor Substrate (IRS-1)
Reductions in IGF-I activity with age are associated with reductions in SkM size and function. However, reduced signalling through the IIS pathway is also associated with increased lifespan and healthspan in model organisms (Clancy et al., 2001; Holzenberger et al., 2002; Barbieri et al., 2003; Tatar et al., 2003; Giannakou & Partridge, 2007; Piper et al., 2008; Selman et al., 2008; Vallejo et al., 2009; Kenyon, 2011; Selman et al., 2011). For example, both female and male mice globally lacking insulin receptor substrate 1 (Irs1−/−) are long lived (Selman et al., 2008, 2011). Female mice lived 32% longer compared to wild-type controls, equating to a mean lifespan of 971 days in the Irs1−/− mice compared with 738 days in wild-type control animals. Interestingly, Irs1−/− mice showed resistance to several parameters associated with aging, including bone, skin, metabolic, immune and motor dysfunction (Selman et al., 2008). Thus, Irs1−/− mice, in common with several other long-lived models, enjoy a greater period of their life free from various age-associated pathologies (Selman and Withers 2011). Importantly, Irs-1−/− mice display reduced growth compared to wild-type animals perhaps due to the important role for IRS-1 in embryonic and postnatal growth (Withers et al., 1998, 1999). Furthermore, mice with growth hormone (GH)/IGF-I defects, while phenotypically growth retarded compared with wild-type littermates, also exhibit enhanced longevity, lower DNA mutation frequencies, higher DNA excision repair and secondary attenuation of IIS (Bates & Holder, 1988; Pell & Bates, 1992; Bartke & Brown-Borg, 2004; Bartke, 2005; Garcia et al., 2008; Garinis et al., 2009; Masternak et al., 2009; Page et al., 2009).
While there are clear benefits of reduced IIS signalling for lifespan and aspects of healthspan, as eluded to above, reductions in SkM mass correspond with decreases in IGF-I with age. Indeed, some studies suggest that bone, cardiac muscle and other tissues display aged characteristics when IGF-I is impaired (Adamo and Farrar, 2006; Anversa, 2005; Ceda et al., 2005; Geusens and Boonen, 2002). Indeed, Irs1−/− mice have reduced body weight and fat mass compared to age-matched controls (Pete et al., 1999; Selman et al., 2008) with reduced gastrocnemius SkM weight that is proportionately greater than the decrease seen in total body weight (Pete et al., 1999). Irs1−/− mice are, however, more resilient to age-associated osteoporosis compared to controls, which may account somewhat for this discrepancy. A recent study using an inducible liver-derived IGF KO mouse, allowing temporal reductions of IGF of 70% in the serum, showed that lower IGF from the age of 1 year resulted in greater oxidative stress in SkM, accelerated bone loss and reduced lifespan (Gong et al., 2014). Indeed, across 31 genetically diverse inbred mouse strains, lower serum IGF-I was associated with enhanced longevity (Yuan et al., 2009). Furthermore, human population studies suggest that reductions in IGF-I at young age but elevations at old age might maximize healthy lifespan, reviewed in Yang et al. (2005). To the authors’ knowledge, the only study to investigate potential mechanisms of SkM adaptation with IRS-1 loss suggested that it did not affect glucose uptake or GLUT1/4 function in electrically stimulated mouse muscle (Dumke et al., 2001). Skeletal muscle mass or synthetic/degradative signalling was, however, not investigated in this study. Overall, it is clear that reductions in IIS enhance lifespan and delay some aging-associated parameters yet perhaps results in small body size that is characterized by both reduced fat mass and potentially, proportionally smaller SkM mass. However, more investigation into SkM mass and the corresponding cellular signalling in Irs1−/− mice into old age is required in the near future to understand the potential crosstalk between the mechanisms that control increased lifespan and healthspan while contributing to reductions in SkM mass with age.
Mammalian target of Rapamycin (mTOR)
In addition to reduced IIS, reduced signalling through the target of rapamycin (TOR) signalling pathway has also been shown to modulate lifespan and increase healthspan in model organisms (Kapahi et al., 2004; Kaeberlein et al., 2005; Powers et al., 2006; Hansen et al., 2007; Harrison et al., 2009; Anisimov et al., 2010; Bjedov et al., 2010; Miller et al., 2011; Robida-Stubbs et al., 2012; Zhang et al., 2014). Longevity in humans is also associated with reduced mTOR signalling (Slagboom et al., 2011; Passtoors et al., 2013). The TOR or mTOR (mammalian target of rapamycin) is, however, a key regulator of SkM growth where it also plays a central role in the crosstalk between growth and metabolism in a wide variety of cell types (Inoki et al., 2003) and SKM (most recently see Hamilton et al., 2014). Mammalian target of rapamycin regulates its hypertrophic effects in SkM through the phosphorylation of downstream effectors such as P70S6 kinase 1 (S6K1) and eIF4E-binding protein1 (4E-BP1) (reviewed in Schiaffino et al., 2013). Their roles in SkM growth following contraction and mechanical load-induced hypertrophy, synergistic ablation, myotube hypertrophy and amino acid sensing are also well defined (Fujita et al., 2007; Drummond et al., 2009; Willett et al., 2009; Goodman et al., 2011; Miyazaki et al., 2011; Philp et al., 2011; Jacobs et al., 2013; Hamilton et al., 2014). In older people, mTOR becomes less responsive to contraction-induced activation (via resistance exercise), compared with young adults (Fry et al., 2011). The activity of mTOR in response to amino acid feeding is also impaired in older individuals, a phenomenon known as ‘anabolic’ resistance (Cuthbertson et al., 2005).
Rapamycin-induced inhibition of mTOR has, however, been shown to increase lifespan in yeast, drosophila and mice (Powers et al., 2006; Harrison et al., 2009; Anisimov et al., 2010; Bjedov et al., 2010; Miller et al., 2011; Robida-Stubbs et al., 2012; Wilkinson et al., 2012). Further, rapamycin diminishes a range of aged-related pathologies (reviewed by Johnson et al., 2013b), and despite a contentious study claiming that it does not (Neff et al., 2013), the wide consensus is that appropriate modulation of mTOR signalling could be an important route of intervention to slow aging and increase healthspan (reviewed by Johnson et al., 2013a). However, in skeletal muscle rapamycin-induced inhibition of mTOR has been shown to impair myogenic differentiation (Willett et al., 2009), blunt the anabolic response to overload and nutrients (Goodman et al., 2011), with muscle-specific inactivation of mTOR leading to myopathy (Risson et al., 2009). These data therefore suggest, perhaps paradoxically, that despite inhibition of mTOR increasing lifespan and improving many age-related pathologies, mTOR signalling plays a critical role in maintaining SkM mass and anabolism. Despite this, the only study that has so far investigated muscle size and function in mice with advancing age, suggests that muscle cross-sectional area and grip/paw strength were unaffected by a 16-month treatment of rapamycin vs. aged-matched controls (Neff et al., 2013).
Similar to rapamycin-induced mTOR inhibition, global deletion of the ribosomal protein S6K1 in mice, a downstream effector of mTOR, also increases lifespan and improves healthspan in mice (Selman et al., 2009). In contrast to rapamycin treatment having no impact on muscle size (Neff et al., 2013), S6K1(−/−) myotubes are smaller, despite having a normal number of nuclei, and their response to a hypertrophic stimuli of IGF-I or nutrients is blunted (Ohanna et al., 2005). Further, deletion of S6K1 in mice induces SkM atrophy even in the presence of high nutrient availability via AMPK activation, where AMPK inhibition in S6K1-deficient myotubes restores SkM growth via increases in myotube diameter and sensitivity to nutrient signals (Aguilar et al., 2007). In aged human SkM, S6K1 is downregulated in response to amino acid feeding (Cuthbertson et al., 2005) and attenuated in old vs. young rodents during recovery from immobilization-induced atrophy (Morris et al., 2004). S6K1 is also reduced in contracting aged SkM in comparison with young muscle, suggesting it plays an important role in SkM protein synthesis, which is hampered with age (Parkington et al., 2004; Kumar et al., 2009). However, surprisingly little is currently known about whether basal muscle maintenance and function is altered in the context of aging in long-lived mTOR mutant or, as discussed, rapamycin-treated mice. Studies examining protein synthesis, protein degradation and SkM function in long-lived mouse models are urgently required if we are to increase our understanding of the potential trade-off between longevity and muscle function. Depicted in Figure 1 (Fig.1).
Fig 1.
