Abstract
The molecular mechanisms behind aging-related declines in muscle function are not well understood, but the growth factor myostatin (MSTN) appears to play an important role in this process. Additionally, epidemiological studies have identified a positive correlation between skeletal muscle mass and longevity. Given the role of myostatin in regulating muscle size, and the correlation between muscle mass and longevity, we tested the hypotheses that the deficiency of myostatin would protect oldest-old mice (28–30 months old) from an aging-related loss in muscle size and contractility, and would extend the maximum lifespan of mice. We found that MSTN+/− and MSTN−/− mice were protected from aging-related declines in muscle mass and contractility. While no differences were detected between MSTN+/+ and MSTN−/− mice, MSTN+/− mice had an approximately 15% increase in maximal lifespan. These results suggest that targeting myostatin may protect against aging-related changes in skeletal muscle and contribute to enhanced longevity.
Keywords: GDF-8, longevity, muscle atrophy, muscle contractility, myostatin, sarcopenia, skeletal muscle
Sarcopenia is the pathological loss in muscle mass and strength that occurs with aging (Gumucio & Mendias, 2013). In mice, muscle mass and force production slowly decreases from adulthood (6–9 months of age) to old age (22–24 months), with a rapid deterioration present once mice reach oldest-old ages (>26–28 months) (Brooks & Faulkner, 1988; Lynch et al., 2001; Graber et al., 2013). There is also an aging-associated increase in collagen accumulation which can diminish force production (Ramaswamy et al., 2011). In humans, muscle mass is positively correlated with a greater longevity (Miller et al., 2002), and the rapid decrease in muscle mass and strength that occurs toward the end of the lifespan can lead to severe disability and reduced quality of life (Fielding et al., 2011).
Myostatin is a negative regulator of skeletal muscle mass, with adult MSTN−/− mice displaying up to a twofold increase in muscle mass (Gumucio & Mendias, 2013). Myostatin induces atrophy by upregulating the E3 ubiquitin ligases atrogin-1 and MuRF-1 and by inhibiting the IGF-1 pathway (Gumucio & Mendias, 2013). As the role of myostatin in regulating muscle function in oldest-old mice had not previously been studied, and there is a positive correlation between muscle mass and longevity in humans (Miller et al., 2002), we tested the hypotheses that oldest-old male myostatin-deficient mice would have improved muscle force production compared to wild-type mice and that the deficiency of myostatin would increase the maximum lifespan of mice.
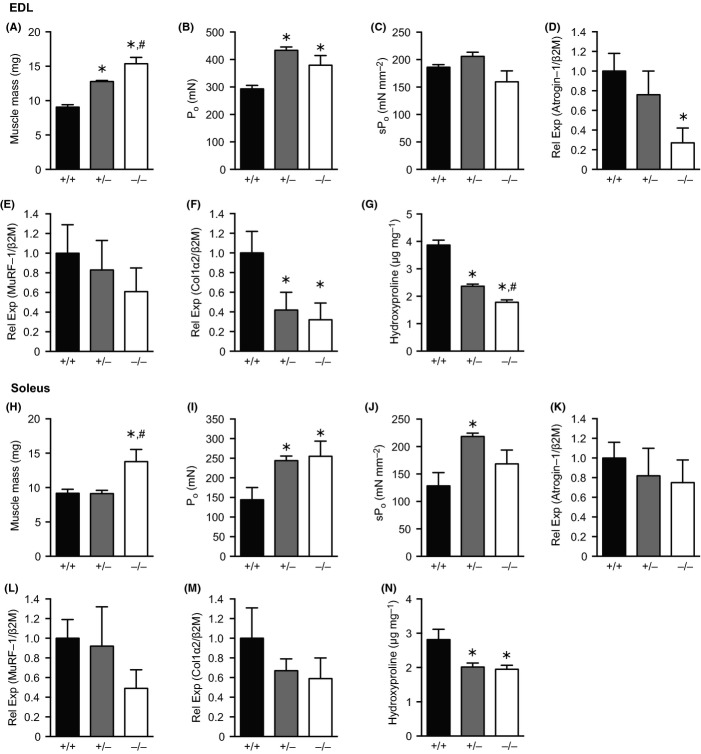
Circulating myostatin protein was not detectable in MSTN−/−mice, while MSTN+/− mice had a 30% decrease (Table S1). For the fast-fibered EDL, MSTN+/− and MSTN−/− mice had a greater mass (Fig.1A) and number of type II muscle fibers (Fig. S1) than controls. Maximum isometric force production (Po) was increased in MSTN+/− and MSTN−/− mice (Fig.1B), although no differences in specific force production (sPo), which is Po normalized to muscle cross-sectional area (CSA), were noted (Fig.1C). Atrogin-1 was decreased in MSTN−/− mice, but no other differences in MuRF-1 were observed (Fig.1D–E).
