Abstract
Chronic pain that responds to antisympathetic treatments and α-adrenergic antagonists is clinically referred to as sympathetically maintained pain. Animal models of neuropathic pain have shown mixed results in terms of antinociceptive effectiveness of antisympathetic agents. The effectiveness of these agents have not been yet investigated in animal models of complex regional pain syndrome-type 1 (CRPS-I). In this study, we examined the effectiveness of antisympathetic agents and sympathetic vasoconstrictor antagonists, as well as agents that are vasodilators, in relieving mechanical allodynia in a recently developed animal model of CRPS-I (chronic postischemia pain or CPIP) produced by three hours of hind paw ischemia-reperfusion injury. Systemic guanethidine, phentolamine, clonidine, and prazosin are effective in reducing mechanical allodynia particularly at 2 days after reperfusion, and less so at 7 days after reperfusion. A nitric oxide donor vasodilator, SIN-1, also reduces mechanical allodynia more effectively at 2 days after reperfusion, but not at 7 days after reperfusion. These results suggest that the pain of CPIP, and possibly also CRPS-I, is relieved by reducing sympathetically mediated vasoconstriction, or enhancing vasodilatation.
Keywords: Complex regional pain syndrome, ischemia-reperfusion injury, neuropathic pain, allodynia, sympathetic block, adrenergic receptors
Complex regional pain syndrome-type I (CRPS-I) is a disorder that occurs after fracture, soft tissue or crush injury.16 Symptoms of CRPS-I include spontaneous burning (cutaneous) and aching (deep) pain, hyperalgesia, allodynia, and disorders of vasomotor and sudomotor regulation.27,57 CRPS-II is similar but also exhibits a clinically verified nerve injury. 74
Although controversial, sympathetic blocks are often reported to relieve CRPS pain.10,29,68 CRPS pain is also relieved with α-adrenergic antagonists such as phentolamine or phenoxybenzamine,48,52 agents that have been used as diagnostic tools for identifying so-called sympathetically maintained pain (SMP).3,76 Although clinical studies have not determined the specific mechanisms of SMP, CRPS-I patients display abnormal responses to sympathetic stimulation75 and enhanced vasoconstrictive responses to norepinephrine (NE),1,4 as well as painful responses to intradermal NE.69 Pain relief in CRPS patients usually follows warming of the affected limb,5,62,70 suggesting that vasodilatation may be important to pain relief by sympathetic blockade in CRPS.70
Sympathectomy also relieves neuropathic pain in animals with spinal nerve ligation (SNL), chronic constriction injury of the sciatic nerve (CCI), and partial sciatic nerve ligation32,65 (PSNL) (however, see Ringkamp et al54) but not cryoneurolysis or sural and tibial nerve transection.24,77 α-Adrenergic antagonists also relieve neuropathic pain in animal models26,34,41 (but see Park et al51; Ringkamp et al55). Mechanisms that have been suggested for SMP in these models of CRPS-II include sympathetic efferent–primary afferent coupling,12 possibly after sympathetic fiber sprouting,40,53 or de novo adrenergic sensitivity in damaged afferents,17,72 dorsal root ganglion cells,18,47 or afferent nociceptors.50,59 De novo adrenergic sensitivity may depend on upregulation of adrenoceptors on primary afferent nerve fibers7,13,79 or other indirect mechanisms such as tissue ischemia due to enhanced vasoconstrictive responsiveness to NE. Importantly, rats with CCI have impaired vasoconstriction to sympathetic stimulation and enhanced vasoconstriction to exogenous NE. 36,37
We recently introduced chronic postischemia pain (CPIP) as an animal model of CRPS-I produced after hind paw ischemia-reperfusion (I-R) injury.14 This animal model shows signs of I-R injury such as no-reflow and vascular abnormalities, as well as nociceptive and vascular hypersensitivity to norepinephrine.38,78 The pain-relieving effects of antisympathetic agents have not previously been tested in animal models of CRPS-I.21,22,71 In this report, we examine whether antisympathetic drugs, and agents that are vasodilators, are effective in reducing painful symptoms in this model. Thus, we test the effectiveness of sympathetic block with guanethidine, or systemic treatments with the α1- and α2-adrenergic antagonists prazosin and yohimbine, the α2-adrenergic agonist clonidine, and a nitric oxide donor, in relieving mechanical allodynia in CPIP rats at 2 and 7 days after reperfusion.
Materials and Methods
Animals
Male Long-Evans hooded rats (275–325 g; Charles River, Quebec, Canada) were housed in groups of 3 to 4, with food and water available ad libitum, on a 12:12-hour light:dark cycle. All treatments and testing procedures were approved by the Animal Care Committee at McGill University, and conformed to the ethical guidelines of the Canadian Council on Animal Care and the International Association for the Study of Pain.
Animal Model of CRPS-I
Chronic postischemia pain was generated after exposure to prolonged hind paw ischemia and reperfusion as described in Coderre et al.14 Briefly, rats were anesthetized over a 3- to 4-hour period with a bolus (40 mg/kg, i.p.) and chronic intraperitoneal infusion of sodium pentobarbital for 2 hours (13 mg/h for the first hour, 6.5 mg/h for the second hour). After induction of anesthesia, a Nitrile 70 Durometer O-ring (O-Rings West, Seattle, WA) with a 7/32-inch internal diameter was placed around the rat’s left hind limb just proximal to the ankle joint for 3 hours. We standardized the position of the O-ring to a point on the limb just proximal to the medial malleolus of the tibia. The termination of sodium pentobarbital anesthesia was timed so that rats recovered fully within 30 to 60 minutes after reperfusion, which occurred immediately after removal of the O-ring. Sham rats received exactly the same treatment, except that the O-ring was cut, so that it only loosely surrounded the ankle and did not occlude blood flow to the hind paw.
Mechanical Sensitivity Nociceptive Testing
Hind paw mechanical thresholds were assessed by measuring the withdrawal response to von Frey filament stimulation according to a modification of the up/down method described by Chaplan et al.11 In brief, rats were placed in a plexiglas box (21 × 16 × 27 cm3) with a wire grid bottom through which the von Frey filaments (nylon monofilaments; Stoelting, Woodale, IL) were applied to the plantar surface of the hind paw. Filaments were applied in either ascending or descending strength as necessary to determine the filament closest to the threshold of response. Each filament was applied once for 10 seconds to the center of the paw between the pads, and a lower intensity hair followed each positive response, whereas a higher intensity hair followed each negative response (until 5 responses were recorded after a first change in response). The minimum stimulus intensity was 0.25 g and the maximum was 15 g. Based on the response pattern and the force of the final filament, the 50% response threshold (grams) was calculated. The resulting pattern of positive and negative responses was tabulated, and the 50% response threshold was interpolated using the formula 50% g threshold = (10[xf+ kδ])/10,000, where xf = the value (in log units) of the final von Frey hair used; k= tabular value11 for pattern of positive/negative responses; and δ = mean difference (in log units) between stimuli (here, 0.224). Hairs were from the standard Semmes-Weinstein series.63
Drugs
Guanethidine, phentolamine, prazosin, yohimbine, and clonidine were all obtained from Sigma (Oakville, Ontario, Canada). 3-Morpholinylsydnoneimine chloride (SIN-1) was obtained from Tocris Bioscience (Ellisville, MO). All agents were diluted in a 0.9% saline vehicle.
Drug Administration Protocol
Mechanical sensitivity was tested before CPIP induction and before drug administration on day 2 or day 7 days after reperfusion (predrug) and at various times after drug administration. To avoid testing rats that did not exhibit allodynia, it was decided a priori that, for all groups, only rats with post-reperfusion (predrug) von Frey thresholds less than 6 g were used in the drug trials (accordingly, the mean and SEM for baseline von Frey thresholds only include rats that were subsequently given drug treatments).