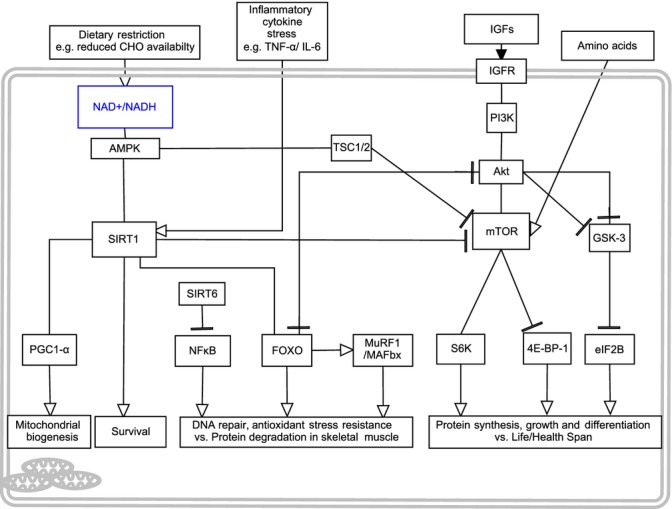
Depicts the extracellular and intracellular signaling molecules involved in the cross-talk between skeletal muscle mass regulation and life/health-span modulation. Genetic or pharmacological suppression of IIS, TOR and Sirtuin pathways increase organism life/health-spans. However, these pathways are fundamental in protein synthesis, growth, differentiation and survival in skeletal muscle into old age. This figure therefore provides the potential molecular and cross-talk modulators for this paradigm of lifespan versus muscle mass maintenance with age.
Sirtuins: divergent roles in the modulation of lifespan vs. skeletal muscle mass
Sirtuins and their roles in aging and longevity
Significant recent research effort has focused on elucidating the various roles of sirtuins (silent information regulator 1–7; Sir1-7) in aging. Sirtuins are a group of seven highly conserved protein deacetylases involved in the process of chromatin remodelling and gene regulation (see Morris, 2013). They have also been shown to have pathophysiological relevance in cancer, obesity, SkM, inflammation and neurodegeneration (Rodriguez & Fraga, 2010; Schug & Li, 2011; Park et al., 2012; Donmez & Outeiro, 2013). There is emerging evidence that these proteins may regulate SkM mass, potentially through alterations in IGF-I and associated signalling (discussed below). The metazoan Sir2 proteins are recognized, somewhat controversially, for their role in regulating lifespan in yeast, worms and fruit flies (Kaeberlein et al., 1999; Burnett et al., 2011; Viswanathan & Guarente, 2011). The rodent homologue of Sir2, SIRT1, does not increase lifespan in mice, although overexpression does improve healthspan (Herranz et al., 2010). More specifically, neural-specific SIRT1 overexpression has been shown to increase lifespan and delay aspects of aging relative to wild-type littermates (Satoh et al., 2013). Downregulation of SIRT1 also induces an aging phenotype (Sommer et al., 2006). Activation, rather than overexpression of SIRT1 using a small molecular activator (resveratrol), reportedly reduces age-related ill health in ad libitum fed old mice, if administered from the middle age, it is, however, without impact on lifespan (Pearson et al., 2008; Miller et al., 2011). Under more pathological conditions, resveratrol administration does extend lifespan, specifically in mice placed on high fat diets (Baur et al., 2006). It is worth stating here that resveratrol has pleiotropic cellular targets and therefore, effects cannot always be directly linked to SIRT activation per se and results should be interpreted with this caveat in mind. Interestingly however, SIRT6, when overexpressed in male mice, has also been attributed to increased lifespan (Kanfi et al., 2012b) and short-lived phentoypes are evident in SIRT6 KO animals (Mostoslavsky et al., 2006).
Sirtuins and their impact on IGF signalling and skeletal muscle
In terms of SkM growth and protein synthesis, evidence exists, implicating SIRT1 and SIRT6 as negative regulators of IGF-I and downstream Akt/mTOR signalling (Ghosh et al., 2010). For example, in mouse neural cells, SIRT1 silencing and overexpression increased and decreased IGF-I and associated Akt signalling, respectively (Sansone et al., 2013). Similarly, SIRT6 overexpression in mice has been associated with a reduction in circulating IGF-I (Kanfi et al., 2012a). An exciting recent link between SIRT1 and IGF-I has been established in a range of nonskeletal muscle human cell types. When stimulated with exogenous IGF-I for prolonged periods, cells exhibited reduced SIRT1 deacetylase activity, increased p53 acetylation and increased senescence, when compared with cells exposed to acute administration of IGF-I exhibiting increased proliferation (Tran et al., 2014). Although speculative, reductions in IGF-I with age could be an attempt to alleviate senescence and maintain SIRT1 activity (Tran et al., 2014). In SkM, our group has shown that the induction of apoptosis, by low-dose tumour necrosis factor-alpha (TNF-α) with the addition of IGF-I, is elevated compared with TNF-α administration alone. Death was associated with increased SIRT1 mRNA levels, which when suppressed using SIRT1 siRNA, culminated in exacerbated, not reduced, apoptosis (Saini et al., 2008, 2012). Overall suggesting that under conditions of both anabolic and catabolic conflicts, SIRT1 was important to the maintenance of survival in skeletal muscle cells. Therefore, SIRT1 appeared fundamental in negatively regulating IGF-I basally, yet in the presence of inflammatory catabolic stress (Saini et al., 2008, 2012), or where IGF-I exposure was prolonged enough to induce cell death (Tran et al., 2014), SIRT1 was important in maintaining survival. It is also worth noting that SRT2104, a synthetic small molecular activator of SIRT1, reduced circulating TNF-α in mice (Mercken et al., 2014). Suggesting a potential regulatory loop between SIRT1 and TNF-α, yet this link in SkM is yet to be directly established. This concept is particularly relevant in aging muscle where chronic low-level TNF-α exposure and changing IGF-I concentrations are strongly associated with muscle wasting in vivo and the pathologies of sarcopenia and cachexia (Li & Reid, 2000; Meadows et al., 2000; Foulstone et al., 2001; Greiwe et al., 2001; Bruunsgaard et al., 2003a,b; Bruunsgaard & Pedersen, 2003; Stewart et al., 2004; Grohmann et al., 2005; Li et al., 2005; Saini et al., 2006, 2008, 2010, 2012).
In addition to its role in regulating IGF-I and survival in the presence of aberrant IGF-I, SIRT1 may also play a role in negatively regulating mTOR. SIRT1 (−/−) mouse embryonic fibroblasts (MEFs) and human HELA cells depleted of SIRT1 using shRNAi resulted in elevated mTOR signalling, which was not abolished by leucine deprivation (Ghosh et al., 2010). In the same study, SIRT1 activators and inhibitors (resveratrol/nicotinamide) reduced and increased mTOR activity, respectively (Ghosh et al., 2010). SIRT1 activation following resveratrol administration in myoblasts inhibited IGF-I-associated signalling (Akt) and abolished leucine-stimulated increases in mTOR (Liu et al., 2010). These studies suggest that any changes in SIRT1 with age in response to catabolic stress or nutrient restriction could potentially impact on mTOR function and result in altered regeneration. Overall, these data present potential negative regulation by SIRT1 on pathways such as Akt/mTOR linked to SkM growth. On the contrary, recent work by Hong et al. (2014) suggested that SIRT1 and SIRT2 deacetylate the substrate of mTOR, S6K, specifically on mTOR-dependant phosphorylation site Thr-389. In this case, acetylation blocked S6K activation and thus, deacetlyation by the sirtuins may actually be involved in the phosphorylation of S6K (Hong et al., 2014). Furthermore, in cardiac muscle, SIRT1 can also deacetylate Akt and PDK, enabling binding to phosphatidylinositol 3,4,5-trisphosphate [PIP(3)], and thus its localization to the membrane where PDK can subsequently facilitate Akt phosphorylation (Sundaresan et al., 2011). Sirtuin activation, however, specifically in SkM tissue or cells through overexpression in rodent models or supplementation of resveratrol/its analogues in humans, requires further investigation to decipher its role in negatively or positively regulating SkM mass. Importantly, based on evidence described above, the reductions in IGF-I seen with age could be an attempt to increase SIRT1 to harness its role in cell survival especially when under a catabolic cytokine stress (e.g. TNF-α) that as mentioned above, is chronically elevated in the circulation and skeletal muscle with age (and discussed in more detail directly below).
Sirtuins and their role in survival and differentiation under catabolic stress in skeletal muscle cells
Despite this apparent trade-off with survival vs. growth, our group has shown that activation of SIRT1 in murine myoblasts following resveratrol administration can begin to rescue differentiation of SkM cells following catabolic stimulation by TNF-α (Saini et al., 2012). This is important when considering that TNF-α is chronically increased in the aging circulation and that it is produced by muscle itself (Greiwe et al., 2001; Bruunsgaard et al., 2003a,b; Bruunsgaard & Pedersen, 2003). In agreement with our group, resveratrol can reverse the negative impact of TNF-α on myotube hypertrophy (Wang et al., 2014). Similarly, activation of SIRT1 using SRT2104 attenuated SkM mass losses of the gastrocnemius and soleus in mice following 2 weeks of hindlimb unloading (Mercken et al., 2014). SRT2104 also extended lifespan, without reducing SkM weight into old age (Mercken et al., 2014). Therefore, as well as an important role in myoblast survival, SIRT1 may also be involved in maintaining adequate differentiation, hypertrophy and attenuating atrophy in vivo during stress stimuli such as those experienced with chronic inflammation or disuse.
