Abstract
NFκB is involved in several pathogenic mechanisms that are believed to underlie the complex regional pain syndrome (CRPS), including ischemia, inflammation and sensitization. Chronic post-ischemia pain (CPIP) has been developed as an animal model that mimics the symptoms of CRPS-I. The possible involvement of NFκB in CRPS-I was studied in CPIP rats. Under sodium pentobarbital anesthesia a tourniquet was placed around the rat left ankle joint, producing 3 h ours of ischemia, followed by rapid reperfusion (IR Injury). NFκB was measured in nuclear extracts of muscle and spinal cord tissue using ELISA. Moreover, the anti-allodynic (mechanical and cold) effect was tested for systemic, intrathecal, or intraplantar treatment with the NFkB inhibitor pyrrolidine dithiocarbamate (PDTC). At 2 and 48 hrs after IR injury, NFκB was elevated in muscle and spinal cord of CPIP rats compared to sham rats. At 7 days, NFκB levels were normalized in muscle, but were still elevated in spinal cord tissue. Systemic PDTC treatment relieved mechanical and cold allodynia in a dose-dependent manner, lasting for at least three hours. Intrathecal -- but not intraplantar -- administration also relieved mechanical allodynia. The results suggest that muscle and spinal NFκB plays a role in the pathogenesis of CPIP and potentially of human CRPS.
Keywords: chronic post-ischemia pain, CPIP, pyrolidine dithiocarbamate, PDTC, ischemia, reperfusion, inflammation, neuropathic pain
Introduction
Complex regional pain syndrome (CRPS) is a painful and disabling complication of an injury, for example, a fracture or sprain, which affects the distal end of the injured extremity. CRPS patients can be classified into 2 subtypes, based on the presence (type II) or absence (type I) of direct nerve injury. The majority of CRPS patients are considered to suffer from type I. CRPS is assumed to evolve from several pathological mechanisms, including oxidative stress,5,29 classic4,7,23,24 and neurogenic4,7 inflammation, and autonomic17 and sensory nerve system alterations.54 A previously described automated analysis of literature has revealed that the transcription factor nuclear factor kappa B (NFkB) is involved in all these disease mechanisms.21 For example, affected limbs of human CRPS patients show signs of chronic ischemia,29,53 which can induce NFkB activation, mediated by the formation of reactive oxygen species (ROS) and peroxinitrite.16,20 Inflammatory mediators, including tumor necrosis factor alpha (TNFa), interleukin-1 (IL-1), and IL-6 have been demonstrated in blister23 and spinal cord fluid1 of CRPS patients, and can activate or are activated themselves by NFkB.9,26,48 Moreover, NFkB interacts with neuropeptides such as calcitonin gene related protein (CGRP)35 and substance P (SP)36 that have been found abnormally expressed during CRPS.7,34 Finally, animal studies have revealed that NFkB is involved in spinal plasticity39 and the development of neuropathic pain.50,51
NFkB resides in the cytosol of many different cell types and can be activated by many triggers, including ultraviolet radiation, free radicals, cytokines, and products of bacterial and viral infections.12 Upon activation, inhibitory kappa B (IkB) protein is cleaved from the NFkB complex, which subsequently forms dimers that are capable of passing through the nuclear membrane. In the nucleus, NFkB promotes the transcription of a wide variety of genes. NFkB has been attracting considerable scientific attention over the past years as a key factor in inflammation, apoptosis, and neuronal-glial interactions.3 Excessive NFkB activity has been attributed to the pathogenesis of several chronic inflammatory disorders and oncological diseases.52 Since 2005, the NFkB pathway inhibitor bortezomib has been applied successfully in the therapy of multiple myeloma and other malignancies.45 An NFkB inhibitor that is frequently applied in research settings is pyrrolidine dithiocarbamate (PDTC). PDTC is a chemical with metal chelating and antioxidant properties, and inhibits NFkB activity by blocking the phosphorylation of IkB.37,42 Systemically administrated PDTC and other dithiocarbamates have been shown to be protective and therapeutic in animal models for ischemia and reperfusion (IR) injury22, acute inflammation,15 and neuropathic pain.31
The chronic posti-schemia pain (CPIP) model is an animal model for the study of molecular mechanisms that underlie the sensory disturbances occurring in rats after IR injury of the hind paw. CPIP rats display several features that resemble human CRPS, including edema, hyperemia, and the development of mechanical and cold allodynia without direct nerve injury.14 The CPIP model has therefore been proposed as animal model for CRPS type-I. The aim of the present study was investigate the involvement of NFkB in CPIP, and potentially the pathogenesis of CRPS, by measuring NFkB levels and assessing the anti-allodynic effect of NFkB inhibition by PDTC in rats after IR injury.
Methods
Study Design
NFkB levels were measured in muscle and spinal cord tissue of CPIP animals and compared to sham animals at 2 hours (CPIP, N = 15; sham, N = 9), 48 hours (CPIP, N = 15; sham, N = 9–10) and 7 days (CPIP, N = 6; Sham, N = 7) after IR injury.
The effect of NFkB inhibition by systemic PDTC administration on allodynia was studied in CPIP rats using 4 treatment groups (saline and 10, 30, and 100 mg/kg of PDTC; 10 rats per group) and in sham rats using 2 treatment groups (saline and 100 mg/kg of PDTC; 10 rats per group). PDTC/saline was administered intraperitoneally (i.p.) 48 hours after IR injury. Animals were tested for mechanical and cold allodynia in the ipsilateral hind paw just before treatment, and at 30, 60, 90, 120, and 180 minutes after treatment.