For sympathetic block experiments, vehicle or guanethidine (30 mg/kg) was injected subcutaneously twice (separated by 24 hours) on either days 2 and 3 or days 7 and 8 after reperfusion. Mechanical allodynia was tested 4 hours after the second injection on day 3 (for days 2–3 guanethidine) or day 8 after reperfusion (for days 7–8 guanethidine). Separate groups of rats that received vehicle or guanethidine injections on days 2 to 3 were tested on days 3, 5, 9, and 14 after reperfusion. Previous studies have shown that even a single dose of systemic guanethidine is sufficient for long-term sympathetic blockade.33,45 For dose-effect studies with phentolamine (1–5 mg/kg), prazosin (1–5 mg/kg), yohimbine (1–5 mg/kg), clonidine (0.01–0.1 mg/kg), and SIN-1 (1–10 mg/kg), the drugs were administered intraperitoneally, and mechanical allodynia was tested 20 minutes later on either day 2 or 7 after reperfusion. Separate rats were tested on days 2 and 7. For time-course studies, rats were tested over a 3-hour period on day 2 after reperfusion with the highest dose of each drug (except guanethidine). All drug dosages were selected on the basis of previous studies examining the effects of these agents on nociception.26,34,44,51,55,64,67 Furthermore, all drug doses that were used were determined not to induce significant abnormalities in the rotorod test. The highest doses of phentolamine, prazosin, and yohimbine used produced a significant inhibition of NE-induced reductions in hind paw blood flow as measured by laser Doppler flowmetry (data not shown). All experiments were performed by using a randomized blocks design, and at the time of testing the experimenter was blind to the animal’s treatment. Six to 7 rats were used per group.
Statistics
Group comparisons were analyzed using a 2-way repeated-measures ANOVA followed by Fisher’s post hoc tests. Preinjury baseline von Frey thresholds are included in the figures for each drug trial but are not included in the statistical analyses.
Results
Approximately 70% of rats subjected to the CPIP procedure displayed mechanical allodynia (von Frey threshold below 6 g) and were used in the drug trials. There were no significant differences between groups in baseline paw-withdrawal thresholds or predrug trial thresholds at 2 days or 7 days after reperfusion.
Effect of Sympathetic Block on CPIP Mechanical Allodynia
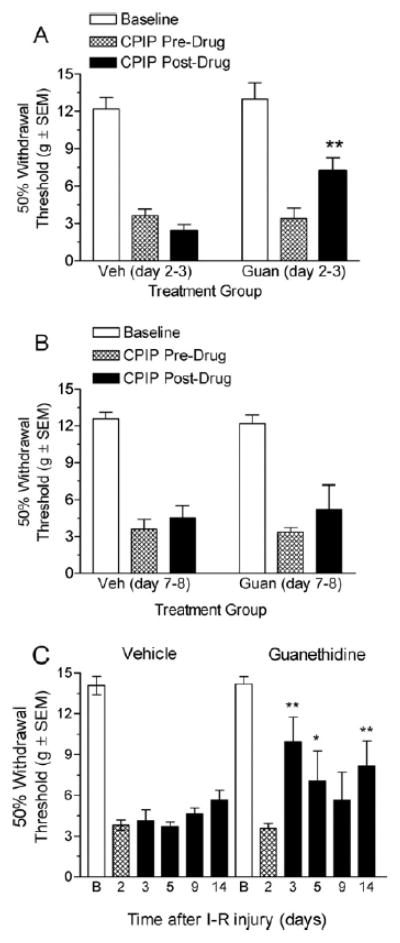
Fig 1A shows von Frey thresholds of CPIP rats before and after guanethidine or vehicle treatment starting on day 2 after reperfusion. Two-way ANOVA reveals significant main effects of group (F1, 23) =5.77, P < .05) and time (F1, 23) = 4.85, P< .05) and a significant group_time interaction (F1, 23) = 19.33, P < .01). The vehicle injection did not produce a significant anti-allodynic effect (P > .05); however, guanethidine-treated rats displayed a significant increase in paw withdrawal threshold after the 2-day guanethidine treatment (P< .01). Continuing daily guanethidine treatment for up to 7 days did not result in any further reduction of mechanical allodynia (data not shown). Fig 1B shows von Frey thresholds of CPIP rats before and after guanethidine or vehicle treatment starting on day 7 after reperfusion. Two-way ANOVA reveals non-significant main effects of group (F1, 23) = 0.05, P > .05) and time (F1, 23) = 1.12, P > .05) and a non-significant group X time interaction (F1, 23) = 0.12, P> .05). This suggests that at this time point on day 8, the 2-day guanethidine treatment did not reduce mechanical allodynia. Fig 1C shows von Frey thresholds of CPIP rats before and for 14 days after vehicle or guanethidine treatment on days 2 and 3 after reperfusion. Two-way ANOVA reveals a significant effect of group (F1, 64) = 7.20; P < .05) and time (F4, 64) = 2.81; P < .05), and a non-significant group _ time interaction (F4, 64) = 1.97, P > .05). The guanethidine treatment resulted in significant increase of paw withdrawal threshold as compared with day 2 predrug baseline, at day 3 (P < .01), day 5 (P < .05), and day 14 (P < .01). The vehicle treatment did not result in any significant increase of paw withdrawal threshold at any of the time points tested.
Figure 1.
A, Effect of sympathetic block with guanethidine on mechanical allodynia in chronic postischemia pain (CPIP) rats at 3 days after reperfusion. CPIP rats display a significant reduction in mechanical allodynia after 2-day guanethidine treatment starting 2 days after reperfusion (**P < .01, as compared with CPIP before drug; n=6 for each group). B, Effect of sympathetic block with guanethidine on mechanical allodynia in CPIP rats at 8 days after reperfusion. CPIP rats did not display a significant reduction in mechanical allodynia after 2-day guanethidine treatment starting 7 days after reperfusion (n= 6 for each group). C, Effect of sympathetic block with guanethidine on mechanical allodynia in CPIP rats at 3, 5, 9, and 14 days after reperfusion. CPIP rats display a significant reduction in mechanical allodynia at 3, 5 and 14 days, after 2-day guanethidine treatment starting 2 days after reperfusion (*P < .05, **P < .01, as compared with CPIP before drug; n= 6 for guanethidine group, n=7 for vehicle group). I-R, ischemia-reperfusion.
Effect of the Mixed α1/α2-Adrenergic Antagonist Phentolamine on CPIP Mechanical Allodynia
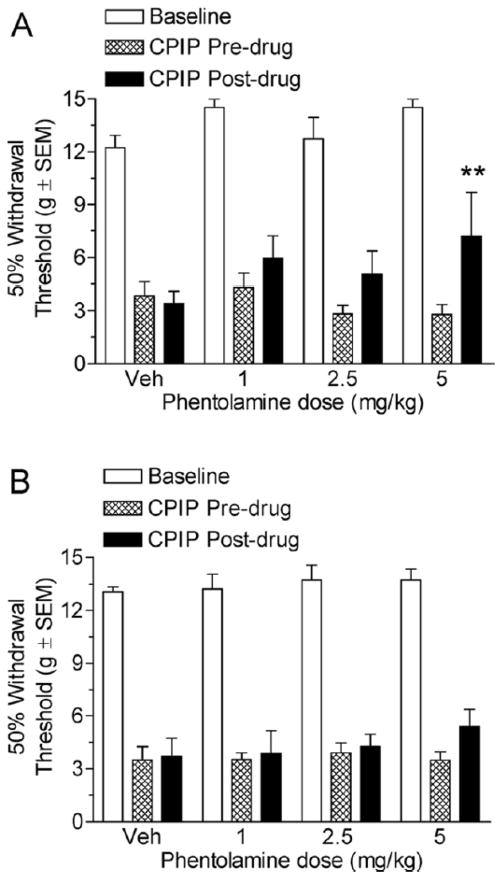
Fig 2A shows the von Frey thresholds of 2-day CPIP rats before and 20 minutes after treatment with vehicle or 1,2.5, and 5 mg/kg phentolamine. Two-way ANOVA revealed a significant main effect of time (pre-post) (F1, 47) = 8.05, P < .05), a non-significant main effect of dose (F3, 47) = 0.58, P < .05), and a non-significant time X dose interaction (F3, 47) = 2.05, P > .05). Neither the vehicle, 1 mg/kg phentolamine, nor 2.5 mg/kg phentolamine injection doses resulted in significant increases in paw withdrawal thresholds (P > .05). However, the time effect was significant because the 5 mg/kg phentolamine significantly increased in paw withdrawal threshold after the drug as compared with before the drug (P < .01). Fig 2B shows the von Frey thresholds of 7-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 2.5, and 5 mg/kg phentolamine. Two-way ANOVA reveals non-significant main effects of time (pre-post) (F1, 47) = 1.36, P > .05) and dose (F3, 47) = 0.52, P > .05) as well as a non-significant dose X time interaction (F3, 47) = 0.43, P > .05). Therefore, it appears that phentolamine is not effective at 7 days after reperfusion, and only the highest dose at 2 days after reperfusion is able to reduce mechanical allodynia in CPIP rats.