Finally, it is important to consider that changes in the [NAD+]/[NADH] ratio occur during skeletal muscle differentiation and this changing ration in turn can regulate SIRT1 (Sartorelli & Caretti, 2005). A reduction in the [NAD+]/[NADH] ratio coincides with skeletal myogenesis, whereas an increase is associated with impaired myogenesis (Fulco et al., 2003). It is clear, however, that differences prevail in terms of derived data. Indeed, Fulco et al. (2008) suggested that increasing SIRT1 activity in mouse and human SkM cells impaired differentiation and myosin heavy chain production (Fulco et al., 2003, 2008), which differs from our work with TNF-α, but complements more recent unpublished work where under control conditions, resveratrol increased proliferation in both control and artificially aged myoblasts but impaired differentiation (Deane CS, Hughes DC, Sharples AP, unpublished). An increase in proliferation, inhibition of p21cip and p27kip and a reduction in differentiation following SIRT1 overexpression in rat myoblasts have also been previously reported (Rathbone et al., 2009). Therefore, despite its proposed negative regulation of IGF-I/Akt/mTOR, SIRT1 seems to be fundamental to SkM cell survival, enabling proliferation and impairing differentiation under control conditions, yet protecting differentiation under conditions of stress. Importantly, the impact of activating SIRT in aged SkM cells/tissue basally or under stress remains to be fully determined especially, we hypothesise, in situations of dietary restriction that directly regulate the NAD/NADH ratio and impact on SIRT expression.
Sirtuins: regulators of longevity and survival vs. activators of protein degradation in SkM via FOXO transcription factors
In addition to its role in SkM proliferation, SIRT1 has also been implicated in controlling protein degradative pathways, specifically via forkhead box protein O (FoxO) transcription factors. These transcription factors are involved in targeting and activating members of the ubiquitin proteasome, such as muscle atrophy F-box (MAFbx/atrogin1), muscle RING finger 2 (MuRF1), and autophagy–lysosome pathways involved in protein degradation (Sandri et al., 2004; Edstrom et al., 2006; Sandri, 2008). SIRTs have been shown to activate both FOXO3a gene expression and deacetylate FOXO3a, thereby activating FOXO DNA binding and elevating the expression of target genes such as p27(Kip1), manganese superoxide dismutase and Bim, proteins associated with cell cycle arrest, oxidative stress and apoptosis respectively (Brunet et al., 2004; Wang et al., 2007; Jacobs et al., 2008). The activation of FOXO transcription factors by the SIRT family appears to, however, impair the ability of FOXO to promote cell apoptosis, instead shifting its function towards oxidative stress resistance and DNA repair (Brunet et al., 2004; Greer & Brunet, 2005; Wang et al., 2007). It is also well established that overexpression of FOXO can extend lifespan in drosophila (Giannakou et al., 2004; Min et al., 2008; Alic et al., 2014). Interestingly, in TNF-α-stimulated SkM cells the activation of SIRT1 via resveratrol restored Akt/mTOR/S6K and 4E-BP1 signalling and reduced FOXO1 but not FOXO3a protein levels, all of which were unchanged basally (Wang et al., 2014). Therefore, the role for SIRT1 activation on FOXO3a in SkM tissue with age requires further investigation. FOXO1 and its role in oxidative stress resistance in aging SkM also requires attention, especially following catabolic stress or dietary restriction where SIRT1 elevation is associated with survival. It is worth mentioning here that class I histone deactylases (HDACs) (sirtuins are class III HDACS) have also been linked with activating FOXO3a and the SkM-atrophy programme (via MAFbx/atrogin-1) during nutrient deprivation and disuse-induced atrophy (Beharry et al., 2014). Potentially this suggests that FOXO1 and FOXO3a are modulated by class I and class III HDACs, respectively, and this may account for some of the discrepancy detailed above. The role of the SIRTs in SkM is intriguing and warrants further investigation, specifically the promotion of longevity via resistance to oxidative stress vs. increased protein degradation with aging.
Sirtuins and NF-Kβ and their roles in longevity and skeletal muscle loss with age
While discussing protein degradation above, it is worth noting that SIRT6 has been associated with modulating lifespan via nuclear factor κB (NF-Kβ) signalling (Yeung et al., 2004; Kanfi et al., 2012b). The inhibition of NF-Kβ delays DNA damage, cellular senescence and oxidative stress during aging (Tilstra et al., 2012). However, in SkM, NF-Kβ is another important protein where cytokine and oxidative stress signalling converge to reduce myoblast differentiation, induce atrophy and increase protein degradation (Langen et al., 2001; Hunter & Kandarian, 2004; Lu et al., 2012). SIRT6 attenuates NF-Kβ signalling through histone deacetylation of NF-Kβ gene promoter regions and suppresses those genes associated with senescence and aging (Kawahara et al., 2009). The deletion of SIRT6 in KO mice also results in shortened lifespan and significantly reduced body weight, suggesting an important developmental and postnatal role for this protein:protein interaction (Mostoslavsky et al., 2006). Studies by our laboratory suggest that inhibition of NF-Kβ can promote delayed myoblast apoptosis in the presence of TNF-α (Stewart et al., 2004). It is, however, worth noting that there was no change in NF-Kβ during disuse atrophy (2 weeks hindlimb suspension) even in the presence of SRT2104 (Mercken et al., 2014). Interestingly, very recent work suggests SIRT activation in murine models via SRT2104 causes a reduction in the ratio of phosphorylated NF-Kβ to total protein (Mercken et al., 2014). This therefore suggests that SIRT1 and SIRT6 may be important in reducing NF-Kβ. Overall, SIRT1 and/or SIRT6 may regulate lifespan as a consequence of reduced IGF-I signalling and potentially attenuate the effects of inflammatory NF-Kβ signalling.
Effect of Dietary Restriction (DR) on longevity and skeletal muscle mass
Calorie restriction is defined as a reduction in energy intake, while maintaining nutrient intake, relative to that consumed normally by individuals with free (ad libitum) access to food (Selman, 2014). For the purposes of this review, dietary restriction (DR) will incorporate both calorie restriction and those interventions in which macro/micronutrients are altered without any overall change in energy intake. DR is the most reproducible intervention, to date, to extend medium and maximum lifespan in various model species (Mair & Dillin, 2008; Speakman & Selman, 2011; Selman, 2014). In mice, there seems to be a strain-specific association with DR and longevity, and in primates, the link between lifespan extension and DR may also be confounded by genetic heterogeneity (reviewed by Selman, 2014). Nevertheless, DR reduces incidence and severity of various pathological conditions in rodents and primates, which are leaner, and display reductions in insulin resistance, glucose intolerance, cognitive decline and immune dysfunction (Masoro et al., 1982; Barger et al., 2003; Selman et al., 2005; Mattison et al., 2012), indicating DR per se is beneficial for health.
Trade-off between cellular energy metabolism and growth in skeletal muscle with dietary restriction
The intuitive impact of chronic DR on SkM mass is that over time, absolute muscle mass decreases. This is not surprising if you consider that in the presence of nutrient restriction, the cell shifts away from growth in an attempt to survive. Further, protein from SkM can provide energy during severe nutrient restriction. One of the first studies to demonstrate this and to establish the molecular link between AMPK energy sensing and cellular growth through mTOR/S6K signalling was that of Inoki and collegues (Inoki et al., 2003). Using various cell types (HEK293, MEF, EEF, LEFs) under starvation conditions, they reported increased AMPK activity and phosphorylated tuberous sclerosis 2 (TSC2). The TSC2 inhibited mTOR and other substrates, including S6K, 4EBP-1 and EIF2, which resulted in reduced cell size and growth rates. The role of TSC2 in this process was confirmed in TSC2 KO cells, which grew and maintained normal size in the presence of starvation. The AMPK activation of TSC2 and inhibition of mTOR therefore appears central in responses to energy deprivation. Fascinating but perhaps unintuitively, given the data thus far, DR appears to delays or prevent age-related loss of SkM mass in rats and rhesus monkeys via attenuation of DNA damage, proteosomal machinery, autophagy, inflammatory signalling and mitochondrial abnormalities (Aspnes et al., 1997; Phillips & Leeuwenburgh, 2005; Hepple et al., 2008; McKiernan et al., 2011). Indeed, short-term DR can potentially increase SkM stem cell availability and subsequent SkM repair following cryo-injury in young and old mice (Cerletti et al., 2012). In a recent in vivo study, chronic DR (by 30% of recommended daily intake) for a period ranging from 4 to 20 years (mean 9.6 years), resulted in reduced IGF-I levels, and a threefold reduction in Akt mRNA/ 30–50% reduction in Akt activity, together with increased FOXO3a and FOXO4 expression (Mercken et al., 2013). These changes in FOXO were reported to modify several genes linked to longevity including genes associated with stress resistance, antioxidants, DNA repair, protein turnover and cell death (Mercken et al., 2013). In SkM however, this shift away from growth towards stress resistance, would potentially reduce protein synthesis and increase degradation over time (Sandri et al., 2004; Edstrom et al., 2006). Furthermore, superoxide dismutase 2 (SOD2) expression, a transcriptional target of FOXOs, was increased under DR, as was DNA damage-binding protein 1 (DDB1), both key regulators of DNA repair. Further, cyclin D2 was significantly downregulated during moderate DR, as a fundamental orchestrator of cell cycle progression for proliferation or growth (Mercken et al., 2013). Interestingly, DR in rats also reduced levels of the inflammatory cytokine TNF-α and associated signalling (Phillips & Leeuwenburgh, 2005). These studies therefore suggest that chronic moderate (∼30%) DR results in transcriptional reprogramming, which shift cellular regulation from growth to maintenance/repair and lifespan activities, while potentially reducing local inflammation. Perhaps most importantly, humans and mice on DR diets had higher lean SkM mass-to-fat mass ratios (Mercken et al., 2013). Therefore, there is potentially an optimal level of DR which has the beneficial effect of longevity, while perhaps preventing growth but not inducing muscle loss. Although overall SkM mass is likely to be reduced by long-term DR, the ratio of lean mass to fat mass may be greater and total body weight maybe reduced, a signature conducive of reduced metabolic disease risk. It remains to be determined, however, whether chronic DR changes SkM strength or the proportions of extracellular matrix to muscle tissue, or alters contractile properties and force per cross-sectional area/muscle quality. Indeed, the influence on force production following DR could be affected by fibre type, as type I fibres were ∼62% larger after DR (30% DR for 12 years) in rhesus monkeys vs. control. Furthermore, in this study it was observed that there was delay in type II fibre atrophy with age (McKiernan et al., 2011). So while data of long-term studies are limited, they do suggest potential for both longevity and muscle health.