Additionally, to investigate the site of PDTC effects, intrathecal or intraplantar administrations (250 mg per rat) were performed in 2 additional groups of animals (N = 10 per group) and compared to saline treatment using both administration routes (N = 10 per group). Intrathecal injections (20 mL volume) were performed by L6 lumbar puncture under brief anesthesia with isofluorane, whereby intrathecal delivery was confirmed by observing an injection induced tail-flick.40 Intraplantar injections (50 mL volume) were performed in the ipsilateral foot of awake animals. Mechanical allodynia was measured in both the ipsi- and the contralateral hindpaw at 30 and 60 minutes after PDTC administration.
All treatment and testing procedures were performed by a single experimenter per test, who was blinded for the CPIP/sham status of rat as well as for the treatment status (PDTC or saline). PDTC was obtained from Sigma-Aldrich (St. Louis, MO) and was freshly dissolved daily in saline.
Animals
Male Long Evans rats (275–300 g, Charles River, Quebec) arrived at least 5 days before the start of experiments. They were kept under a 12 hour/12 hour light-dark cycle (lights on at 7:00 h) with free access to food and water. All experiments were performed during the light cycle. Methods were approved by the Animal Care Committee at the McGill University, and conformed to the ethical guidelines of the Canadian Council on Animal Care.
CPIP
CPIP was induced by ischemia and reperfusion (IR) injury of the left hind paw as described by Coderre et al.14 Briefly, animals were anesthetized over a 3-hour period with a bolus (55 mg/kg, i.p.) and chronic i.p. infusion of sodium pentobarbital for 2 hours (27.5 mg/kg/h). After induction of anesthesia, a Nitrile 70 Durometer O-ring (O-rings West, Seattle, WA) with a 5.5mm internal diameter was placed around the rat’s left ankle joint. After 3 hours the O-ring was cut, allowing reperfusion of the hind limb. Sham animals underwent anesthesia similar to the CPIP animals, but an O-ring was not placed around the ankle.
Tissue Sampling and Preparation
Animals were euthanized by decapitation under anesthesia with isofluorane. Immediately, muscle samples of the superficial plantar layer (one each from the Flexor Hallucis Brevis, Flexor Digiti Minimi Brevis and Flexor Digitorium Brevis, each weighting between 29 and 50 mg) and spinal cord samples at L5-L6 (each weighting 12 to 20 mg) were obtained and quickly frozen in isopentane, kept on dry ice, and stored at −80°C until processing. Spinal cord samples were sectioned to isolate the dorsal half, which contained predominantly the dorsal horns, and sectioned again at the midline to isolate ipsilateral and contralateral tissue. Samples were thawed at 4°C and homogenized either mechanically (muscle) or by sonification (spinal cord) in 12 mL/mg tissue of RIPA buffer containing 50 mM Tris-HCl, 150 mM NaCl, 1mM EDTA, 1% Igepal (Sigma-Aldrich), 1% Sodium deoxycholate and .1% SDS (Ph 7.4), to which was added a 1% protease inhibitor cocktail (Sigma-Aldrich). Tissue homogenates were centrifuged at 3,000 g for 10 minutes, and the supernatant was collected and processed for nuclear fraction extraction following the recommended procedure of a commercially produced extraction kit (Chemicon Nuclear Extraction Kit; Millipore Corp Billerica, MA). Briefly, after spinning at 250 g for 5 minutes, samples were diluted 1/5 (vol/vol) in cytoplasmic lysis buffer and incubated at 4°C for 20 minutes. The homogenates were mechanically sheared by repeatedly drawing and ejecting each sample through a series of 25 ga, 26 ga and finally 27 ga needles and centrifuged at 8,000 g for 20 minutes at 4°C. Subsequently, the pellets were resuspended in nuclear extraction buffer, mechanically disrupted with a 27 ga needle, incubated for 60 minutes, and then centrifuged at 16,000 g for 6 minutes at 4°C, in order to obtain the supernatant that contained the nuclear fraction. Nuclear fractions were concentrated by centrifugal filtration at 14,000 g for 20 minutes using cellulose filters with a 30 kDa cut-off (Microcon YM-30; Millipore Corp). Nuclear fractions remaining after filtration were collected and diluted in buffer to a final volume of 100 mL for muscle and 50 mL for spinal samples. Total sample protein content was determined by the Bradford method.10 Nuclear extracts were stored at −80°C until further analysis.
Measurement of NFkB by ELISA
NFkB measurements were performed using a commercially supplied NFkB transcription factor binding assay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s suggested protocol. Briefly, duplicate 10 mL samples of nuclear extract were first incubated overnight at 4°C in wells precoated with a dsDNA sequence corresponding to the NFkB consensus motif. The N-terminus of NFkB is highly conserved (92% sequence homology between rat and human p50 subunit); thus, the NFkB consensus motif of the assay should bind both human and rat p50. After 5 washes, the samples were incubated overnight at 4°C with a rabbit polyclonal antibody to the p50 subunit of NFkB at a final dilution of 1:100 (sc-7178; Santa Cruz Biotechnology, Santa Cruz, CA). The detection antibody is an epitope corresponding to amino acids 120–239 mapping at the N-terminus of NFkB of human origin recommended for the detection of NFkB p50 and p105 and has high cross-reactivity between mouse, rat, and human. Subsequently, samples were incubated for 60 minutes with an HRP-conjugated goat antirabbit secondary antibody (Cayman Chemical), followed by colorimetric detection (measured as absorbance at 450 nm, Versamax; Molecular Devices, Sunnyvale, CA). After background subtraction, absorbance measures were referred to a standard curve obtained from a series of duplicate wells containing measured amounts of human recombinant p50 (Cayman Chemical) and then converted to an estimate of the quantity of p50/well, which was normalized by dividing the p50 estimate by the total amount of protein measured in the sample.