Fig 1.
Muscle contractility, hydroxyproline and gene expression values of EDL muscles (A through G) and soleus muscles (H through N) from 28- to 30-month old MSTN+/+, MSTN+/− and MSTN−/− mice. (A, H): Wet mass. (B, I): Maximum isometric force (Po). C, J: Specific force (sPo). Gene expression for (D, K) Atrogin-1, (E, L) MuRF-1 and (F, M) Type 1 collagen. G, N: Hydroxyproline content. Values are mean ± SE; N = 6 mice per genotype. Differences tested with a one-way ANOVA and Fisher’s LSD post hoc sorting. *, different from MSTN+/+ (P < 0.05). #, different from MSTN+/− (P < 0.05).
For mixed-fiber soleus muscles, MSTN−/− mice had increased mass (Fig.1H). No change in the percent distribution of fiber types or fiber CSA was observed, although there was an increase in the number of fibers in MSTN+/− and MSTN−/− mice (Fig. S1). Interestingly, despite both MSTN+/− and MSTN−/− mice demonstrating a substantial increase in Po (Fig.1I), only the MSTN+/− mice had an increase in sPo (Fig.1J). No differences in atrogin-1 or MuRF-1 expression were observed (Fig.1K–L). The differences between muscle mass and Po across the three genotypes are also similar to previous reports in adult animals, but sPo was only elevated in adult MSTN−/− mice (Mendias et al., 2006) unlike in the current study. Combined, these results suggest the prolonged deficiency of myostatin protects against the aging-associated decrease in Po without having a negative impact on sPo in oldest-old mice. Further, as fiber loss is considered to be the primary contributor to aging-associated muscle atrophy (Gumucio & Mendias, 2013), there appears to be a protective effect of myostatin deficiency on the primary cause of aging-related muscle weakness.
We next evaluated changes in the muscle ECM, as myostatin can directly induce collagen expression in muscle and fibroblast cells (Mendias et al., 2006, 2008). Hydroxyproline, which is a marker of collagen, and type I collagen expression were reduced in EDL muscles of MSTN+/− and MSTN−/− mice (Fig.1F–G). For soleus muscles, MSTN+/− and MSTN−/− mice had a reduction in hydroxyproline, although no change in type I collagen expression was detected (Fig.1M–N). This reduction in collagen is consistent with findings in adult animals (Mendias et al., 2006), and the reduction in collagen levels in oldest-old mice may contribute to their improved contractile properties.
In the lifespan study, there were no differences between the survival curves of MSTN+/+ and MSTN−/− mice, but MSTN+/− mice had significant different survival curves from MSTN+/+ and MSTN−/− mice (Fig.2A). MSTN+/− mice also had an increase in maximal lifespan and maximum age (Fig.2B). Approximately 2/3 of mice could be submitted to necropsy, and the only pathological finding of significance was gross cardiomegaly. No differences in relative heart mass were present between MSTN+/+ and MSTN+/− mice, but there was an increase observed in MSTN−/− mice (Fig.2B). Although we did not evaluate female mice in this study, we do not anticipate sex-specific differences in these findings.
Fig 2.