Despite this body of work, several other studies oppose these findings. For example, although different to sustained DR, Lee and Goldberg investigated the impact of acute fasting in mice and showed that this resulted in a reduction in SIRT1 activity and an increase in the atrogenes MuRF-1 and atrogin-1, which ultimately led to a significant decrease in SkM mass (Lee & Goldberg, 2013). Dietary restriction (−30%) for 6 weeks, in combination with exercise, also reduced gastrocnemius SkM weight and cross-sectional area in comparison with similarly exercised mice under ad libitum feeding (Park et al., 2013). However, it should be noted that this study did not include a DR or ad libitum alone group. This does, however, highlight the temporal role of short-duration fasting vs. longer duration DR and the modulation of SIRT1 (McKiernan et al., 2012; Mercken et al., 2013). DR in combination with physical activity and its impact on SkM phenotypes therefore requires further investigation. Finally, it is unlikely that DR is a pragmatic intervention for humans, given that there is a considerable level of motivation and restraint required, where DR mimetics maybe more practical as reviewed previously by Selman et al. (Selman, 2014).
Roles of amino acid feeding or high-protein diets in association with calorie restriction: potential impact on skeletal muscle mass vs. disease and longevity
One of the issues with DR is the contribution of total calories from carbohydrates vs. proteins. Most studies do not differentiate between the two. It is well established that protein intake can enhance muscle protein synthesis in a dose-responsive manner in young and old adults (Cuthbertson et al., 2005; Moore et al., 2009). Furthermore, increasing dietary protein can help maintain SkM mass during periods of disuse (reviewed in Wall & van Loon, 2013) and induce greater increases in skeletal muscle hypertrophy following chronic supplementation when combined with exercise (resistance) vs. exercise alone (meta-analysis Cermak et al., 2012). Indeed, there is substantial support to suggest that with DR, overall weight loss is no different with higher protein intakes vs. DR alone (Sacks et al., 2009; de Souza et al., 2012). With some acute trials showing that fat mass decreases while SkM is spared (Krieger et al., 2006), importantly, exercise in combination with higher protein content in DR diets seems to have a SkM maintaining effect (Garthe et al., 2011; Josse et al., 2011; Mojtahedi et al., 2011), without negative impact on markers of mitochondrial biogenesis, albeit after acute fasting in humans (Taylor et al., 2013). Interestingly, undertaking DR that is protein rich reduces both body mass and percentage body fat, with associated reductions in circulating insulin and IGF-I levels (Maestu et al., 2010), alluding to potential benefits for lifespan while potentially maintaining SkM mass. Supplementation with branched-chain amino acids (BCAAs) such as leucine, isoluecine, valine or metabolites of leucine such as β-hydroxy-β-methylbutyrate (HMB) have become a favoured intervention as they have been shown to activate mTOR and protein synthesis in SkM to a greater extent compared with other essential/nonessential amino acids (Atherton et al., 2010; Pimentel et al., 2011; Churchward-Venne et al., 2012; Salles et al., 2013). Leucine alone can activate protein synthesis in humans to the same extent as whey protein and mixed essential amino acids plus leucine when administered 1–3 h postresistance exercise (Churchward-Venne et al., 2012). However, the requirement for whey protein for optimal protein synthesis 3–5 h postexercise is acknowledged (Churchward-Venne et al., 2012; Phillips, 2014). Previously, Mourier and colleagues observed that DR in human males (wrestlers) when combined with supplementation of mixed BCAAs led to a reduction in total body mass and fat mass (−17.3%), although SkM mass was unchanged (Mourier et al., 1997). This suggests a potential role for BCAAs in maintaining SkM mass under DR conditions. Furthermore, a recent study highlighted that HMB attenuated the loss of SkM mass observed following DR in murine exercise models (Park et al., 2013). Mice underwent exercise at 6 m.min−1 run for 1 h, three times a week alone or combined with HMB and/or DR. The HMB animals had higher lean mass than the training alone group. Grip strength decreased under DR, but was maintained in DR mice supplemented with HMB. Interestingly, gastrocnemius mass and myofibre cross-sectional area were greater with HMB in the presence of a DR diet compared to DR alone, albeit there were no data reported for either ad libitum or HMB alone supplemented mice (Park et al., 2013). This latter finding was also associated with the reduced ubquitin ligase, MAFbx, alluding to reduced protein degradation. Surprisingly however, Akt and mTOR mRNA were elevated under DR conditions in SkM. Speculation based on evidence presented in above sections suggests this may be due to increased SIRT1, yet this hypothesis requires further investigation. Therefore, in the light of the above discussion it would be prudent to investigate, on a background of DR, how AMPK and SIRT1 (energy sensing) change in the presence of BCAAs and the way in which they impact on Akt/mTOR (growth) via the molecular modulators of TSC1/TSC2 (discussed above and seen in Fig.1).
Finally, it is important to note that increased protein intake, especially BCAAs, stimulates targets such as mTOR and S6K, which are downstream of IIS, the precise signalling which is reportedly suppressed to enable longevity and to reduce age-related disease. This therefore contributes to the recently debated paradigm whereby downstream IIS signalling is still activated, yet independently of IGF binding to its receptor, and thus protein synthesis in SkM mass may be maintained with increased protein intake during aging. However, it has been conversely suggested that increased protein intake may increase incidence of diseases, such as cancer, and thus impact negatively on longevity (Renehan et al., 2004). Indeed, it is known that cancer patients who do not respond to chemotherapy or are end-stage patients have reduced protein diets that, while potentially adding to the chronically inflamed milieu that causes SkM loss (cachexia), can slow tumour progression. Examples include animal models where DR can attenuate tumorigenesis via inhibition of mTOR, whereas leucine feeding can increase pancreatic tumour growth in both lean and overweight mice (Vellai et al., 2003; Bjornsti & Houghton, 2004; Hursting et al., 2010; Lashinger et al., 2011; Liu et al., 2014). Restricting the amino acid methionine can also limit tumour growth, and both methionine and essential amino acid restriction increase lifespan in rodents (Richie et al., 1994; Miller et al., 2005; Emran et al., 2014; Sinha et al., 2014). Overall, these studies suggest caution for cancer patients and amino acid supplementation, even those who suffer with muscle loss (Liu et al., 2014). The role of higher protein diets with age and the impact on disease risk and early mortality have recently received a high level of attention. Cohorts of 6381 adults aged 50 and over were studied for their habitual dietary intake and macronutrient composition with corresponding disease and mortality incidence (Levine et al., 2014). Between the ages of 60 and 65, those who reported high animal-derived protein intake had a 75% increased risk in overall mortality and a fourfold increase in cancer risk during the subsequent 18 years. If aged over 65 years of age, however, higher protein intake was associated with reduced cancer risk, but a fivefold increased risk of diabetes. These results therefore suggested that a low-protein diet is potentially beneficial in midlife; however, the benefits reduce with age. In an attempt to compliment these studies with mechanisms, high-protein diets were implemented in middle-aged mice, where the increase in GH/IGF signalling observed was associated with increased progression of tumours. The authors did, however, suggest that low protein impacted negatively on SkM mass in aged mice (Levine et al., 2014). In agreement with this study, an investigation published in the same issue as that by Levine et al. using a Geometric Framework approach to investigate the contributions of protein-to-carbohydrate ratios and their association with increased longevity in mice, suggested that healthy aging is not as a consequence of high-protein low calorie diets, but low-protein (especially BCAAs) diets, with the remaining macronutrients being made up of carbohydrate rather than fat (Solon-Biet et al., 2014). Also, data by Levine et al. have been scrutinized in terms of the methodological design. For example, 24-h dietary recalls suggesting up to 18 years of habitual diet are potentially not appropriate to account for lifelong habitual dietary intake. Furthermore, the grouping of the low- to high-protein categories [based on Institute of Medicines’ (IOM) Acceptable Macronutrient Distribution Range] has also received attention, where the low-protein group would probably be classed as protein deficient. It is also worth stating that in the total cohort (50 years and over), the level of protein intake was not associated with differences in all-cause, cancer or CVD mortality. Importantly, however the study did find a significant association between the subjects aged 50–55, higher protein consumption and cancer/mortality. Amongst 2253 subjects, the risk of cancer and mortality was increased in the high-protein subjects who also had higher IGF-I serum levels. It is indeed, established that people in the highest circulating IGF-I quintiles are at the highest risk of developing cancer (Hankinson et al., 1998; Kaaks et al., 2000; Giovannucci et al., 2003) and the role of IGF-I and associated signalling in cancer cells and tumour development is fairly robust (Pollak et al., 2004; Guevara-Aguirre et al., 2011). It is important to note that these are similar pathways to growth/amino acid stimuli required for SkM maintenance with age. The future paradigm we should be addressing would therefore be the trade-off between maintenance of SkM mass vs. longevity, potentially at the expense of age-related diseases.