Mechanical and Cold Allodynia
Mechanical allodynia was assessed by measuring the 50% withdrawal response to stimulation with von Frey filaments, according to a modified ethod as described by Chaplan et al.11 Briefly, rats were placed in Plexiglas cages with a wire grid bottom. Filaments (Stoelting, Wood Dale, IL) were applied to the plantar surface of the hind paw for approximately 5 seconds in either ascending or descending strength, to determine the filament closest to the threshold of response. CPIP rats that had not developed mechanical allodynia at 48 hours post-IR injury (nonresponders, 50% threshold > 10 g) were excluded from further measurements of mechanical allodynia after PDTC treatment.
Cold allodynia was assessed using a modification of the acetone drop method as described by Choi et al.13 A drop of acetone was placed on the plantar surface of the foot and the response was measured as the amount of seconds of nociceptive behavior observed during the first minute after acetone application. Again, nonresponders for cold allodynia at 48 hours post-IR injury (pain behavior for 1 second or less) were excluded from further measurements of cold allodynia after PDTC treatment.
Mechanical and cold allodynia were tested in the same animals, with mechanical allodynia always tested first. When both sides were tested, the contralateral side was tested before the ipsilateral side.
Statistics
All statistical analyses were performed using the statistical package for social sciences (SPSS v.12.0; SPSS Inc, Chicago, IL) Significance was established at P < .05. Data were plotted as the mean 6 standard error of the mean (SEM).
NFkB in tissue from the ipsi- and contralateral side of CPIP rats was compared to sham rats using a Mann-Whitney U test. Ipsi- vs contralateral differences within rats were compared using a Wilcoxon signed rank test.
Baseline mechanical and cold allodynia test results were compared with one-way ANOVA. Post-treatment differences between groups were analyzed using repeated measures ANOVA with a Greenhous-Geisser correction for sphericity. In post hoc analyses, each treatment group was compared to the saline control group applying a Bonferoni correction. In CPIP rats only, decreases in cold allodynia relative (percentage) to pretreatment values were calculated and compared to the saline control group. For mechanical allodynia, a delta area under the curve (DAUC) relative to pretreatment values was calculated over the period of observation and compared with the saline group using one-way ANOVA followed by a post hoc LSD test. Pre-and posttreatment values within 1 treatment group were compared using a Wilcoxon signed rank test.
Results
NFkB in Muscle and Spinal Cord
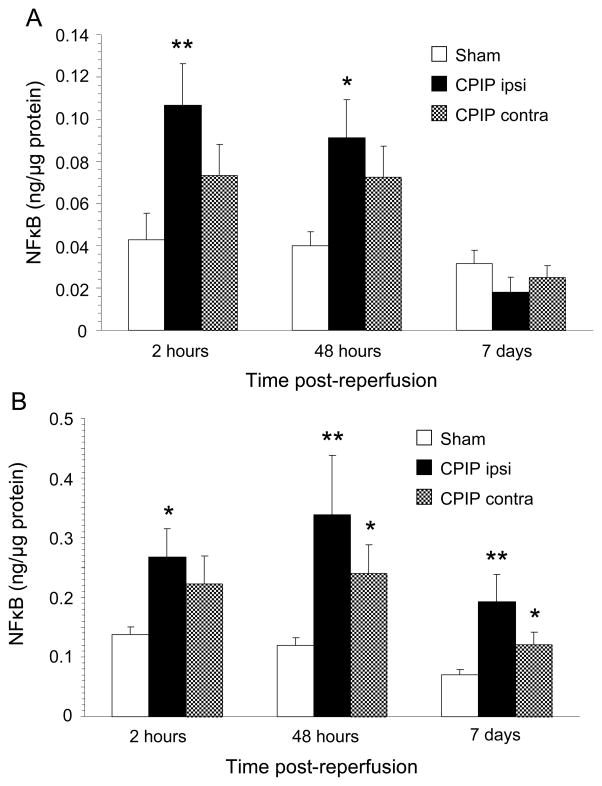
The results of NFkB measurements in muscle and spinal cord are depicted in Fig 1. Because in sham rats NFkB levels at both sides were similar (P = .162 for muscle and P = .694 for spinal cord), the right and left side measures of sham rats were combined in the comparison to CPIP rats. At both 2 and 48 hours after IR injury, NFkB was increased compared to sham rats in muscle (P = .004 at 2 hours and P = .020 at 48 hours) as well as spinal cord (P = .027 at 2 hours and P = .001 at 48 hours) from the ipsilateral side of CPIP rats. At 7 days after IR injury, NFkB levels in muscle did not differ between CPIP and sham rats. However, in spinal cord, the ipsilateral NFkB levels from CPIP rats were still elevated (P = .001). Remarkably, also on the contralateral side of CPIP rats, NFkB seemed to be increased compared to shams, although this difference was only statistically significant for the spinal cord at 48 hours and 7 days after IR injury. Moreover, within CPIP rats there was no significant difference in NFkB levels between ipsi- and contralateral sides in both muscle and spinal cord.
Fig. 1.
A) NFκB levels in muscle from the ipsilateral (ipsi) and contralateral (contra) hind paw of CPIP rats compared to shams at 2 hrs, 48 hrs and 7 days post-reperfusion, measured by ELISA. CPIP ipsilateral at 2 and 48 hrs: N = 15, at 7 days: N = 6. CPIP contralateral at 2 and 48 hrs: N = 15, at 7 days: N = 6. Sham at 2 and 48 hrs: N = 18, at 7 days N = 14. *p < 0.05, **p < 0.005.
B) NFκB levels in spinal cord from the ipsilateral (ipsi) and contralateral (contra) side of CPIP rats compared to shams at 2 hrs, 48 hrs and 7 days post reperfusion, measured by ELISA. CPIP ipsilateral at 2 and 48 hrs: N = 15, at 7 days: N = 6. CPIP contralateral at 2 and 48 hrs: N =15, at 7 days: N = 6. Sham at 2 and 48 hrs: N = 18, at 7 days N=14. *p < 0.05, **p < 0.005.