Figure 2.
A, Effect of systemic phentolamine in chronic postischemia pain (CPIP) rats at 2 days after reperfusion. CPIP rats display a significant reduction in mechanical allodynia with 5 mg/kg i.p. phentolamine administration but not with the 1 or 2.5 mg/kg doses (**P < .01 as compared with CPIP before drug; n=6 for each group). B, Effect of systemic phentolamine in CPIP rats at 7 days after reperfusion. CPIP rats did not display a significant reduction in mechanical allodynia with either 1, 2.5, or 5 mg/kg phentolamine doses at 7 days after reperfusion (n=6 for each group).
Effect of the α1-Adrenergic Antagonist Prazosin on CPIP Mechanical Allodynia
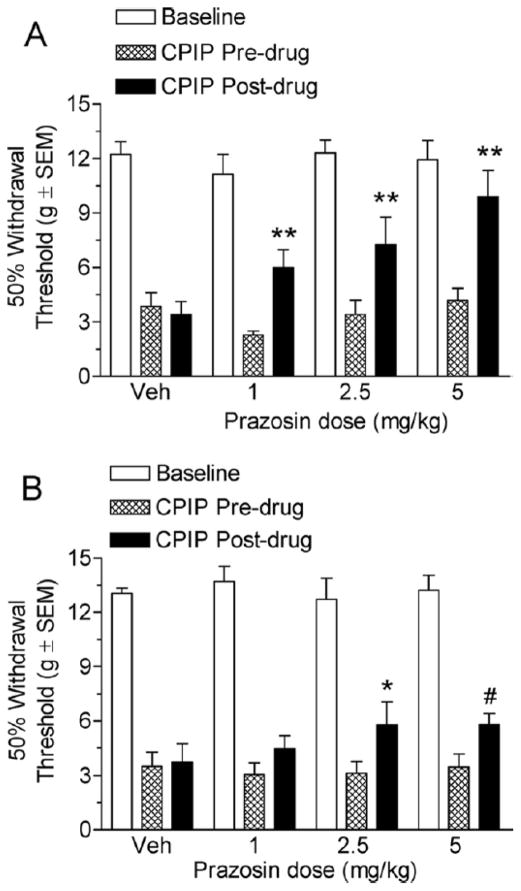
Fig 3A shows the von Frey thresholds of 2-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 2.5, and 5 mg/kg prazosin. Two-way ANOVA reveals significant main effects of time (pre-post) (F1, 47) = 28.27, P < .0001) and dose (F3, 47) = 3.90, P < .05) and a significant time X dose interaction (F3, 47) = 4.59, P < .05). The vehicle injection did not result in a significant increase in paw withdrawal threshold (P > .05). All 3 prazosin doses, 1, 2.5, and 5 mg/kg, resulted in significant increases in paw withdrawal thresholds (P < 0.01). Fig 3B shows the von Frey thresholds of 7-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 2.5, and 5 mg/kg prazosin. Two-way ANOVA reveals a significant main effect of time (pre-post) (F1, 47) = 8.80, P < .01), a non-significant main effect of dose (F3, 47) = 0.67, P > .05), and a non-significant time X dose interaction (F3, 47) = 0.95, P > .05). The vehicle injection did not result in a significant increase in paw withdrawal threshold (P > .05). The time effect was significant because the 2.5 mg/kg dose significantly increased the paw withdrawal thresholds after the drug as compared with before the drug (P > .05). However, neither the 1 mg/kg nor the 5 mg/kg prazosin doses were able to significantly increase the paw withdrawal thresholds relative to predrug levels (P < .05). However, rats that received the 5 mg/kg dose of prazosin did have significantly higher von Frey thresholds than rats that received vehicle (P < .05). It appears that prazosin is able to reduce mechanical allodynia at both 2 and 7 days after reperfusion, and is more effective at 2 days after reperfusion in CPIP rats.
Figure 3.
A, Effect of systemic prazosin in chronic postischemia pain (CPIP) rats at 2 days after reperfusion. CPIP rats display a significant reduction in mechanical allodynia with 1, 2.5, and 5 mg/kg prazosin administration (**P < .01 as compared with CPIP before drug; n= 6 for each group). B, Effect of systemic prazosin in CPIP rats at 7 days after reperfusion. CPIP rats display a significant reduction with the 2.5 and 5 mg/kg doses, but not the 1 mg/kg prazosin dose at 7 days after reperfusion (*P <.05 as compared with CPIP before drug, #P <.05 as compared with CPIP after vehicle; n=6 for each group).
Effect of the α2-Adrenergic Antagonist Yohimbine on CPIP Mechanical Allodynia
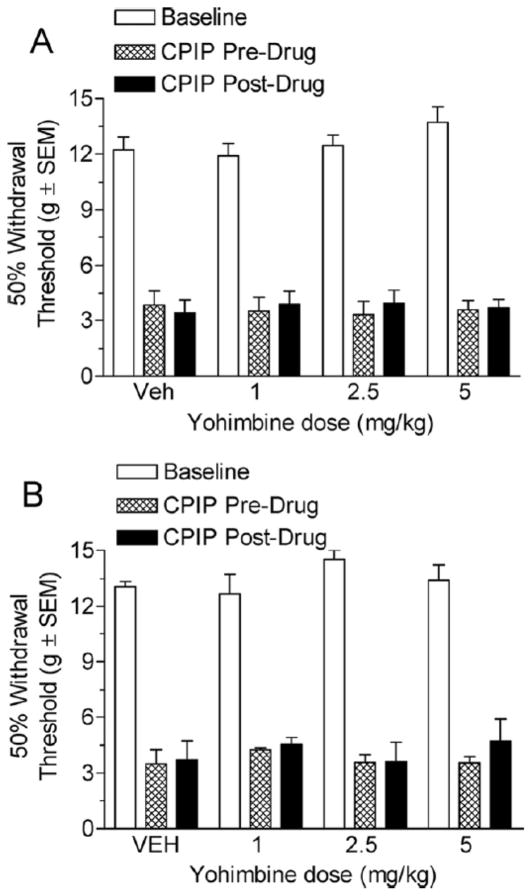
Fig 4A shows the von Frey thresholds of 2-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 2.5, and 5 mg/kg yohimbine. Two-way ANOVA shows non-significant main effects of time (pre-post) (F1, 47) = 0.61, P >.05) and dose (F3, 47 ) =0.01, P >.05) as well as a Non-significant time X dose interaction (F3, 47 ) =1.02, P >.05). This suggests that none of these doses of yohimbine are able to relieve mechanical allodynia at 2 days after reperfusion. Fig 4B shows the von Frey thresholds of 7-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 2.5, and 5 mg/kg yohimbine. Two-way ANOVA shows non-significant main effects of time (prepost) (F1, 47) = 0.56, P > .05) and dose (F3, 47) = 0.66, P > .05) as well as a non-significant time X dose interaction (F3, 47) =0.19, P >.05). Therefore, it appears that yohimbine is unable to reduce mechanical allodynia in CPIP rats either at 2 days or 7 days after reperfusion.