Conclusion
The understanding of aging and the development of interventions to increase healthy lifespan have been greatly aided by the development of genetic mutants for IIS, TOR and sirtuin pathways as well as the use of pharmacological agents known to act on these pathways. However, all of these pathways are fundamental in regulating the trade-off between survival and maintenance vs. growth, particularly in skeletal muscle where age-associated losses in SkM mass and function are observed with advancing age. This provides a paradigm in which there is potentially reduced regenerative capacity within SkM tissue with age in an attempt to promote longevity of the organism and survival within the tissue. Optimizing dietary restriction (DR) or using DR mimetics in combination with amino acid administration may be critical interventions to help attenuate SkM loss with advancing age, while enabling healthy aging.
Author contributions
Sharples, AP is the corresponding author who instigated/conceptualized the review and wrote the first draft of the manuscript, amended all drafts and worked extensively on the final draft as well as in creating the final figure to complete the manuscript. Hughes, DC and Deane, CS wrote sections of the review with Sharples, AP and significantly inputted to the writing and reading of the manuscript. Saini A, Selman C and Stewart, CE contributed extensively to editing the manuscript while contributing both very valuable comments and important insights and additions throughout.
Funding
No funding information provided.
Conflict of interest
None declared.
References
- Adamo ML, Farrar RP. Resistance training, and IGF involvement in the maintenance of muscle mass during the aging process. Ageing Res. Rev. 2006;5:310–331. doi: 10.1016/j.arr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Aguilar V, Alliouachene S, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudiere E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab. 2007;5:476–487. doi: 10.1016/j.cmet.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Alic N, Tullet JM, Niccoli T, Broughton S, Hoddinott MP, Slack C, Gems D, Partridge L. Cell-nonautonomous effects of dFOXO/DAF-16 in aging. Cell Rep. 2014;6:608–616. doi: 10.1016/j.celrep.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Shanti N, Stewart CE. Inhibitory effects of IL-6 on IGF-1 activity in skeletal myoblasts could be mediated by the activation of SOCS-3. J. Cell. Biochem. 2011;113:923–933. doi: 10.1002/jcb.23420. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am. J. Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P. Aging and longevity: the IGF-1 enigma. Circ. Res. 2005;97:411–414. doi: 10.1161/01.RES.0000182212.09147.56. [DOI] [PubMed] [Google Scholar]
- Aspnes LE, Lee CM, Weindruch R, Chung SS, Roecker EB, Aiken JM. Caloric restriction reduces fiber loss and mitochondrial abnormalities in aged rat muscle. FASEB J. 1997;11:573–581. doi: 10.1096/fasebj.11.7.9212081. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Barbieri M, Bonafè M, Franceschi C, Paolisso G. Insulin/IGF-I-signaling pathway: an evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1064–E1071. doi: 10.1152/ajpendo.00296.2003. [DOI] [PubMed] [Google Scholar]
- Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp. Gerontol. 2003;38:1343–1351. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- Bartke A, Brown-Borg H. Life extension in the dwarf mouse. Curr. Top. Dev. Biol. 2004;63:189–225. doi: 10.1016/S0070-2153(04)63006-7. [DOI] [PubMed] [Google Scholar]
- Bates PC, Holder AT. The anabolic actions of growth hormone and thyroxine on protein metabolism in Snell dwarf and normal mice. J. Endocrinol. 1988;119:31–41. doi: 10.1677/joe.0.1190031. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccafico S, Riuzzi F, Puglielli C, Mancinelli R, Fulle S, Sorci G, Donato R. Human muscle satellite cells show age-related differential expression of S100B protein and RAGE. Age (Dordr) 2010;33(4):523–541. doi: 10.1007/s11357-010-9197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry AW, Sandesara PB, Roberts BM, Ferreira LF, Senf SM, Judge AR. HDAC1 activates FoxO and is both sufficient and required for skeletal muscle atrophy. J. Cell Sci. 2014;127:1441–1453. doi: 10.1242/jcs.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbassat CA, Maki KC, Unterman TG. Circulating levels of insulin-like growth factor (IGF) binding protein-1 and -3 in aging men: relationships to insulin, glucose, IGF, and dehydroepiandrosterone sulfate levels and anthropometric measures. J. Clin. Endocrinol. Metab. 1997;82:1484–1491. doi: 10.1210/jcem.82.5.3930. [DOI] [PubMed] [Google Scholar]
- Bigot A, Jacquemin V, Debacq-Chainiaux F, Butler-Browne GS, Toussaint O, Furling D, Mouly V. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol. Cell. 2008;100:189–199. doi: 10.1042/BC20070085. [DOI] [PubMed] [Google Scholar]
- Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am. J. Med. 2003a;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Ladelund S, Pedersen AN, Schroll M, Jorgensen T, Pedersen BK. Predicting death from tumour necrosis factor-alpha and interleukin-6 in 80-year-old people. Clin. Exp. Immunol. 2003b;132:24–31. doi: 10.1046/j.1365-2249.2003.02137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett C, Valentini S, Cabreiro F, Goss M, Somogyvari M, Piper MD, Hoddinott M, Sutphin GL, Leko V, McElwee JJ, Vazquez-Manrique RP, Orfila AM, Ackerman D, Au C, Vinti G, Riesen M, Howard K, Neri C, Bedalov A, Kaeberlein M, Soti C, Partridge L, Gems D. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–485. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceda GP, Dall’Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, Grimaldi W, Ceresini G, Corradi F, Ferrucci L, Valenti G, Hoffman AR. Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J. Endocrinol. Invest. 2005;28:96–100. [PubMed] [Google Scholar]
- Cerletti M, Jang YC, Finley LW, Haigis MC, Wagers AJ. Short-term calorie restriction enhances skeletal muscle stem cell function. Cell Stem Cell. 2012;10:515–519. doi: 10.1016/j.stem.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am. J. Clin. Nutr. 2012;96:1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J. Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- Cracknell R. 2013. The Ageing Populationed [WWW document]. URL http://www.parliament.uk/business/publications/research/key-issues-for-the-new-parliament/value-for-money-in-public-services/the-ageing-population/ [accessed on 2010]
- Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Deane CS, Hughes DC, Sculthorpe N, Lewis MP, Stewart CE, Sharples AP. Impaired hypertrophy in myoblasts is improved with testosterone administration. J. Steroid Biochem. Mol. Biol. 2013;138C:152–161. doi: 10.1016/j.jsbmb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Donmez G, Outeiro TF. SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol. Med. 2013;5:344–352. doi: 10.1002/emmm.201302451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J. Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumke CL, Wetter AC, Arias EB, Kahn CR, Cartee GD. Absence of insulin receptor substrate-1 expression does not alter GLUT1 or GLUT4 abundance or contraction-stimulated glucose uptake by mouse skeletal muscle. Horm. Metab. Res. 2001;33:696–700. doi: 10.1055/s-2001-19141. [DOI] [PubMed] [Google Scholar]
- Edstrom E, Altun M, Hagglund M, Ulfhake B. Atrogin-1/MAFbx and MuRF1 are downregulated in aging-related loss of skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61:663–674. doi: 10.1093/gerona/61.7.663. [DOI] [PubMed] [Google Scholar]
- Emran S, Yang M, He X, Zandveld J, Piper MD. Target of rapamycin signalling mediates the lifespan-extending effects of dietary restriction by essential amino acid alteration. Aging (Albany NY) 2014;6:390–398. doi: 10.18632/aging.100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulstone EJ, Meadows KA, Holly JM, Stewart CE. Insulin-like growth factors (IGF-I and IGF-II) inhibit C2 skeletal myoblast differentiation and enhance TNF alpha-induced apoptosis. J. Cell. Physiol. 2001;189:207–215. doi: 10.1002/jcp.10017. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Savage PB, Crown AL, Holly JM, Stewart CE. Adaptations of the IGF system during malignancy: human skeletal muscle versus the systemic environment. Horm. Metab. Res. 2003a;35:667–674. doi: 10.1055/s-2004-814159. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Savage PB, Crown AL, Holly JM, Stewart CE. Role of insulin-like growth factor binding protein-3 (IGFBP-3) in the differentiation of primary human adult skeletal myoblasts. J. Cell. Physiol. 2003b;195:70–79. doi: 10.1002/jcp.10227. [DOI] [PubMed] [Google Scholar]