Systemic PDTC Treatment
Paw-withdrawal thresholds of the ipsilateral hind paw at baseline did not differ between CPIP (N= 40) and sham (N = 20) rats (13.2 ± 3.6 g and 13.4 ± 3.0 g respectively, P = .823). At 48 hours after IR injury, CPIP rats developed a decrease in paw-withdrawal threshold (mean 50% von Frey threshold of 6.83 ± 3.64 g) compared to shams (mean 50% von Frey threshold of 11.88 ± 3.42 g) (P < .0001). Within the CPIP group, 32 rats (80%) displayed a 50% von Frey threshold < 10 and were regarded as responders for mechanical allodynia.
Acetone responses at baseline were similar between CPIP and sham rats (1.075 ± 1.8 seconds and 1.10 ± 1.6 seconds, respectively, P = .957). Compared to shams (2.05 ± 3.1 seconds), acetone responses were increased in CPIP rats at 48 hrs after IR injury (3.78 ± 4.3 seconds), although the difference was not significant (P = .113). In the CPIP group, 26 rats (65%) displayed pain behavior for more than 1 second, and were considered as responders for cold allodynia.
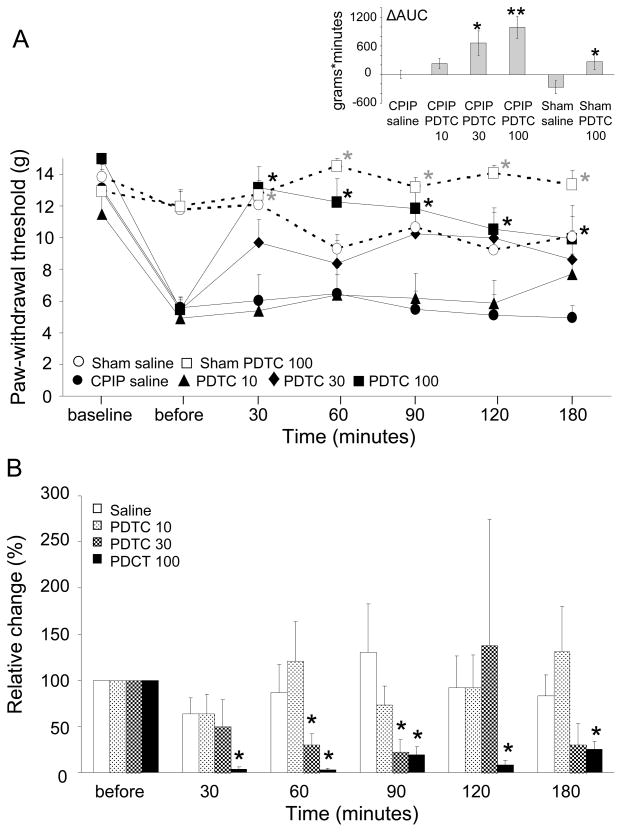
The effect of systemic PDTC treatment at 48 hours after IR injury on mechanical allodynia is displayed in Fig 2A. A significant main effect of time was observed (F(4,121) = 14.3, P < .001), as well as a significant main effect of treatment (F(3,28) = 8.1, P < .001), but there was no significant time X treatment interaction (F(13,121) = 1.29, P = 0.229). In post hoc analyses, CPIP rats that had received the highest dosage of PDTC (100 mg/kg) showed a decrease in mechanical hypersensitivity compared to the saline group (P = .02) and the group that received the lowest dose of PDTC (10 mg/kg, P = .02). The ΔAUC showed an effect of treatment for the highest (100 mg/kg, P = .002) and middle (30 mg/kg, P = .021) doses of PDTC, compared to saline controls. In the sham rats, no significant effect of time was observed, but there was a significant main effect of treatment (F(1,17) = 7.3, P = .015) and a significant time X treatment interaction (F(6,102) = 3.1, P = .008). Also within sham rats, the ΔAUC differed between the PDTC and the saline-treated group (P = .028).
Fig. 2.
A) Mechanical paw-withdrawal threshold in CPIP rats after systemic saline or PDTC treatment 48 hrs post-reperfusion. CPIP saline: N = 7; CPIP PDTC 10 mg/kg: N = 9; CPIP PDTC 30 mg/kg: N = 9; CPIP PDTC 100 mg/kg: N = 7; Sham saline: N = 10; Sham CPIP: N = 10. * p < 0.05 **p < 0.005.
B) Relative changes in cold water responses in the ipsilateral hind paw of CPIP rats after systemic saline or PDTC treatment 48 hrs post-reperfusion, compared to cold water responses before treatment. CPIP saline: N = 9; CPIP PDTC 10 mg/kg: N = 6; CPIP PDTC 30 mg/kg: N = 4; CPIP PDTC 100 mg/kg: N = 7. *p < 0.05.
Regarding the absolute values for cold allodynia, a significant main effect of time was observed (F(5,104) = 5.7, P < .001), but the main effect of treatment (F(3,22) = 2.6, P = .079) and the time X treatment interaction (F(14,104) = 1.7, P = .057) just failed to reach significance. In sham rats, there was no significant main effect of time (F(3,50) = 1.8, P = .164) or treatment (F(1,17) = 0.8, P = .377), nor was there a significant time X treatment interaction (F(3,50) = 1.2, P = 0.318). For CPIP rats only, the relative (percentage) decrease in cold allodynia after systemic PDTC treatment is depicted in Fig 2B. A significant main effect of treatment was observed (F(3,22) = 4, P = .020), and post hoc analyses demonstrated a decrease in cold allodynia in rats that had received the highest dose of PDTC compared to saline treatment (P = .035) and the lowest PDTC dose (P = .045).