Figure 4.
A, Effect of systemic yohimbine in chronic postischemia pain (CPIP) rats at 2 days after reperfusion. CPIP rats did not display a significant reduction in mechanical allodynia with either the 1, 2.5, or 5 mg/kg yohimbine dose (n=6 for each group). B, Effect of systemic yohimbine in CPIP rats at 7 days after reperfusion. CPIP rats did not display a significant reduction in mechanical allodynia with either 1, 2.5, or 5 mg/kg yohimbine dose at 7 days after reperfusion (n=6 for each group).
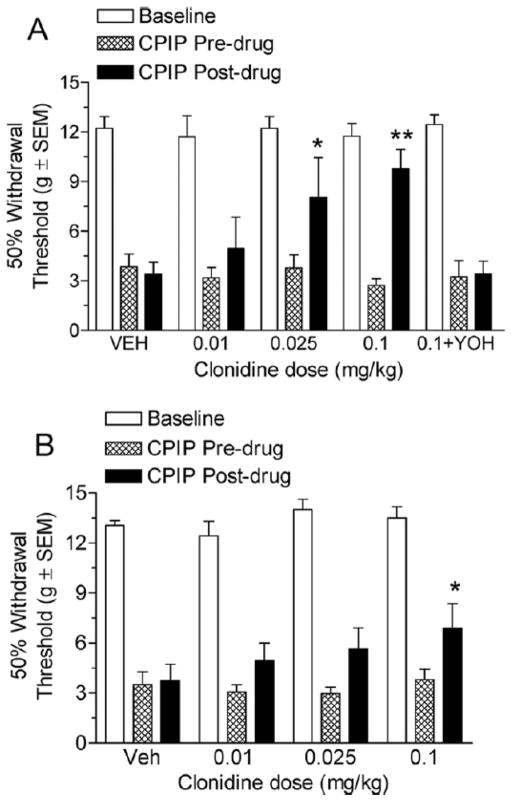
Effect of the α2-Adrenergic Agonist Clonidine on CPIP Mechanical Allodynia
Fig 5A shows the von Frey thresholds of 2-day CPIP rats before and 20 minutes after treatment with vehicle or 0.01, 0.025, and 0.1 mg/kg clonidine, as well as pretreatment with a 5 mg/kg yohimbine dose before a 0.1 mg/kg clonidine injection. Two-way ANOVA reveals a significant main effect of time (pre-post) (F1, 59) = 12.34, P < .01), a non-significant main effect of dose (F4, 59) = 2.39, P > .05), and a significant time X dose interaction (F4, 59) = 3.55, P < .05). Neither the vehicle injection nor the 0.01 mg/kg clonidine dose resulted in a significant increase in paw withdrawal threshold (P > .05). Both the 0.025 mg/kg and the 0.1 mg/kg clonidine doses resulted in significant increases in paw withdrawal thresholds (P < .05 and P < .01, respectively). Further, pretreatment with yohimbine fully inhibited any increase in paw withdrawal threshold observed with the highest dose of clonidine (P > .05), confirming that the effects are α2-adrenergic receptor mediated. Fig 5B shows the von Frey thresholds of 7-day CPIP rats before and 20 minutes after treatment with vehicle or 0.01, 0.025, and 0.1 mg/kg clonidine. Two-way ANOVA shows a significant main effect of time (pre-post) (F1, 47) = 9.34, P < .01), a non-significant main effect of dose (F3, 47) = 1.1, P > .05), and a non-significant time X dose interaction (F3, 47) = 0.97, P > .05). Neither the vehicle injection, the 0.01 mg/kg, nor the 0.025 mg/kg clonidine doses resulted in significant increases in paw withdrawal threshold (P > .05). The time effect was significant, however, since the 0.1 mg/kg clonidine dose significantly increased in paw withdrawal thresholds after the drug as compared with before the drug (P < .05). Therefore, it appears that clonidine is able to significantly reduce mechanical allodynia at 2 days, and lesser so at 7 days, after reperfusion.
Figure 5.
A, Effect of systemic clonidine in chronic postischemia pain (CPIP) rats at 2 days after reperfusion. CPIP rats display significant reductions in mechanical allodynia with the 0.025 and 0.1 mg/kg clonidine doses but not with the 0.01 mg/kg dose. The effect of the highest dose of clonidine was reversed by yohimbine (*P < .05 and **P < .01, as compared with CPIP before drug; n= 6 for each group). B, Effect of systemic clonidine in CPIP rats at 7 days after reperfusion. CPIP rats display a significant reduction in mechanical allodynia with a 0.1 mg/kg clonidine dose but not with the 0.01 or 0.025 mg/kg dose at 7 days after reperfusion. (*P < .05 as compared with CPIP before drug; n=6 for each group).
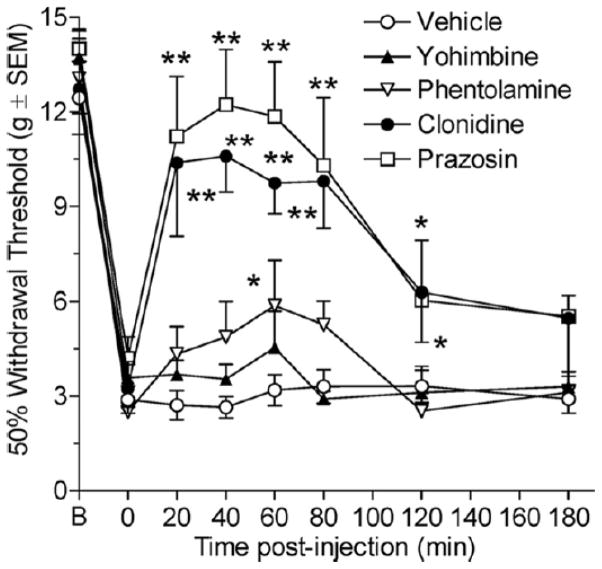
Time Course of Anti-allodynia of Adrenergic Agents on CPIP
Fig 6 shows an anti-allodynia time course of the highest dose of the above drugs (except guanethidine) in 2-day CPIP rats at 20, 40, 60, 80, 120, and 180 minutes after drug injection. Two-way ANOVA revealed significant main effects of group (F4, 209) = 14.40, P < .0001) and time (F6, 209) = 3.29, P < .0001) as well as a significant group X time interaction (F24, 209) = 2.92, P < .0001). None of the post-injection paw withdrawal thresholds in the vehicle group are significantly different from the pre-injection paw withdrawal threshold. For phentolamine, paw withdrawal thresholds are significantly increased compared with vehicle at the 60-minute time point (P < .05). For prazosin, paw withdrawal thresholds are significantly increased compared with vehicle at the 20-, 40-, 60-, 80- (P < .01), and 120-minute (P < .05) time points. For yohimbine, paw-withdrawal thresholds fail to differ significantly from vehicle at any time point. Finally, for clonidine, paw withdrawal thresholds are significantly increased compared with vehicle at the 20-, 40-, 60-, 80- (P < .01), and 120-minute (P < .05) time points. Therefore, phentolamine, prazosin, and clonidine, but not yohimbine, are able to relieve mechanical allodynia with peak anti-allodynia observed approximately 60 minutes after injection.