- Foulstone EJ, Huser C, Crown AL, Holly JM, Stewart CE. Differential signalling mechanisms predisposing primary human skeletal muscle cells to altered proliferation and differentiation: roles of IGF-I and TNFalpha. Exp. Cell Res. 2004;294:223–235. doi: 10.1016/j.yexcr.2003.10.034. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet. Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J. Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AM, Busuttil RA, Calder RB, Dolle ME, Diaz V, McMahan CA, Bartke A, Nelson J, Reddick R, Vijg J. Effect of Ames dwarfism and caloric restriction on spontaneous DNA mutation frequency in different mouse tissues. Mech. Ageing Dev. 2008;129:528–533. doi: 10.1016/j.mad.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garinis GA, Uittenboogaard LM, Stachelscheid H, Fousteri M, van Ijcken W, Breit TM, van Steeg H, Mullenders LH, van der Horst GT, Bruning JC, Niessen CM, Hoeijmakers JH, Schumacher B. Persistent transcription-blocking DNA lesions trigger somatic growth attenuation associated with longevity. Nat. Cell Biol. 2009;11:604–615. doi: 10.1038/ncb1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int. J. Sport Nutr. Exerc. Metab. 2011;21:97–104. doi: 10.1123/ijsnem.21.2.97. [DOI] [PubMed] [Google Scholar]
- Geusens PP, Boonen S. Osteoporosis and the growth hormone-insulin-like growth factor axis. Horm. Res. 2002;58(Suppl 3):49–55. doi: 10.1159/000066483. [DOI] [PubMed] [Google Scholar]
- Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem. Sci. 2007;32:180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, Willett WC. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol. Biomarkers Prev. 2003;12:84–89. [PubMed] [Google Scholar]
- Gong Z, Kennedy O, Sun H, Wu Y, Williams GA, Klein L, Cardoso L, Matheny RW, Jr, Hubbard GB, Ikeno Y, Farrar RP, Schaffler MB, Adamo ML, Muzumdar RH, Yakar S. Reductions in serum IGF-1 during aging impair health span. Aging Cell. 2014;13:408–418. doi: 10.1111/acel.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You JS, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J. Physiol. 2011;589:5485–5501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Grohmann M, Sabin M, Holly J, Shield J, Crowne E, Stewart C. Characterization of differentiated subcutaneous and visceral adipose tissue from children the influences of TNF-α and IGF-I. J. Lipid Res. 2005;46:93–103. doi: 10.1194/jlr.M400295-JLR200. [DOI] [PubMed] [Google Scholar]
- Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci. Transl. Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton DL, Philp A, MacKenzie MG, Patton A, Towler MC, Gallagher IJ, Bodine SC, Baar K. Molecular brakes regulating mTORC1 activation in skeletal muscle following synergist ablation. Am. J. Physiol. Endocrinol. Metab. 2014;307:E365–373. doi: 10.1152/ajpendo.00674.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Qin M, Nakamoto H, Goto S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1231–R1237. doi: 10.1152/ajpregu.90478.2008. [DOI] [PubMed] [Google Scholar]
- Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010;1:3. doi: 10.1038/ncomms1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidestrand M, Richards-Malcolm S, Gurley CM, Nolen G, Grimes B, Waterstrat A, Zant GV, Peterson CA. Sca-1-expressing nonmyogenic cells contribute to fibrosis in aged skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2008;63:566–579. doi: 10.1093/gerona/63.6.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holly JM, Perks CM, Stewart CE. Overview of insulin-like growth factor physiology. Growth Horm. IGF Res. 2000;10(Suppl A):S8–S9. doi: 10.1016/s1096-6374(00)90003-0. [DOI] [PubMed] [Google Scholar]
- Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2002;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- Hong S, Zhao B, Lombard DB, Fingar DC, Inoki K. Cross-talk between sirtuin and mammalian target of rapamycin complex 1 (mTORC1) signaling in the regulation of S6 kinase 1 (S6K1) phosphorylation. J. Biol. Chem. 2014;289:13132–13141. doi: 10.1074/jbc.M113.520734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- Hunter RB, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Smith SM, Lashinger LM, Harvey AE, Perkins SN. Calories and carcinogenesis: lessons learned from 30 years of calorie restriction research. Carcinogenesis. 2010;31:83–89. doi: 10.1093/carcin/bgp280. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Pennington JD, Bisht KS, Aykin-Burns N, Kim H-S, Mishra M, Sun L, Nguyen P, Ahn B-H, Leclerc J. SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci. 2008;4:291. doi: 10.7150/ijbs.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, You JS, Frey JW, Goodman CA, Gundermann DM, Hornberger TA. Eccentric contractions increase the phosphorylation of tuberous sclerosis complex-2 (TSC2) and alter the targeting of TSC2 and the mechanistic target of rapamycin to the lysosome. J. Physiol. 2013;591:4611–4620. doi: 10.1113/jphysiol.2013.256339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PL, Stewart CE, Rotwein P. Insulin-like growth factor binding protein-5 modulates muscle differentiation through an insulin-like growth factor-dependent mechanism. J. Cell Biol. 1996;133:683–693. doi: 10.1083/jcb.133.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M. Preserving youth: does rapamycin deliver? Sci. Transl. Med. 2013a;5:211 fs240. doi: 10.1126/scitranslmed.3007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013b;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse AR, Atkinson SA, Tarnopolsky MA, Phillips SM. Increased consumption of dairy foods and protein during diet- and exercise-induced weight loss promotes fat mass loss and lean mass gain in overweight and obese premenopausal women. J. Nutr. 2011;141:1626–1634. doi: 10.3945/jn.111.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Sommersberg B. Plasma androgens, IGF-1, body size, and prostate cancer risk: a synthetic review. Prostate Cancer Prostatic Dis. 2000;3:157–172. doi: 10.1038/sj.pcan.4500421. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012a;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012b;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY. SIRT6 links histone H3 lysine 9 deacetylation to NF-κB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am. J. Clin. Nutr. 2006;83:260–274. doi: 10.1093/ajcn/83.2.260. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Lashinger LM, Malone LM, McArthur MJ, Goldberg JA, Daniels EA, Pavone A, Colby JK, Smith NC, Perkins SN, Fischer SM, Hursting SD. Genetic reduction of insulin-like growth factor-1 mimics the anticancer effects of calorie restriction on cyclooxygenase-2-driven pancreatic neoplasia. Cancer Prev. Res. (Phila.) 2011;4:1030–1040. doi: 10.1158/1940-6207.CAPR-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau MM, Stewart CE, Liu Z, Bhatt H, Rotwein P, Stewart CL. Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev. 1994;8:2953–2963. doi: 10.1101/gad.8.24.2953. [DOI] [PubMed] [Google Scholar]
- Lee D, Goldberg AL. SIRT1 protein, by blocking the activities of transcription factors FoxO1 and FoxO3, inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 2013;288:30515–30526. doi: 10.1074/jbc.M113.489716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008;11(1):163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J, Passarino G, Kennedy BK, Wei M, Cohen P, Crimmins EM, Longo VD. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J. Biol. Chem. 2010;285:36387–36394. doi: 10.1074/jbc.M110.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KA, Lashinger LM, Rasmussen AJ, Hursting SD. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014;2:6. doi: 10.1186/2049-3002-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Proto JD, Guo L, Tang Y, Lavasani M, Tilstra JS, Niedernhofer LJ, Wang B, Guttridge DC, Robbins PD, Huard J. NF-kappaB negatively impacts the myogenic potential of muscle-derived stem cells. Mol. Ther. 2012;20:661–668. doi: 10.1038/mt.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestu J, Eliakim A, Jurimae J, Valter I, Jurimae T. Anabolic and catabolic hormones and energy balance of the male bodybuilders during the preparation for the competition. J. Strength Cond. Res. 2010;24:1074–1081. doi: 10.1519/JSC.0b013e3181cb6fd3. [DOI] [PubMed] [Google Scholar]
- Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu. Rev. Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proc. Natl Acad. Sci. USA. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:516–521. doi: 10.1093/gerona/glp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny RW, Merritt E, Zannikos SV, Farrar RP, Adamo ML. Serum IGF-I-deficiency does not prevent compensatory skeletal muscle hypertrophy in resistance exercise. Exp. Biol. Med. (Maywood) 2009;234:164–170. doi: 10.3181/0808-RM-251. [DOI] [PubMed] [Google Scholar]