Intrathecal and Intraplantar PDTC Treatment
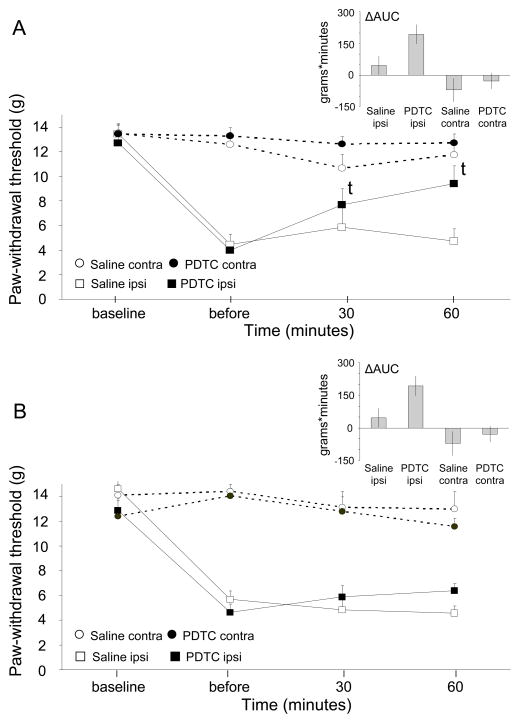
At baseline, there was no difference in mechanical sensitivity between the right and left hind paw (13.5 ± 2.15g and 13.3 ± 2.20 g, P = .689), while after IR injury, 26 out of 40 rats (65%) had developed mechanical allodynia. The effects of intrathecal and intraplantar PDTC treatment at 48 hours after IR injury on mechanical allodynia are depicted in Fig 3. For intrathecal treatment (Fig 3A), there was a significant main effect of time (F(2,27) = 21.6, P < .001), but there was no significant main effect of treatment (F(1.14) = 2.6, P = .132) or time X treatment interaction (F(2,27) = 2.6, P = .094). However, in the PDTC treatment group, the mean 50% VF threshold was increased at both 30 minutes (P = .017) and 60 minutes (P = .012) posttreatment, compared to the pretreatment threshold. No pre- vs post differences were observed in the saline-treatment group (P = .687). The ΔAUC was significantly larger for the PDTC group compared to the saline group (P = .034).
Fig. 3.
A) Mechanical paw-withdrawal thresholds in CPIP rats after intrathecal treatment with PDTC 48 hrs post-reperfusion. Saline ipsilateral (ipsi): N = 8; PDTC ipsilateral: N = 8; Saline contralateral (contra): N = 8; PDTC contralateral N=8; t p < 0.05) in mean paw-withdrawal threshold compared to before treatment.
B) Mechanical paw-withdrawal thresholds in CPIP rats after intraplantar treatment with PDTC 48 hrs post-reperfusion. Saline ipsilateral (ipsi): N = 5; PDTC ipsilateral: N = 5; Saline contralateral (contra): N = 5; PDTC contralateral: N = 5.
For intraplantar PDTC treatment (Fig 3B), a significant main effect of time was observed (F(2,14) = 45.9, P < .001), but there was no significant main effect of treatment (F(1,8) = 0, P = .980) or time X treatment interaction (F(2,14) = 1.9, P = .195). The mean 50% von Frey thresholds did not differ significantly between before and after treatment. Additionally, the DAUC was not significantly different.
Discussion
We investigated the involvement of NFkB in CPIP. NFkB was increased in muscle and spinal cord from CPIP rats compared to shams at both 2 and 48 hours after IR injury. At 7 days after IR injury, NFkB was equalized to shams in muscle, but was still elevated in spinal cord. Systemic PDTC administration at 48 hours after IR injury relieved mechanical and cold allodynia in a dose-dependent manner. Mechanical allodynia was also relieved upon intrathecal treatment, but not upon intraplantar treatment.
Considering previous studies, a role of NFkB in CPIP was a plausible expectation. First, several studies in different tissue types have demonstrated increased NFkB activity early after hypoxia, for example, in myocardial tissue, brain, hepatic tissue, and skeletal muscle.37,38,42,49 In all of these studies, the extent of the damage caused by IR injury could be attenuated by administration of an NFkB inhibitor. Second, CPIP rats display signs of inflammation.14 IR injury is known to provoke a well-documented cascade of inflammatory events,8 and NFkB is an important mediator in such inflammatory responses. Third, CPIP rats develop neuropathic painlike symptoms, including mechanical and cold allodynia. Previously, increased NFkB activity has been demonstrated in animal neuropathic pain models, while these symptoms can be relieved by an NFkB inhibitor.32,47,51
The hypothetical involvement of NFkB in CPIP is confirmed by our present observations. NFkB elevation in muscle tissue is consistent with a previous report of muscular NFkB activation upon ischemia by arterial clamping.37 On the contrary, increases in spinal NFkB levels following peripheral IR injury is to our knowledge a new finding, although spinal NFkB activation has been reported following peripheral nerve section44 and nerve inflammation.25,33 These peripheral triggers can induce an intraspinal cytokine release, a process that may be mediated by NFkB.47,51 However, neuropathic painlike symptoms and spinal NFkB activation in CPIP rats are subsequent to IR injury instead of traumatic nerve injury or direct immunological stimulation.14 Presumably, IR injury can induce pathological responses in the central nervous system (CNS) similar to those induced by mechanical or inflammatory nerve damage, through direct activation of nociceptors by either reactive oxygen species (ROS) or ROS-induced inflammatory reactions. The observation in CPIP rats of prolonged spinal NFkB activity (until at least 7 days after IR injury), when peripheral levels were normalized, suggests that eventually the CNS pathology becomes independent of its initial peripheral trigger. This is consistent with previous observations showing that CPIP rats display ongoing mechanical allodynia at 7 days after IR injury, while plasma extravasation in the affected hind paw had been normalized within 24 hours.14
NFkB activity is also increased in the contralateral muscle tissue of CPIP rats, although not as profoundly as on the ipsilateral side, and within CPIP rats, a significant difference between the ipsi- and contralateral sides was not observed. A possible explanation may be the spread of free radicals and subsequently activated inflammatory mediators from the side of IR injury to the opposite side by blood circulation. In support of this possibility, contralateral allodynia has been found in the CPIP rats in the past,14 although not consistently in all studies.41 It may be that contralateral allodynia depends on spinal sensitization which may be mediated by NFkB that is increased in the contralateral spinal cord dorsal horn.