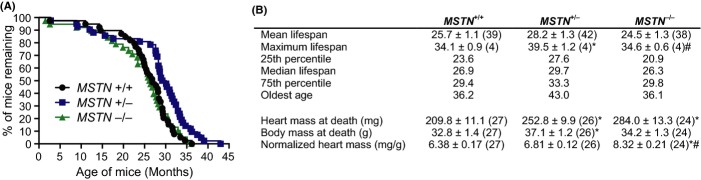
Lifespan data for MSTN+/+, MSTN+/− and MSTN−/− mice. (A) Survival curves of mice. Log-rank analysis of survival curves: MSTN+/+ vs. MSTN−/−, P = 0.85; MSTN+/+ vs. MSTN+/−, P = 0.003; MSTN+/− vs. MSTN−/−, P = 0.003. (B) Summary longevity and cardiac data. Values are mean ± SE (N). Differences tested with a one-way ANOVA and Fisher’s LSD post hoc sorting. *, different from MSTN+/+ (P < 0.05). #, different from MSTN+/− (P < 0.05).
Mouse strains with loss of function in the growth hormone/IGF-1 axis have a smaller body size and enhanced lifespan (Blagosklonny, 2013). The current results are the first to identify a loss of function gene mutation in mice that results in an increase in muscle and body mass along with enhanced longevity. The mechanism behind the increased longevity of MSTN+/− mice is not known, but inhibition of myostatin can reduce systemic inflammatory proteins and body fat (Gumucio & Mendias, 2013). Given the increase in relative heart mass, the contribution of aging-associated cardiomegaly to mortality (Lakatta & Levy, 2003) and that inhibition of myostatin can increase heart mass (Bish et al., 2011), it is possible that positive effects of increased skeletal muscle mass on the longevity of MSTN−/− mice was offset by cardiac pathologies.
Most genetic models of enhanced longevity in mice have identified an inverse relationship between body mass and longevity, which has lead to the observation that ‘big mice die young’ (Blagosklonny, 2013). However, the results from the current study support the epidemiological observations in humans that when it comes to skeletal muscle mass and longevity, bigger may be better.
Acknowledgments
None.
Funding
This work was supported by National Institutes of Health grants R01-AR063649 and P30-AG13283.
Conflict of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site.
Data S1 Experimental procedures.
Fig. S1 Histology for EDL muscles (A through C) and soleus muscles (D through H) from 28 to 30 month old MSTN+/+, MSTN+/− and MSTN−/− mice.
Table S1 Morphological and contractile properties measurements.
Table S2 Lifespan data.
References
- Bish LT, George I, Maybaum S, Yang J, Chen JM, Sweeney HL. Myostatin is elevated in congenital heart disease and after mechanical unloading. PLoS One. 2011;6:e23818. doi: 10.1371/journal.pone.0023818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Big mice die young but large animals live longer. Aging (Albany NY) 2013;5:227–233. doi: 10.18632/aging.100551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. (Lond) 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber TG, Ferguson-Stegall L, Kim J-H, Thompson LV. C57BL/6 neuromuscular healthspan scoring system. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1326–1336. doi: 10.1093/gerona/glt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12–21. doi: 10.1007/s12020-012-9751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J. Physiol. (Lond) 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendias CL, Marcin JE, Calerdon DR, Faulkner JA. Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J. Appl. Physiol. 2006;101:898–905. doi: 10.1152/japplphysiol.00126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendias CL, Bakhurin KI, Faulkner JA. Tendons of myostatin-deficient mice are small, brittle, and hypocellular. Proc. Natl Acad. Sci. USA. 2008;105:388–393. doi: 10.1073/pnas.0707069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Crotty M, Giles LC, Bannerman E, Whitehead C, Cobiac L, Daniels LA, Andrews G. Corrected arm muscle area: an independent predictor of long-term mortality in community-dwelling older adults? J. Am. Geriatr. Soc. 2002;50:1272–1277. doi: 10.1046/j.1532-5415.2002.50316.x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy KS, Palmer ML, Van der Meulen JH, Renoux A, Kostrominova TY, Michele DE, Faulkner JA. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J. Physiol. (Lond) 2011;589:1195–1208. doi: 10.1113/jphysiol.2010.201921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Experimental procedures.
Fig. S1 Histology for EDL muscles (A through C) and soleus muscles (D through H) from 28 to 30 month old MSTN+/+, MSTN+/− and MSTN−/− mice.
Table S1 Morphological and contractile properties measurements.
Table S2 Lifespan data.