- Matthews L, Hammer R, Behringer R, D’Ercole A, Bell G, Brinster R, Palmiter R. Growth, enhancement of transgenic mice expressing human insulin-like growth factor-I. Endocrinology. 1988;123:2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Lopez M, Beasley TM, Aiken JM, Anderson RM, Weindruch R. Caloric restriction delays aging-induced cellular phenotypes in rhesus monkey skeletal muscle. Exp. Gerontol. 2011;46:23–29. doi: 10.1016/j.exger.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman RJ, Aiken E, Evans TD, Beasley TM, Aiken JM, Weindruch R, Anderson RM. Cellular adaptation contributes to calorie restriction-induced preservation of skeletal muscle in aged rhesus monkeys. Exp. Gerontol. 2012;47:229–236. doi: 10.1016/j.exger.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J. Cell. Physiol. 2000;183:330–337. doi: 10.1002/(SICI)1097-4652(200006)183:3<330::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Crosby SD, Lamming DW, JeBailey L, Krzysik-Walker S, Villareal DT, Capri M, Franceschi C, Zhang Y, Becker K, Sabatini DM, de Cabo R, Fontana L. Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell. 2013;12:645–651. doi: 10.1111/acel.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang Y, Becker KG, Khraiwesh H, Gonzalez-Reyes JA, Villalba JM, Baur JA, Elliott P, Westphal C, Vlasuk GP, Ellis JL, Sinclair DA, Bernier M, de Cabo R. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7:199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, McCarthy JJ, Fedele MJ, Esser KA. Early activation of mTORC1 signalling in response to mechanical overload is independent of phosphoinositide 3-kinase/Akt signalling. J. Physiol. 2011;589:1831–1846. doi: 10.1113/jphysiol.2011.205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtahedi MC, Thorpe MP, Karampinos DC, Johnson CL, Layman DK, Georgiadis JG, Evans EM. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:1218–1225. doi: 10.1093/gerona/glr120. [DOI] [PubMed] [Google Scholar]
- Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 2009;89:161–168. doi: 10.3945/ajcn.2008.26401. [DOI] [PubMed] [Google Scholar]
- Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic. Biol. Med. 2013;56:133–171. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- Morris RT, Spangenburg EE, Booth FW. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J. Appl. Physiol. 2004;96:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Mian OS, Muirhead A, Birch KM, Narici MV. Muscle strength, volume and activation following 12-month resistance training in 70-year-old males. Eur. J. Appl. Physiol. 2005a;95:197–204. doi: 10.1007/s00421-005-1342-3. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J. Appl. Physiol. 2005b;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng HL, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- Mourier A, Bigard AX, Legrand H, Guezennec CY, De Kerviler E, Roger B. Combined effects of caloric restriction and branched-chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int. J. Sports Med. 1997;18:47–55. doi: 10.1055/s-2007-972594. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L. Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Invest. 2013;123:3272. doi: 10.1172/JCI67674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MS, Carlson ME, Conboy IM. Differentiation rather than aging of muscle stem cells abolishes their telomerase activity. Biotechnol. Prog. 2009;25:1130–1137. doi: 10.1002/btpr.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- Page MM, Salmon AB, Leiser SF, Robb EL, Brown MF, Miller RA, Stuart JA. Mechanisms of stress resistance in Snell dwarf mouse fibroblasts: enhanced antioxidant and DNA base excision repair capacity, but no differences in mitochondrial metabolism. Free Radic. Biol. Med. 2009;46:1109–1118. doi: 10.1016/j.freeradbiomed.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S. A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc. Natl Acad. Sci. USA. 2002;99:9213–9218. doi: 10.1073/pnas.142166599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BS, Henning PC, Grant SC, Lee WJ, Lee SR, Arjmandi BH, Kim JS. HMB attenuates muscle loss during sustained energy deficit induced by calorie restriction and endurance exercise. Metabolism. 2013;62:1718–1729. doi: 10.1016/j.metabol.2013.06.005. [DOI] [PubMed] [Google Scholar]
- Parkington JD, LeBrasseur NK, Siebert AP, Fielding RA. Contraction-mediated mTOR, p70S6k, and ERK1/2 phosphorylation in aged skeletal muscle. J. Appl. Physiol. 2004;97:243–248. doi: 10.1152/japplphysiol.01383.2003. [DOI] [PubMed] [Google Scholar]
- Passtoors WM, Beekman M, Deelen J, van der Breggen R, Maier AB, Guigas B, Derhovanessian E, van Heemst D, de Craen AJ, Gunn DA, Pawelec G, Slagboom PE. Gene expression analysis of mTOR pathway: association with human longevity. Aging Cell. 2013;12:24–31. doi: 10.1111/acel.12015. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell JM, Bates PC. Differential actions of growth hormone and insulin-like growth factor-I on tissue protein metabolism in dwarf mice. Endocrinology. 1992;130:1942–1950. doi: 10.1210/endo.130.4.1547721. [DOI] [PubMed] [Google Scholar]
- Pete G, Fuller CR, Oldham JM, Smith DR, D’Ercole AJ, Kahn CR, Lund PK. Postnatal growth responses to insulin-like growth factor I in insulin receptor substrate-1-deficient mice. Endocrinology. 1999;140:5478–5487. doi: 10.1210/endo.140.12.7219. [DOI] [PubMed] [Google Scholar]
- Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44(Suppl 1):S71–S77. doi: 10.1007/s40279-014-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J. Appl. Physiol. 2011;110:561–568. doi: 10.1152/japplphysiol.00941.2010. [DOI] [PubMed] [Google Scholar]
- Pietrangelo T, Puglielli C, Mancinelli R, Beccafico S, Fano G, Fulle S. Molecular basis of the myogenic profile of aged human skeletal muscle satellite cells during differentiation. Exp. Gerontol. 2009;44:523–531. doi: 10.1016/j.exger.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM, Oller do Nascimento CM, de Mello MT, Tufik S, Santos RV. beta-Hydroxy-beta-methylbutyrate (HMbeta) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr. Metab. (Lond) 2011;8:11. doi: 10.1186/1743-7075-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Selman C, McElwee JJ, Partridge L. Separating cause from effect: how does insulin/IGF signalling control lifespan in worms, flies and mice? J. Intern. Med. 2008;263:179–191. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- Player DJ, Martin NR, Passey SL, Sharples AP, Mudera V, Lewis MP. Acute mechanical overload increases IGF-I and MMP-9 mRNA in 3D tissue-engineered skeletal muscle. Biotechnol. Lett. 2014;36:1113–1124. doi: 10.1007/s10529-014-1464-y. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand. J. Med. Sci. Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J. Am. Geriatr. Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Rathbone CR, Booth FW, Lees SJ. Sirt1 increases skeletal muscle precursor cell proliferation. Eur. J. Cell Biol. 2009;88:35–44. doi: 10.1016/j.ejcb.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman JA. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Risson V, Mazelin L, Roceri M, Sanchez H, Moncollin V, Corneloup C, Richard-Bulteau H, Vignaud A, Baas D, Defour A, Freyssenet D, Tanti JF, Le-Marchand-Brustel Y, Ferrier B, Conjard-Duplany A, Romanino K, Bauche S, Hantai D, Mueller M, Kozma SC, Thomas G, Ruegg MA, Ferry A, Pende M, Bigard X, Koulmann N, Schaeffer L, Gangloff YG. Muscle inactivation of mTOR causes metabolic and dystrophin defects leading to severe myopathy. J. Cell Biol. 2009;187:859–874. doi: 10.1083/jcb.200903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RM, Fraga MF. Aging and cancer: are sirtuins the link? Future Oncol. 2010;6:905–915. doi: 10.2217/fon.10.57. [DOI] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J. Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Rossi P, Marzani B, Giardina S, Negro M, Marzatico F. Human skeletal muscle aging and the oxidative system: cellular events. Curr. Aging Sci. 2008;1:182–191. doi: 10.2174/1874609810801030182. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, Leboff MS, Rood JC, de Jonge L, Greenway FL, Loria CM, Obarzanek E, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N. Engl. J. Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini A, Al-Shanti N, Stewart CE. Waste management – cytokines, growth factors and cachexia. Cytokine Growth Factor Rev. 2006;17:475–486. doi: 10.1016/j.cytogfr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Saini A, Al-Shanti N, Faulkner SH, Stewart CE. Pro- and anti-apoptotic roles for IGF-I in TNF-alpha-induced apoptosis: a MAP kinase mediated mechanism. Growth Factors. 2008;26:239–253. doi: 10.1080/08977190802291634. [DOI] [PubMed] [Google Scholar]
- Saini A, Al-Shanti N, Stewart C. C2 skeletal myoblast survival, death, proliferation and differentiation: regulation by Adra1d. Cell. Physiol. Biochem. 2010;25:253–262. doi: 10.1159/000276559. [DOI] [PubMed] [Google Scholar]
- Saini A, Al-Shanti N, Sharples AP, Stewart CE. Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Exp. Physiol. 2012;97:400–418. doi: 10.1113/expphysiol.2011.061028. [DOI] [PubMed] [Google Scholar]
- Salles J, Chanet A, Giraudet C, Patrac V, Pierre P, Jourdan M, Luiking YC, Verlaan S, Migné C, Boirie Y. 1, 25 (OH) 2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. Mol. Nutr. Food Res. 2013;57:2137–2146. doi: 10.1002/mnfr.201300074. [DOI] [PubMed] [Google Scholar]
- Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology. 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone L, Reali V, Pellegrini L, Villanova L, Aventaggiato M, Marfe G, Rosa R, Nebbioso M, Tafani M, Fini M, Russo MA, Pucci B. SIRT1 silencing confers neuroprotection through IGF-1 pathway activation. J. Cell. Physiol. 2013;228:1754–1761. doi: 10.1002/jcp.24334. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr. Opin. Genet. Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scime A, Rudnicki MA. Anabolic potential and regulation of the skeletal muscle satellite cell populations. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9:214–219. doi: 10.1097/01.mco.0000222102.21385.7d. [DOI] [PubMed] [Google Scholar]