Figure 6.
Three-hour time course of anti-allodynic effects produced by systemic phentolamine, prazosin, yohimbine, and clonidine in 2-day chronic postischemia pain (CPIP) rats. Phentolamine (5 mg/kg) significantly reduced mechanical allodynia at 60 minutes after injection. Prazosin (5 mg/kg) significantly reduced mechanical allodynia at 20, 40, 60, 80, and 120 minutes after injection. Yohimbine (5 mg/kg) did not significantly reduce mechanical allodynia at any time measured. Clonidine (0.1 mg/kg) significantly reduced mechanical allodynia at 20, 40, 60, 80, and 120 minutes after injection (* P <.05 and ** P <.01, as compared with corresponding vehicle time point; n=6 for each group).
Effect of a Nitric Oxide Donor Vasodilator (SIN-1) on CPIP Mechanical Allodynia
Fig 7A shows the von Frey thresholds of 2-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 3, and 10 mg/kg of SIN-1. Two-way ANOVA revealed a significant main effect of time (F1, 53) = 24.02, P < .0001), a non-significant main effect of dose (F3, 53) = 2.54, P > 05), and a significant time X dose interaction (F3, 53) = 4.87, P < .01). The vehicle injection did not result in a significant increase in paw withdrawal threshold (P > .05). All 3 doses, 1, 2.5 (P < .05), and 10 mg/kg (P < .01), resulted in significant increases in paw withdrawal thresholds. Fig 7B shows the von Frey thresholds of 7-day CPIP rats before and 20 minutes after treatment with vehicle or 1, 3, and 10 mg/kg of SIN-1. Two-way ANOVA revealed non-significant main effects of time (F1, 47) = 2.75, P > 0.05) and dose (F3, 47) = 1.05, P > .05) as well as a non-significant time X dose interaction (F3, 47) = 2.89, P > .05). Therefore, SIN-1 is able to reduce mechanical allodynia at 2 days but not at 7 days after reperfusion in CPIP rats.
Figure 7.
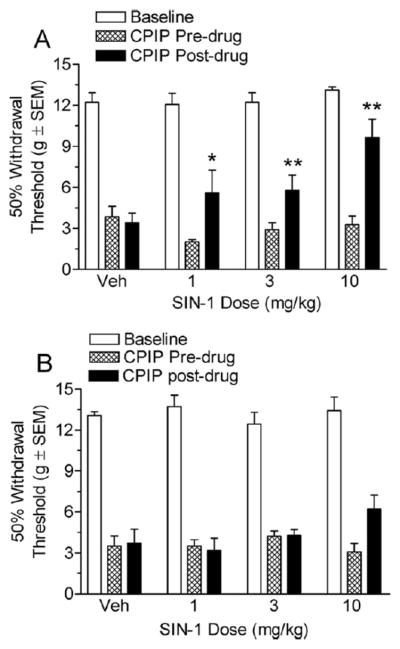
A, Effect of systemic SIN-1 in chronic post-ischemia pain (CPIP) rats at 2 days after reperfusion. CPIP rats display significant reductions in mechanical allodynia with either 1, 3, or 10 mg/kg doses (*P < .05 and **P < .01, as compared with CPIP before drug; n= 7 for each group). B, Effect of systemic SIN-1 in CPIP rats at 7 days after reperfusion. CPIP rats did not display significant reductions in mechanical allodynia with either 1, 3, or 10 mg/kg of SIN-1 at 7 days after reperfusion (n=6 for each group).
Discussion
A contribution of the sympathetic system to pain mechanisms has been hypothesized in a variety of animal models of nerve injury and inflammation. Clinically, it has been suggested that it could be useful to consider subtypes of CRPS-I classified as SMP and sympathetically mindependent pain (SIP).9,56 However, the effectiveness of sympathectomy and sympathetic blockade as standardized treatment for CRPS has been questioned.35,42 In animal models, sympathectomy has been shown to partially reduce mechanical allodynia after chronic constriction injury, sciatic nerve ligation, and spinal nerve ligation.32,33,43,65 In CPIP rats, we found that sympathetic block with guanethidine partially reduced mechanical allodynia for up to 14 days when administered on days 2 and 3 after reperfusion; however, this effect was not significant when guanethidine was administered on days 7 and 8 after reperfusion. This is consistent with the clinical literature in which sympathetic blocks or sympathectomy may only be partially effective for the overall CRPS population, but also that they are particularly more effective early in the disease.8,66
Systemic administration of the nonspecific α-adrenergic antagonist phentolamine also partially reduces mechanical allodynia in CPIP rats at 2 days after reperfusion but, again, not significantly at 7 days after reperfusion. Along with sympathetic blocks, the response to phentolamine has been used clinically to identify SMP.52 Our results also resemble those in studies using rat spinal nerve ligation, in which both sympathectomy and systemic phentolamine reduce pain behaviors,33,79 but this has not been consistently demonstrated.54,55 It has been suggested that for CRPS patients, the response to phentolamine predicts response to sympathetic blocks76 and regional guanethidine.3 CPIP rats are similar to CRPS patients because their allodynia is relieved both by phentolamine and guanethidine. Phenoxybenzamine, another nonselective α1-antagonist, is also effective in CRPS, 48 although we did not study it here.
We found that the α1-adrenergic antagonist prazosin, but not the α2-antagonist yohimbine, effectively reduces mechanical allodynia, with almost a complete reversal for the 5 mg/kg prazosin dose in 2-day CPIP rats. This strongly suggests that the α1-receptor contributes to the mechanical allodynia in CPIP. Lee et al41 found that α1-adrenergic antagonists, but not α2-adrenergic antagonists, also reduce mechanical allodynia in rats with segmental spinal injury. Phentolamine and α1- but not α2-adrenergic antagonists also reduce cold allodynia in rats with S1/S2 spinal nerve ligation.34 Heat hyperalgesia in CCI rats is also reduced by α1-adrenergic antagonists.26 Therefore, it appears that the CPIP model of CRPS -I displays similarities to animal models of CRPS-II, with each showing characteristics of SMP, and a critical role for α1-receptors in SMP. As for the clinical use of these agents in CRPS, there are no carefully controlled trials with selective α1-or α2-adrenergic antagonists.57
The finding that yohimbine did not produce anti-allodynia in our model and has even been found to exacerbate cold allodynia in rats with spinal nerve ligation34 suggests that antagonists of α2-adrenergic receptors may have detrimental effects rather than beneficial ones in these models. Since many α2-adrenergic receptors are autoreceptors mediating negative feedback on NE release from sympathetic postganglionic neurons (SPGNs),28,60 α2-antagonists are known to enhance SPGN NE release.39 The enhanced NE release would increase vasoconstriction, which we expect would be detrimental in CPIP rats, which already have poor blood flow.38 In a similar fashion, the action of phentolamine as an α2-adrenergic antagonist may also be reducing its antiallodynic actions at the α1-adrenergic receptor, providing a possible explanation for why phentolamine is less effective than prazosin in CPIP rats.
Like prazosin, systemic clonidine is particularly effective in CPIP rats at doses that do not produce defects on the rotorod. Studies have shown that systemic clonidinecan produce analgesic effects in humans23 and topical administration relieves SMP in CRPS patients.15 Systemic clonidine also partially relieves mechanical allodynia in rodents with CCI of the sciatic nerve,30 and topical application has also been shown to be antinociceptive in animal studies.19 It is tempting to speculate that the analgesic effects of clonidine in CPIP rats are based on its known negative feedback effects on NE release from SPGNs.28,60 The α2-agonist clonidine may also be particularly effective due to a combination of spinal analgesic and local vasodilatory effects.20,25 We have previously shown that the I-R injury associated with CPIP produces persistent tissue ischemia, indicated by reduced muscle perfusion,38 as well as vasoconstrictor hyper-responsiveness, indicated by an enhanced reduction in hind paw blood flow after close arterial injection of NE.78 The results here are consistent with a vasoactive role for NE from sympathetic efferents, in which activity at α1-receptors is pronociceptive due to vasoconstriction in already ischemic tissue. Blocking the α1-receptor with prazosin, therefore, results in vasodilatation and pain relief.