The anti-allodynic effect of systemic PDTC administration was clear and dose-dependent. Results from intrathecal administration were less pronounced, and no effect was obtained by intraplantar treatment. Presumably, at 48 hours after IR injury, mechanical allodynia is mainly caused by the enhanced central, and not the peripheral, NFkB activity. PDTC passes the blood brain barrier and systemic doses will likely produce CNS effects, whereas low concentration intraplantar injections should not. However, it is also possible that we were unable to determine the effective dose for local treatment. In sham animals, we observed a slightly decreasing 50% von Frey threshold during the course of the experiment (Fig 1A), which we believe can be attributed to sensitization by repeated testing. This sensitization did not occur in sham rats that were systemically treated with PDTC. PDTC may have prevented mechanical sensitization upon repeated mechanical stimulation by reducing NFkB activity, which was also measured at lower levels in the peripheral and central tissues of sham rats.
In CPIP rats, we observed a central and peripheral increase of NFkB activity together with allodynia that was relieved by administration of the NFkB inhibitor PDTC. However, we did not show directly the relationship between PDTC administration and decreasing NFkB activity. Although this might have completed the study, we considered such experiments of limited additional value, since both a central and peripheral decrease in NFkB activity upon systemic PDTC treatment have already been demonstrated convincingly by others. For example, Lille et al have demonstrated that systemic PDTC administration results in diminished NFkB binding activity in muscle tissue,37 and Nurmi et al have demonstrated blocking of the otherwise increased NFkB activity upon middle cerebral artery occlusion when rats were pretreated with systemically administered PDTC.42
Few animal models have been used to study the molecular mechanisms that potentially underlie CRPS. Some of these rely on direct nerve injury and are therefore more representative for CRPS type II and not for CRPS type I.28,30 Animal models that claim to mimic CRPS-I involve tibia fracture and casting,19 local infusion of a free radical donor,53 interarterial infusion of SP,18 and IR injury (CPIP model).14 The IR injury or CPIP model that was used in the present study resembles human CRPS-I in several aspects. CPIP rats display features that represent both inflammatory and neuropathic painlike symptoms of human CRPS. Moreover, CPIP rats express sympathetically maintained pain,55 a phenomenon that in the past has been considered almost pathognomic for CRPS, although currently it is acknowledged to be present in only a subset of CRPS patients. Similar to CRPS-I patients, CPIP rats receive poor pain relief from classical anti-inflammatory and anti-neuropathic pain treatments,41 but respond well to treatment with free radical scavengers.14 The major advantage of the CPIP model above others is that the CRPS-resembling features occur after a physical (not chemical) injury without microscopic evidence of direct nerve injury.
However, despite these parallels between CPIP rats and human CRPS, one can still argue about the convenience of the CPIP model for the study of disease mechanisms of CRPS. A major issue of concern is that human CRPS is most commonly preceded by a fracture or sprain and not by an evident ischemic injury. However, ischemia is actually relevant in at least part of the cases of human CRPS, as is demonstrated by findings consistent with oxidative stress in CRPS-affected limbs,29,53 and by the beneficial effect of treatment with free radical scavengers.43 Like IR injury, fracture or sprain can cause an acute inflammatory response, which may induce sensitization leading to allodynia and microvascular changes that may result in prolonged ischemia. The actual contribution of acute and chronic ischemia to the entire pathogenesis of CRPS may vary from patient to patient, as the disease onset and course of CRPS tend to be very heterogeneous. However, the connected inflammatory responses and mechanisms of sensitization may be similar, which makes the CPIP model useful for the study of mediators in these processes. Obviously, extrapolation of the results to human CRPS should be done with reserve, and the model may be representative for a subset of human CRPS cases only. However, our findings may at least provide new clues to the mechanisms of a long poorly understood disorder.
Inhibitors of NFkB have been suggested as interesting new therapeutics for (chronic) inflammatory disorders, such as asthma,6 rheumatoid diseases,46 and inflammatory bowel disorders.2 It was hypothesized that CRPS may be another candidate disorder for such treatment, since NFkB is likely involved in both peripheral (ischemia, inflammation) and central (sensitization) disease mechanisms that are assumed to underlie CRPS.21 The present study using the CPIP model strengthened this idea, since it demonstrated that NFkB is indeed involved in the development of allodynia without direct nerve injury, as is the case in CRPS-I. However, because of serious immunological consequences, there may be safety issues associated with targeting NFkB in human patients,52 and current therapeutic indications have been restricted to oncology. On the other hand, some nonspecific modulators of NFkB activity, including N-acetylcysteine14,43 and corticosteroids,19,27,41 have already been demonstrated to be beneficial in attenuating CRPS in animal models as well as in human trials. Currently, these drugs are not applied generally for CRPS treatment, but these new mechanistic insights warrant further clinical studies to establish their clinical benefits.
In conclusion, we have demonstrated increased NFkB activity in muscle and spinal cord, as well as a therapeutic effect of NFkB inhibition on allodynia, in CPIP rats. We believe that these results encourage further exploration of the role of NFkB in the pathogenesis of inflammatory pain disorders like CRPS-I. Potentially this will reveal new treatment opportunities for such disorders.