- Selman C. Dietary restriction and the pursuit of effective mimetics. Proc. Nutr. Soc. 2014;73:260–270. doi: 10.1017/S0029665113003832. [DOI] [PubMed] [Google Scholar]
- Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech. Ageing Dev. 2005;126:783–793. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson IC, Thornton JM, Gems D, Partridge L, Withers DJ. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- Selman C, Tullet JM, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson IC, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Partridge L, Withers DJ. Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS One. 2011;6:e16144. doi: 10.1371/journal.pone.0016144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman C, Withers DJ. Mammalian models of extended healthy lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:99–107. doi: 10.1098/rstb.2010.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples AP, Stewart CE. Myoblast models of skeletal muscle hypertrophy and atrophy. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:230–236. doi: 10.1097/MCO.0b013e3283457ade. [DOI] [PubMed] [Google Scholar]
- Sharples AP, Al-Shanti N, Stewart CE. C2 and C2C12 murine skeletal myoblast models of atrophic and hypertrophic potential: relevance to disease and ageing? J. Cell. Physiol. 2010;225:240–250. doi: 10.1002/jcp.22252. [DOI] [PubMed] [Google Scholar]
- Sharples AP, Al-Shanti N, Lewis MP, Stewart CE. Reduction of myoblast differentiation following multiple population doublings in mouse C(2) C(12) cells: a model to investigate ageing? J. Cell. Biochem. 2011;112:3773–3785. doi: 10.1002/jcb.23308. [DOI] [PubMed] [Google Scholar]
- Sharples AP, Player DJ, Martin NR, Mudera V, Stewart CE, Lewis MP. Modelling in-vivo skeletal muscle ageing in-vitro using three dimensional bioengineered constructs. Aging Cell. 2012;8:1474–9726. doi: 10.1111/j.1474-9726.2012.00869.x. [DOI] [PubMed] [Google Scholar]
- Sharples AP, Al-Shanti N, Hughes DC, Lewis MP, Stewart CE. The role of insulin-like-growth factor binding protein 2 (IGFBP2) and phosphatase and tensin homologue (PTEN) in the regulation of myoblast differentiation and hypertrophy. Growth Horm. IGF Res. 2013;23:53–61. doi: 10.1016/j.ghir.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Sinha R, Cooper TK, Rogers CJ, Sinha I, Turbitt WJ, Calcagnotto A, Perrone CE, Richie JP., Jr Dietary methionine restriction inhibits prostatic intraepithelial neoplasia in TRAMP mice. Prostate. 2014;74:1663–1673. doi: 10.1002/pros.22884. [DOI] [PubMed] [Google Scholar]
- Slagboom PE, Beekman M, Passtoors WM, Deelen J, Vaarhorst AA, Boer JM, van den Akker EB, van Heemst D, de Craen AJ, Maier AB, Rozing M, Mooijaart SP, Heijmans BT, Westendorp RG. Genomics of human longevity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:35–42. doi: 10.1098/rstb.2010.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Poliak N, Upadhyay S, Ratovitski E, Nelkin BD, Donehower LA, Sidransky D. DeltaNp63alpha overexpression induces downregulation of Sirt1 and an accelerated aging phenotype in the mouse. Cell Cycle. 2006;5:2005–2011. doi: 10.4161/cc.5.17.3194. [DOI] [PubMed] [Google Scholar]
- de Souza RJ, Bray GA, Carey VJ, Hall KD, LeBoff MS, Loria CM, Laranjo NM, Sacks FM, Smith SR. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am. J. Clin. Nutr. 2012;95:614–625. doi: 10.3945/ajcn.111.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Selman C. The free-radical damage theory: accumulating evidence against a simple link of oxidative stress to ageing and lifespan. BioEssays. 2011;33:255–259. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Pell JM. Point:Counterpoint: IGF is/is not the major physiological regulator of muscle mass. Point: IGF is the major physiological regulator of muscle mass. J. Appl. Physiol. 2010;108:1820–1821. doi: 10.1152/japplphysiol.01246.2009. discussion 1823–1824; author reply 1832. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Rotwein P. Growth, differentiation, and survival: multiple physiological functions for insulin-like growth factors. Physiol. Rev. 1996a;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Rotwein P. Insulin-like growth factor-II is an autocrine survival factor for differentiating myoblasts. J. Biol. Chem. 1996b;271:11330–11338. doi: 10.1074/jbc.271.19.11330. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Bates PC, Calder TA, Woodall SM, Pell JM. Potentiation of insulin-like growth factor-I (IGF-I) activity by an antibody: supportive evidence for enhancement of IGF-I bioavailability in vivo by IGF binding proteins. Endocrinology. 1993;133:1462–1465. doi: 10.1210/endo.133.3.7689959. [DOI] [PubMed] [Google Scholar]
- Stewart CE, James PL, Fant ME, Rotwein P. Overexpression of insulin-like growth factor-II induces accelerated myoblast differentiation. J. Cell. Physiol. 1996;169:23–32. doi: 10.1002/(SICI)1097-4652(199610)169:1<23::AID-JCP3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Mihai R, Holly JM. Increased tyrosine kinase activity but not calcium mobilization is required for ceramide-induced apoptosis. Exp. Cell Res. 1999a;250:329–338. doi: 10.1006/excr.1999.4546. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Newcomb PV, Savage PB, Dickens M, Tavare J, Holly JM. Increased, not decreased activation of the insulin-like growth factor (IGF) receptor signalling pathway during ceramide-induced apoptosis. Growth Horm. IGF Res. 1999b;9:131–142. doi: 10.1054/ghir.1999.0098. [DOI] [PubMed] [Google Scholar]
- Stewart CE, Newcomb PV, Holly JM. Multifaceted roles of TNF-alpha in myoblast destruction: a multitude of signal transduction pathways. J. Cell. Physiol. 2004;198:237–247. doi: 10.1002/jcp.10387. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci. Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Taylor C, Bartlett JD, van de Graaf CS, Louhelainen J, Coyne V, Iqbal Z, Maclaren DP, Gregson W, Close GL, Morton JP. Protein ingestion does not impair exercise-induced AMPK signalling when in a glycogen-depleted state: implications for train-low compete-high. Eur. J. Appl. Physiol. 2013;113:1457–1468. doi: 10.1007/s00421-012-2574-7. [DOI] [PubMed] [Google Scholar]
- Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc. Natl Acad. Sci. USA. 1994;91:11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra JS, Robinson AR, Wang J, Gregg SQ, Clauson CL, Reay DP, Nasto LA, St Croix CM, Usas A, Vo N. NF-κB inhibition delays DNA damage–induced senescence and aging in mice. J. Clin. Invest. 2012;122:2601. doi: 10.1172/JCI45785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D, Bergholz J, Zhang H, He H, Wang Y, Zhang Y, Li Q, Kirkland JL, Xiao Z-X. Insulin-like growth factor-1 regulates the SIRT1-p53 pathway in cellular senescence. Aging Cell. 2014;13:669–678. doi: 10.1111/acel.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Michel JJ, Bale LK, Lemster BH, Borghesi L, Conover CA. Resistance to age-dependent thymic atrophy in long-lived mice that are deficient in pregnancy-associated plasma protein A. Proc. Natl Acad. Sci. USA. 2009;106:11252–11257. doi: 10.1073/pnas.0807025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Guarente L. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature. 2011;477:E1–E2. doi: 10.1038/nature10440. [DOI] [PubMed] [Google Scholar]
- Wall BT, van Loon LJ. Nutritional strategies to attenuate muscle disuse atrophy. Nutr. Rev. 2013;71:195–208. doi: 10.1111/nure.12019. [DOI] [PubMed] [Google Scholar]
- Wang F, Nguyen M, Qin F, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Wang DT, Yin Y, Yang YJ, Lv PJ, Shi Y, Lu L, Wei LB. Resveratrol prevents TNF-alpha-induced muscle atrophy via regulation of Akt/mTOR/FoxO1 signaling in C2C12 myotubes. Int. Immunopharmacol. 2014;19:206–213. doi: 10.1016/j.intimp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Stokes CM, Wang X, Sakaue H, Ogawa W, Kasuga M, Proud CG. Activation of translation initiation factor eIF2B by insulin requires phosphatidyl inositol 3-kinase. FEBS Lett. 1997;410:418–422. doi: 10.1016/s0014-5793(97)00579-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, Woodward MA, Miller RA. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett M, Cowan JL, Vlasak M, Coldwell MJ, Morley SJ. Inhibition of mammalian target of rapamycin (mTOR) signalling in C2C12 myoblasts prevents myogenic differentiation without affecting the hyperphosphorylation of 4E-BP1. Cell. Signal. 2009;21:1504–1512. doi: 10.1016/j.cellsig.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Gutierrez JS, Towery H, Burks DJ, Ren JM, Previs S, Zhang Y, Bernal D, Pons S, Shulman GI, Bonner-Weir S, White MF. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. doi: 10.1038/36116. [DOI] [PubMed] [Google Scholar]
- Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat. Genet. 1999;23:32–40. doi: 10.1038/12631. [DOI] [PubMed] [Google Scholar]
- Yang J, Anzo M, Cohen P. Control of aging and longevity by IGF-I signaling. Exp. Gerontol. 2005;40:867–872. doi: 10.1016/j.exger.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, Diaz V, Sloane L, Maslin K, Treaster S, Rendon S, van Remmen H, Ward W, Javors M, Richardson A, Austad SN, Fischer K. Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]