In the CPIP rats, we hypothesize that the I-R injury results in persistent tissue ischemia that contributes to the pain. Some of the known changes of I-R injury include vascular abnormalities such as persistent ischemia, dependent on either no-reflow due to capillary clogging,6 or arterial vasospasms due to sympathetic vasoconstrictor hyper-responsiveness and/or endothelial cell dysfunction. Indeed, after I-R injury there is both an upregulation and hyper-responsiveness of vascular adrenoceptors61 as well as a reduced production and vasodilatory function of nitric oxide.31,58
If persistent tissue ischemia contributes to CPIP pain,38 then CPIP pain should also be relieved by agents that enhance vasodilatation. In this study, we found that allodynia in CPIP rats was dose-dependently reduced by systemic administration of the nitric oxide donor SIN-1. We propose that by increasing the levels of nitric oxide, SIN-1 induces vasodilatation that relieves pain-producing vasospasms and ischemia in CPIP rats. Indeed, it has been shown that blood flow can be improved in ischemic tissue with exogenous NO.46,73 Further, our results show that anti-allodynic doses of SIN-1, as well as another NO donor (sodium nitroprusside), also attenuate NE-evoked pain in CPIP rats.78 Therefore, it seems likely that NO-mediated vasodilation can reduce persistent tissue ischemia, and this may be a useful treatment for SMP.
In the CPIP rats, we find that antisympathetic and vasodilatory drugs are more effective at 2 days after reperfusion rather than 7 days after reperfusion. The temporal reduction in effectiveness may depend partly on a shift in the reliance of persistent ischemia on arterial vasospasms to no-reflow, a phenomenon that develops quickly after prolonged ischemia or repeated I-R injury.2,38,49 In CRPS-I patients, a slower development of no-reflow may also result in chronic tissue ischemia and pain that is more resistant to relief by sympathetic blockers.
In conclusion, CPIP rats show evidence of SMP (as defined clinically), since their mechanical allodynia is relieved by both sympathetic block and systemic phentolamine treatment. Furthermore, the responses to prazosin, clonidine, and the nitric oxide donor SIN-1 demonstrates that pain relief in CPIP rats can be produced by agents that decrease sympathetic vasoconstriction or enhance vasodilatation. We conclude that SMP mechanisms may involve exaggerated sympathetically mediated vascular contractility and persistent tissue ischemia.
Perspective.
The results of this study indicate that sympathetic block, or administration of α1-adrenergic antagonists, clonidine, or a nitric oxide donor, relieve allodynia in an animal model of CRPS-I. Thus, the pain of CRPS-I may depend on enhanced vasoconstrictor responsiveness, which may be relieved by blocking sympathetic efferent-dependent vasoconstriction, or by enhancing nitric oxide–dependent vasodilatation.
Acknowledgments
Supported by grants from CIHR, NSERC, FRSQ, and the Louise-Edwards Foundation to T.J.C. D.N.X. is supported by a NSERC doctoral fellowship. T.J.C. has received research and travel support from Pfizer Corporation and is a member of the Scientific Advisory Board of Pain Ceptor Pharma.
References
- 1.Ackerman WE, Munir MA, Zhang J-M. Assessment of laser Doppler imaging for the diagnosis of complex regional pain syndrome I. J Neuro Symp Pal. 2005;1:13–20. [Google Scholar]
- 2.Allen DM, Chen LE, Seaber AV, Urbaniak JR. Pathophysiology and related studies of the no reflow phenomenon in skeletal muscle. Clin Orthop Relat Res. 1995;314:122–133. [PubMed] [Google Scholar]
- 3.Arnér S. Intravenous phentolamine test: Diagnostic and prognostic use in reflex sympathetic dystrophy. Pain. 1991;46:17–22. doi: 10.1016/0304-3959(91)90028-V. [DOI] [PubMed] [Google Scholar]
- 4.Arnold JM, Teasell RW, MacLeod AP, Brown JE, Carruthers SG. Increased venous alpha-adrenoceptor responsiveness in patients with reflex sympathetic dystrophy. Ann Intern Med. 1993;118:619–621. doi: 10.7326/0003-4819-118-8-199304150-00008. [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Maier C. Reflex sympathetic dystrophy: Skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain. 1996;67:317–326. doi: 10.1016/0304-3959(96)03136-3. [DOI] [PubMed] [Google Scholar]
- 6.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 7.Birder LA, Perl ER. Expression of alpha2-adrenergic receptors in rat primary afferent neurones after peripheralnerve injury or inflammation. J Physiol. 1999;515:533–542. doi: 10.1111/j.1469-7793.1999.533ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonica JJ. Causalgia and other reflex sympathetic dystrophies. In: Bonica JJ, editor. The Management of Pain. Philadelphia, PA: Lea & Febiger; 1990. pp. 220–243. [Google Scholar]
- 9.Campbell JN, Raja SN, Meyer RA. Painful sequelae of nerve injury. In: Dubner R, Gebhart GF, Bond MR, editors. Proceedings of the Vth World Congress on Pain. Amsterdam, The Netherlands, Elsevier. 1988. pp. 135–143. [Google Scholar]
- 10.Cepeda MS, Lau J, Carr DB. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: A narrative and systematic review. Clin J Pain. 2002;18:216–233. doi: 10.1097/00002508-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Michaelis M, Jänig W, Devor M. Adrenoreceptor subtype mediating sympathetic-sensory coupling in injured sensory neurons. J Neurophysiol. 1996;76:3721–3730. doi: 10.1152/jn.1996.76.6.3721. [DOI] [PubMed] [Google Scholar]
- 13.Cho HJ, Kim DS, Lee NH, Kim JK, Lee KM, Han KS, Kang YN, Kim KJ. Changes in the alpha2-adrenergicd receptor subtypes gene expression in rat dorsal root ganglion in an animal model of neuropathic pain. Neuro Report. 1997;8:3119–3122. doi: 10.1097/00001756-199709290-00022. [DOI] [PubMed] [Google Scholar]
- 14.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): A novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Davis KD, Treede RD, Raja SN, Meyer RA, Campbell JN. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–317. doi: 10.1016/0304-3959(91)90221-I. [DOI] [PubMed] [Google Scholar]
- 16.De Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: A population-based study. Pain. 2007;129:12–20. doi: 10.1016/j.pain.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Devor M, Jänig W. Activation of myelinated afferents ending in a neuroma by stimulation of the sympathetic supply in the rat. Neurosci Lett. 1981;24:43–47. doi: 10.1016/0304-3940(81)90356-6. [DOI] [PubMed] [Google Scholar]