Perspective.
Using the CPIP model, we demonstrate that NFkB is involved in the development of allodynia after a physical injury (ischemia and reperfusion) without direct nerve trauma. Since CPIP animals exhibit many features of human CRPS-I, this observation indicates a potential role for NFkB in human CRPS.
References
- 1.Alexander GM, van Rijn MA, van Hilten JJ, Perreault MJ, Schwartzman RJ. Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain. 2005;116(3):213–219. doi: 10.1016/j.pain.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Birklein F, Schmelz M, Schifter S, Weber M. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–2184. doi: 10.1212/wnl.57.12.2179. [DOI] [PubMed] [Google Scholar]
- 5.Birklein F, Weber M, Ernst M, Riedl B, Neundorfer B, Handwerker HO. Experimental tissue acidosis leads to increased pain in complex regional pain syndrome (CRPS) Pain. 2000;87:227–234. doi: 10.1016/S0304-3959(00)00286-4. [DOI] [PubMed] [Google Scholar]
- 6.Birrell MA, Hardaker E, Wong S, McCluskie K, Catley M, De Alba J, Newton R, Haj-Yahia S, Pun KT, Watts CJ, Shaw RJ, Savage TJ, Belvisi MG. Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med. 2005;172:962–971. doi: 10.1164/rccm.200412-1647OC. [DOI] [PubMed] [Google Scholar]
- 7.Blair SJ, Chinthagada M, Hoppenstehdt D, Kijowski R, Fareed J. Role of neuropeptides in pathogenesis of reflex sympathetic dystrophy. Acta Orthop Belg. 1998;64:448–451. [PubMed] [Google Scholar]
- 8.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 9.Boone DL, Lee EG, Libby S, Gibson PJ, Chien M, Chan F, Madonia M, Burkett PR, Ma A. Recent advances in understanding NF-kappaB regulation. Inflamm Bowel Dis. 2002;8:201–212. doi: 10.1097/00054725-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 13.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 14.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Chatterjee PK, Mazzon E, Dugo L, Serraino I, Britti D, Mazzullo G, Caputi AP, Thiemermann C. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–747. [PubMed] [Google Scholar]
- 17.Gibbs GF, Drummond PD, Finch PM, Phillips JK. Unravelling the Pathophysiology of Complex Regional Pain Syndrome: Focus on sympathetically maintained pain. Clin Exp Pharmacol Physiol. 2008;35:717–724. doi: 10.1111/j.1440-1681.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- 18.Gradl G, Finke B, Schattner S, Gierer P, Mittlmeier T, Vollmar B. Continuous intra-arterial application of substance P induces signs and symptoms of experimental complex regional pain syndrome (CRPS) such as edema, inflammation and mechanical pain but no thermal pain. Neuroscience. 2007;148:757–765. doi: 10.1016/j.neuroscience.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Hattori Y, Hattori S, Sato N, Kasai K. High-glucoseinduced nuclear factor kappaB activation in vascular smooth muscle cells. Cardiovasc Res. 2000;46:188–197. doi: 10.1016/s0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- 21.Hettne KM, de Mos M, de Bruijn AG, Weeber M, Boyer S, van Mulligen EM, Cases M, Mestres J, van der Lei J. Applied information retrieval and multidisciplinary research: New mechanistic hypotheses in Complex Regional Pain Syndrome. J Biomed Discov Collab. 2007;2:2. doi: 10.1186/1747-5333-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. Faseb J. 1999;13:1845–1854. [PubMed] [Google Scholar]
- 23.Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, Zijistra FJ. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm. 2002;11:47–51. doi: 10.1080/09629350210307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huygen FJ, Ramdhani N, van Toorenenbergen A, Klein J, Zijlstra FJ. Mast cells are involved in inflammatory reactions during complex regional pain syndrome type 1. Immunol Lett. 2004;91:147–154. doi: 10.1016/j.imlet.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Inoue G, Ochiai N, Ohtori S, Nakagawa K, Gemba T, Doya H, Ito T, Koshi T, Moriya H, Takahashi K. Injection of nuclear factor-kappa B decoy into the sciatic nerve suppresses mechanical allodynia and thermal hyperalgesia in a rat inflammatory pain model. Spine. 2006;31:2904–2908. doi: 10.1097/01.brs.0000248424.46652.67. [DOI] [PubMed] [Google Scholar]
- 26.Jongeneel CV. Regulation of the TNF alpha gene. Prog Clin Biol Res. 1994;388:367–381. [PubMed] [Google Scholar]
- 27.Kalita J, Vajpayee A, Misra UK. Comparison of prednisolone with piroxicam in complex regional pain syndrome following stroke: A randomized controlled trial. Qjm. 2006;99:89–95. doi: 10.1093/qjmed/hcl004. [DOI] [PubMed] [Google Scholar]
- 28.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104:75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 29.Koban M, Leis S, Schultze-Mosgau S, Birklein F. Tissue hypoxia in complex regional pain syndrome. Pain. 2003;104:149–157. doi: 10.1016/s0304-3959(02)00484-0. [DOI] [PubMed] [Google Scholar]
- 30.Kurvers H, Daemen M, Slaaf D, Stassen F, van den Wildenberg F, Kitslaar P, de Mey J. Partial peripheral neuropathy and denervation induced adrenoceptor supersensitivity. Functional studies in an experimental model. Acta Orthop Belg. 1998;64:64–70. [PubMed] [Google Scholar]
- 31.Laughlin TM, Bethea JR, Yezierski RP, Wilcox GL. Cytokine involvement in dynorphin-induced allodynia. Pain. 2000;84:159–167. doi: 10.1016/s0304-3959(99)00195-5. [DOI] [PubMed] [Google Scholar]