- 18.Devor M, Jänig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation innerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Dogrul A, Uzbay IT. Topical clonidine antinociception. Pain. 2004;111:385–391. doi: 10.1016/j.pain.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Figueroa XF, Poblete MI, Boric MP, Mendizabal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br J Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gradl G, Finke B, Schattner S, Gierer P, Mittlmeier T, Vollmar B. Continuous intra-arterial application of substance P induces signs and symptoms of experimental complex regional pain syndrome (CRPS) such as edema, inflammation and mechanical pain but no thermal pain. Neuroscience. 2007;148:757–765. doi: 10.1016/j.neuroscience.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Guo TZ, Wei T, Kingery WS. Glucocorticoid inhibition of vascular abnormalities in a tibia fracture rat model of complex regional pain syndrome type I. Pain. 2006;121:158–167. doi: 10.1016/j.pain.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Hall JE, Uhrich TD, Ebert TJ. Sedative, analgesic and cognitive effects of clonidine infusions in humans. Br J Anaesth. 2001;86:5–11. doi: 10.1093/bja/86.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Han DW, Kweon TD, Kim KJ, Lee JS, Chang CH, Lee YW. Does the tibial and sural nerve transection model represent sympathetically independent pain? Yonsei Med J. 2006;47:847–851. doi: 10.3349/ymj.2006.47.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermann D, Schlereth T, Vogt T, Birklein F. Clonidine induces nitric oxide-and prostaglandin-mediated vasodilation in healthy human skin. J Appl Physiol. 2005;99:2266–2270. doi: 10.1152/japplphysiol.00271.2005. [DOI] [PubMed] [Google Scholar]
- 26.Hord AH, Denson DD, Stowe B, Haygood RM. Alpha-1 and alpha-2 Adrenergic antagonists relieve thermal hyperalgesia in experimental mononeuropathy from chronic constriction injury. Anesth Analg. 2001;92:1558–1562. doi: 10.1097/00000539-200106000-00042. [DOI] [PubMed] [Google Scholar]
- 27.Jänig W, Baron R. Complex regional pain syndrome: Mystery explained? Lancet Neurol. 2003;2:687–697. doi: 10.1016/s1474-4422(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 28.Kahan T. Prejunctional adrenergic receptors and sympathetic neurotransmission: Studies in canine skeletal muscle vasculature in situ. Acta Physiol Scand Suppl. 1987;560:1–38. [PubMed] [Google Scholar]
- 29.Kaplan R, Claudio M, Kepes E, Gu XF. Intravenous guanethidine in patients with reflex sympathetic dystrophy. Acta Anaesthesiol Scand. 1996;40:1216–1222. doi: 10.1111/j.1399-6576.1996.tb05553.x. [DOI] [PubMed] [Google Scholar]
- 30.Kayser V, Desmeules J, Guilbaud G. Systemic clonidine differentially modulates abnormal reactions to mechanical and thermal stimuli in rats with peripheral mononeuropathy. Pain. 1995;60:275–285. doi: 10.1016/0304-3959(94)00125-x. [DOI] [PubMed] [Google Scholar]
- 31.Khanna A, Cowled PA, Fitridge RA. Nitric oxide and skeletal muscle reperfusion injury: Current controversies (research review) J Surg Res. 2005;128:98–107. doi: 10.1016/j.jss.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 32.Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- 34.Kim SK, Min B-I, Kim JH, Hwang BG, Yoo GY, Park DS, Na HS. Effects of α1- and α2-adrenoreceptor antagonists on cold allodynia in a rat tail model of neuropathic pain. Brain Res. 2005;1039:207–210. doi: 10.1016/j.brainres.2005.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Kingery WS. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–139. doi: 10.1016/S0304-3959(97)00049-3. [DOI] [PubMed] [Google Scholar]
- 36.Kurvers H, Daemen M, Slaaf D, Stassen F, van den Wildenberg F, Kitslaar P, de Mey J. Partial peripheral neuropathy and denervation induced adrenoceptor supersensitivity: Functional studies in an experimental model. Acta Orthop Belg. 1998;64:64–70. [PubMed] [Google Scholar]
- 37.Kurvers HA, Tangeider GJ, De Mey JG, Slaaf DW, Beuk RJ, van den Wildenberg FA, Kitslaar PJ, Reneman RS, Jacobs MJ. Skin blood flow abnormalities in rat model of neuropathic pain: Result of decreases sympathetic vasoconstrictor outflow? J Auton Nerv Syst. 1997;63:19–29. doi: 10.1016/s0165-1838(96)00127-0. [DOI] [PubMed] [Google Scholar]
- 38.Laferrière A, Millecamps M, Xanthos DN, Xiao W, Bennett GJ, Coderre TJ. Chronic post-ischemia pain: A novel animal model suggests that ischemia-reperfusion (I-R) in jury, no-reflow and chronic tissue ischemia contribute to CRPS-I. Eur J Pain. 2007;11:S61–S62. [Google Scholar]
- 39.Langer SZ. Presynaptic regulation of the release of catecholamines. Pharmacol Rev. 1980;32:337–362. [PubMed] [Google Scholar]
- 40.Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Exp Brain Res. 1998;120:432–438. doi: 10.1007/s002210050416. [DOI] [PubMed] [Google Scholar]
- 41.Lee DH, Liu X, Kim HT, Chung K, Chung JM. Receptor subtype mediating the adrenergic sensitivity of pain behavior and ectopic discharges in neuropathic Lewis rats. J Neurophysiol. 1999;81:2226–2233. doi: 10.1152/jn.1999.81.5.2226. [DOI] [PubMed] [Google Scholar]
- 42.Mailis A, Furlan A. Sympathectomy for neuropathic pain. Cochrane Database Syst Rev. 2003;2:CD002918. doi: 10.1002/14651858.CD002918. [DOI] [PubMed] [Google Scholar]
- 43.Malmberg AB, Basbaum AI. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: Behavioral and neuroanatomical correlates. Pain. 1998;76:215–222. doi: 10.1016/s0304-3959(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 44.Martin LJ, Persinger MA. Thermal analgesia induced by 30-min exposure to 1 microT burst-firing magnetic fields is strongly enhanced in a dose-dependent manner by the alpha2 agonist clonidine in rats. Neurosci Lett. 2004;366:226–229. doi: 10.1016/j.neulet.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 45.Maxwell RA, Plummer AJ, Schneider F, Povalski H, Daniel AI. Pharmacology of [2-(octahydro-1-azocinyl)- ethyl]-guanidine sulfate (Su-5864) J Pharmacol Exp Ther. 1960;128:22–29. [PubMed] [Google Scholar]
- 46.Meldrum DG, Stephenson LL, Zamboni WA. Effects of L-NAME and L-arginine on ischemia-reperfusion injury in rat skeletal muscle. Plast Reconstr Surg. 1999;103:935–940. doi: 10.1097/00006534-199903000-00025. [DOI] [PubMed] [Google Scholar]
- 47.Michaelis M, Devor M, Jänig W. Sympathetic modulation of activity in rat dorsal root ganglion neurons changes over time following peripheral nerve injury. J Neurophysiol. 1996;76:753–763. doi: 10.1152/jn.1996.76.2.753. [DOI] [PubMed] [Google Scholar]
- 48.Muizelaar JP, Klever M, Hertogs IA, DeLange DC. Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia): Management with the calcium channel blocker nifedipine and/or the alpha-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg. 1997;99:26–30. doi: 10.1016/s0303-8467(96)00594-x. [DOI] [PubMed] [Google Scholar]
- 49.Nanobashvili J, Neumayer C, Fuegl A, Blumer R, Prager M, Sporn E, Polterauer P, Malinski T, Huk I. Development of ‘no-reflow’ phenomenon in ischemia/reperfusion injury: Failure of active vasomotility and not simply passive vasoconstriction. Eur Surg Res. 2003;35:417–424. doi: 10.1159/000072226. [DOI] [PubMed] [Google Scholar]
- 50.O’Halloran KD, Perl ER. Effects of partial nerve injury on the responses of C-fiber polymodal nociceptors to adrenergic agonists. Brain Res. 1997;759:233–240. doi: 10.1016/s0006-8993(97)00261-8. [DOI] [PubMed] [Google Scholar]
- 51.Park SK, Chung K, Chung JM. Effects of purinergic and adrenergic antagonists in a rat model of painful peripheral neuropathy. Pain. 2000;87:171–179. doi: 10.1016/S0304-3959(00)00277-3. [DOI] [PubMed] [Google Scholar]
- 52.Raja SN, Treede RD, Davis KD, Campbell JN. Systemic alpha-adrenergic blockade with phentolamine: A diagnostic test for sympathetically maintained pain. Anesthesiology. 1991;74:691–698. doi: 10.1097/00000542-199104000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–244. doi: 10.1016/s0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- 54.Ringkamp M, Eschenfelder S, Grethel EJ, Häbler HJ, Meyer RA, Jänig W, Raja SN. Lumbar sympathectomy failed to reverse mechanical allodynia- and hyperalgesia-like behavior in rats with L5 spinal nerve injury. Pain. 1999;79:143–153. doi: 10.1016/s0304-3959(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 55.Ringkamp M, Grethel EJ, Choi Y, Meyer RA, Raja SN. Mechanical hyperalgesia after spinal nerve ligation in rat is not reversed by intraplantar or systemic administration of adrenergic antagonists. Pain. 1999;79:135–141. doi: 10.1016/s0304-3959(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 56.Roberts W. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- 57.Rowbotham MC. Pharmacologic management of complex regional pain syndrome. Clin J Pain. 2006;22:425–429. doi: 10.1097/01.ajp.0000194281.74379.01. [DOI] [PubMed] [Google Scholar]
- 58.Rubin BB, Romaschin A, Walker PM, Gute DC, Korthuis RJ. Mechanisms of postischemic injury in skeletal muscle: Intervention strategies. J Appl Physiol. 1996;80:369–387. doi: 10.1152/jappl.1996.80.2.369. [DOI] [PubMed] [Google Scholar]
- 59.Sato J, Perl ER. Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science. 1991;251:1608–1610. doi: 10.1126/science.2011742. [DOI] [PubMed] [Google Scholar]
- 60.Saelens DA, Williams PB. Evidence for prejunctional alpha- and beta-adrenoceptors in the canine saphenous vein: Influence of frequency of stimulation and external calcium concentration. J Cardiovasc Pharmacol. 1983;5:598–603. doi: 10.1097/00005344-198307000-00014. [DOI] [PubMed] [Google Scholar]
- 61.Sapienza P, Edwards JD, Mingoli JS, McGregor PE, Cavallari N, Agrawal DK. Ischemia-induced peripheral arterial, vasospasm: Role of alpha 1 -and alpha 2-adrenoceptors. J Surg Res. 1996;62:192–196. doi: 10.1006/jsre.1996.0194. [DOI] [PubMed] [Google Scholar]
- 62.Schürmann M, Gradl G, Wizgal I, Tutic M, Moser C, Azad S, Beyer A. Clinical and physiologic evaluation of stellate ganglion blockade for complex regional pain syndrome type I. Clin J Pain. 2001;17:94–100. doi: 10.1097/00002508-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Semmes J, Weinstein S, Ghent L, Teuber H-L. Somatosensory Changes After Penetrating Brain Wounds in Man. Cambridge, MA: Harvard University Press; 1960. [Google Scholar]
- 64.Shannon HE, Lutz EA. Effects of the I(1) imidazoline/alpha(2)-adrenergic receptor agonist moxonidine in comparison with clonidine in the formalin test in rats. Pain. 2000;85:161–167. doi: 10.1016/s0304-3959(99)00260-2. [DOI] [PubMed] [Google Scholar]
- 65.Shir Y, Seltzer Z. Effects of sympathectomy in a model of causalgiform pain produced by partial sciatic nerve injury in rats. Pain. 1991;45:309–320. doi: 10.1016/0304-3959(91)90056-4. [DOI] [PubMed] [Google Scholar]
- 66.Singh B, Moodley J, Shaik AS, Robbs JV. Sympathectomy for complex regional pain syndrome. J Vasc Surg. 2003;37:508–511. doi: 10.1067/mva.2003.78. [DOI] [PubMed] [Google Scholar]
- 67.Sluka KA, Chandran P. Enhanced reduction in hyperalgesia by combined administration of clonidine and TENS. Pain. 2002;100:183–190. doi: 10.1016/s0304-3959(02)00294-4. [DOI] [PubMed] [Google Scholar]
- 68.Stanton-Hicks M. Complex regional pain syndrome. Anesth Clin North Am. 2003;21:733–744. doi: 10.1016/s0889-8537(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 69.Torebjörk E, Wahren L, Wallin G, Hallin R, Koltzenburg M. Noradrenaline-evoked pain in neuralgia. Pain. 1995;63:11–20. doi: 10.1016/0304-3959(95)00140-N. [DOI] [PubMed] [Google Scholar]
- 70.Treede RD, Davis KD, Campbell JN, Raja SN. The plasticity of cutaneous hyperalgesia during sympathetic ganglion blockade in patients with neuropathic pain. Brain. 1992;115:607–621. doi: 10.1093/brain/115.2.607. [DOI] [PubMed] [Google Scholar]
- 71.Van der Laan L, Oven WJ, Verhofstad AA, Tan EC, ter Laak HJ, Gabreels-Festen A, Hendriks T, Goris RJ. Soft tissue repair capacity after oxygen-derived free radical-induced damage in one hindlimb of the rat. J Surg Res. 1997;72:60–69. doi: 10.1006/jsre.1997.5167. [DOI] [PubMed] [Google Scholar]
- 72.Wall PD, Gutnick M. Ongoing activity in peripheral nerves: The physiology and pharmacology of impulses originating from a neuroma. Exp Neurol. 1974;43:580–593. doi: 10.1016/0014-4886(74)90197-6. [DOI] [PubMed] [Google Scholar]
- 73.Wang WZ, Anderson G, Fleming JT, Peter FW, Franken RJ, Acland RD, Barker J. Lack of nitric oxide contributes to vasospasm during ischemia/reperfusion injury. Plast Reconstr Surg. 1997;99:1099–1108. doi: 10.1097/00006534-199704000-00028. [DOI] [PubMed] [Google Scholar]
- 74.Wasner G, Backonja MM, Baron R. Traumatic neuralgias: complex regional pain syndromes (reflex sympathetic dystrophy and causalgia): Clinical characteristics, pathophysiological mechanisms and therapy. Neurol Clin. 1998;16:851–868. doi: 10.1016/s0733-8619(05)70101-8. [DOI] [PubMed] [Google Scholar]
- 75.Wasner G, Schattschneider J, Heckmann K, Maier C, Baron R. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): Mechanisms and diagnostic value. Brain. 2001;124:587–599. doi: 10.1093/brain/124.3.587. [DOI] [PubMed] [Google Scholar]
- 76.Wehnert Y, Muller B, Larsen B, Kohn D. Sympathetically maintained pain (SMP): Phentolamine test vs sympathetic nerve blockade: Comparison of two diagnostic methods. Orthopade. 2002;31:1076–1083. doi: 10.1007/s00132-002-0361-6. [DOI] [PubMed] [Google Scholar]
- 77.Willenbring S, Beauprie IG, DeLeo JA. Sciatic cryoneurolysis in rats: A model of sympathetically independent pain, I: Effects of sympathectomy. Anesth Analg. 1995;81:544–548. doi: 10.1097/00000539-199509000-00021. [DOI] [PubMed] [Google Scholar]
- 78.Xanthos DN, Bennett GJ, Coderre TJ. Norepinephrine-induced nociception and vasoconstrictor hypersensitivity in rats with chronic post-ischemia pain. Pain. 2007 Dec 11; doi: 10.1016/j.pain.2007.10.031. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie J, Ho Lee Y, Wang C, Mo Chung J, Chung K. Differential expression of alpha1-adrenoceptor subtype mRNAs in the dorsal root ganglion after spinal nerve ligation. Mol Brain Res. 2001;93:164–172. doi: 10.1016/s0169-328x(01)00201-7. [DOI] [PubMed] [Google Scholar]