- 32.Ledeboer A, Gamanos M, Lai W, Martin D, Maier SF, Watkins LR, Quan N. Involvement of spinal cord nuclear factor kappaB activation in rat models of proinflammatory cytokine-mediated pain facilitation. Eur J Neurosci. 2005;22:1977–1986. doi: 10.1111/j.1460-9568.2005.04379.x. [DOI] [PubMed] [Google Scholar]
- 33.Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur J Neurosci. 2004;19:3375–3381. doi: 10.1111/j.0953-816X.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 34.Leis S, Weber M, Isselmann A, Schmelz M, Birklein F. Substance-P-induced protein extravasation is bilaterally increased in complex regional pain syndrome. Exp Neurol. 2003;183:197–204. doi: 10.1016/s0014-4886(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Hou L, Hua Z, Wang X. Interleukin-1beta induces beta-calcitonin gene-related peptide secretion in human type II alveolar epithelial cells. Faseb J. 2004;18:1603–1605. doi: 10.1096/fj.04-1737fje. [DOI] [PubMed] [Google Scholar]
- 36.Lieb K, Fiebich BL, Berger M, Bauer J, Schulze-Osthoff K. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 37.Lille ST, Lefler SR, Mowlavi A, Suchy H, Boyle EM, Jr, Farr AL, Su CY, Frank N, Mulligan DC. Inhibition of the initial wave of NF-kappaB activity in rat muscle reduces ischemia/reperfusion injury. Muscle Nerve. 2001;24:534–541. doi: 10.1002/mus.1037. [DOI] [PubMed] [Google Scholar]
- 38.Matsui N, Kasajima K, Hada M, Nagata T, Senga N, Yasui Y, Fukuishi N, Akagi M. Inhibiton of NF-kappaB activation during ischemia reduces hepatic ischemia/reperfusion injury in rats. J Toxicol Sci. 2005;30:103–110. doi: 10.2131/jts.30.103. [DOI] [PubMed] [Google Scholar]
- 39.Mattson MP, Meffert MK. Roles for NF-kB in nerve cell survival, plasticity and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 40.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 41.Millecamps M, Coderre TJ. Rats with chronic post-ischemia pain exhibit an analgesic sensitivity profile similar to human patients with complex regional pain syndrome - type I. Eur J Pharmacol. 2008;583:97–102. doi: 10.1016/j.ejphar.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nurmi A, Lindsberg PJ, Koistinaho M, Zhang W, Juettler E, Karjalainen-Lindsberg ML, Weih F, Frank N, Schwaninger M, Koistinaho J. Nuclear factor-kappaB contributes to infarction after permanent focal ischemia. Stroke. 2004;35:987–991. doi: 10.1161/01.STR.0000120732.45951.26. [DOI] [PubMed] [Google Scholar]
- 43.Perez RS, Zuurmond WW, Bezemer PD, Kuik DJ, van Loenen AC, de Lange JJ, Zuidhof AJ. The treatment of complex regional pain syndrome type I with free radical scavengers: A randomized controlled study. Pain. 2003;102:297–307. doi: 10.1016/S0304-3959(02)00414-1. [DOI] [PubMed] [Google Scholar]
- 44.Pollock G, Pennypacker KR, Memet S, Israel A, Saporta S. Activation of NF-kappaB in the mouse spinal cord following sciatic nerve transection. Exp Brain Res. 2005;165:470–477. doi: 10.1007/s00221-005-2318-6. [DOI] [PubMed] [Google Scholar]
- 45.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC. Bortezomib or highdose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 46.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Sakaue G, Shimaoka M, Fukuoka T, Hiroi T, Inoue T, Hashimoto N, Sakaguchi T, Sawa Y, Morishita R, Kiyono H, Noguchi K, Mashimo T. NF-kappa B decoy suppresses cytokine expression and thermal hyperalgesia in a rat neuropathic pain model. Neuroreport. 2001;12:2079–2084. doi: 10.1097/00001756-200107200-00008. [DOI] [PubMed] [Google Scholar]
- 48.Sehgal PB. Regulation of IL6 gene expression. Res Immunol. 1992;143:724–734. doi: 10.1016/0923-2494(92)80011-9. [DOI] [PubMed] [Google Scholar]
- 49.Stansfield WE, Moss NC, Willis MS, Tang R, Selzman CH. Proteasome inhibition attenuates infarct size and preserves cardiac function in a murine model of myocardial ischemia reperfusion injury. Ann Thorac Surg. 2007;84:120–125. doi: 10.1016/j.athoracsur.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 50.Sun T, Song WG, Fu ZJ, Liu ZH, Liu YM, Yao SL. Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-kappaB. Br J Anaesth. 2006;97:553–558. doi: 10.1093/bja/ael209. [DOI] [PubMed] [Google Scholar]
- 51.Tegeder I, Niederberger E, Schmidt R, Kunz S, Guhring H, Ritzeler O, Michaelis M, Geisslinger G. Specific Inhibition of IkappaB kinase reduces hyperalgesia in inflammatory and neuropathic pain models in rats. J Neurosci. 2004;24:1637–1645. doi: 10.1523/JNEUROSCI.3118-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uwe S. Anti-inflammatory interventions of NF-kappaB signaling: Potential applications and risks. Biochem Pharmacol. 2008;75:1567–1579. doi: 10.1016/j.bcp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 53.van der Laan L, ter Laak HJ, Gabreels-Festen A, Gabreels F, Goris RJ. Complex regional pain syndrome type I (RSD): Pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–25. doi: 10.1212/wnl.51.1.20. [DOI] [PubMed] [Google Scholar]
- 54.Watkins LR, Maier SF. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- 55.Xanthos DN, Coderre TJ. Sympathetic vasoconstrictor antagonism and vasodilatation relieve mechanical allodynia in rats with chronic postischemia pain. J Pain. 2008;9:423–433. doi: 10.1016/j.jpain.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]