Abstract
Tissue-engineering techniques have been successful in developing cartilage-like tissues in vitro using cells from animal sources. The successful translation of these strategies to the clinic will likely require cell expansion to achieve sufficient cell numbers. Using a two-dimensional (2D) cell migration assay to first identify the passage at which chondrocytes exhibited their greatest chondrogenic potential, the objective of this study was to determine a more optimal culture medium for developing three-dimensional (3D) cartilage-like tissues using human cells. We evaluated combinations of commonly used growth factors that have been shown to promote chondrogenic growth and development. Human articular chondrocytes (AC) from osteoarthritic (OA) joints were cultured in 3D environments, either in pellets or encapsulated in agarose. The effect of growth factor supplementation was dependent on the environment, such that matrix deposition differed between the two culture systems. ACs in pellet culture were more responsive to bone morphogenetic protein (BMP2) alone or combinations containing BMP2 (i.e. BMP2 with PDGF or FGF). However, engineered cartilage development within agarose was better for constructs cultured with TGFβ3. These results with agarose and pellet culture studies set the stage for the development of conditions appropriate for culturing 3D functional engineered cartilage for eventual use in human therapies.
Keywords: osteoarthritis, chondrocytes, pellet culture, agarose, tissue engineering, articular cartilage
1. Introduction
Adult articular cartilage is an avascular, load-bearing tissue that has limited capacity to heal following injury or degeneration. Osteoarthritis is the leading cause of disability in Americans and is associated with medical costs > ~$130 billion (Kotlarz et al., 2009; Luo et al., 2004). Successful biological repair of osteoarthritic (OA) or injured cartilage may reduce the need for artificial joint replacement by providing long-term pain relief and maintaining the mechanical function of the healthy native tissue. Cultivating engineered tissue in vitro has shown promising results for development of engineered cartilage using animal chondrocytes and stem cells (O’Connell et al., 2012a, 2012b; Pei et al., 2008, 2009; Rodrigues et al., 2011; Sampat et al., 2011). For clinical applications, autologous chondrocytes arguably represent the ideal cell source. Aside from the usual concerns of donor site morbidity, however, developing functional engineered cartilage using cells isolated from diseased (e.g. arthritic) tissue is not trivial (Selmi et al., 2008). The successful translation of engineered cartilage to the clinic will necessitate cell expansion to achieve sufficient cell numbers. To date, the culture conditions required for expanding and cultivating engineered cartilage with human cells that produce native properties remain elusive. Current methods for human cells reported in the literature employ various passage conditions and culture media formulae to support growth of chondrocytes (e.g. (Adesida et al., 2012; Alves da Silva et al., 2011; Bian et al., 2011; de Mara et al., 2013; Ghone and Grayson, 2012; Handorf and Li, 2011; Matsumoto et al., 2012; Mendelson et al., 2011; Park et al., 2011; Perrier et al., 2011; Wu et al., 2012; Yin et al., 2011; Yoo et al., 2011)).
In this study, we first evaluated the effect of passaging on cell migration and movement in two-dimensional (2D) culture to guide an appropriate condition (i.e. passage number) for developing three-dimensional (3D) engineered cartilage. The use of direct current (DC) electric fields (EFs) of strengths in the range 1–10 V/cm is known to induce directed movement (galvanotaxis) and shape change (galvanotropism) in a number of terminally differentiated musculoskeletal cells, including chondrocytes, fibroblasts, osteoblasts, osteoclasts and meniscal fibrochondrocytes (Chao et al., 2000; Ferrier et al., 1986; Gunja et al., 2010, 2012; Sillman et al., 2003). Endogenously generated electric field gradients of this strength have been shown to guide cell migration in developing embryos (Robinson, 1985) and at the cut surfaces of wounds (Soong et al., 1990). Using the well-established bovine model for cartilage research (Hung et al., 2004; O’Connell et al., 2012a, 2012b; Sampat et al., 2011), our laboratory has recently demonstrated that calf synovial-derived stem cells (SDSCs), expanded in the presence of a growth factor cocktail comprised of TGFβ-1, FGF-2 and PDGF-ββ, display passage-dependent cell surface marker expression and migration behaviour in response to applied EFs (Tan et al., 2014). Specifically, late-passage cells (e.g. passages 3 and 4), with sufficient chemical priming, responded to DC EFs with cathodal migration, which is the same direction in which chondrocytes travel (Chao et al., 2000), and were capable of creating cartilaginous-like tissue with elevated amounts of collagen II and GAG deposition (Tan et al., 2014). Importantly, we have also observed similar findings for human MSCs with passage (unpublished). In this manner, we believe that galvanotaxis provides a useful 2D assay for identifying cell populations that exhibit a high propensity to produce cartilage-like tissue when cultured in 3D conditions.
Monolayer and pellet cultures with human chondrocytes have demonstrated that chondrogenesis can be improved by using various growth factors and their combinations. Bone morphogenetic protein-2 (BMP2) has been suggested to increase aggrecan and collagen type II expression and to prevent dedifferentiation of chondrocytes (Luyten et al., 1992; Sailor et al., 1996; Stewart et al., 2000; Theodoropoulos et al., 2011). Previous studies demonstrated beneficial effects of TGFβ on collagen type II production and gene expression (Chiang et al., 2011). Furthermore, combining growth factors, such as TGFβ with BMP2, has shown potential to further enhance aggrecan and collagen gene expression (Mendelson et al., 2011; Perrier et al., 2011). While these studies provide valuable information about the cellular response to chemical stimuli, the culture conditions of these relatively short duration studies (i.e. 7–14 days) that monitor gene expression and matrix production in pellet culture may not be indicative of conditions that will lead to long-term functional cartilage development. A successful biological repair strategy for damaged or injured cartilage will need to exhibit functional tissue properties that can withstand and distribute the high compressive joint loads experienced by the native tissue.
With the growing success in developing functional engineered cartilage in vitro using chondrocytes and stem cells derived from various animal models (Farrell et al., 2013; Huey et al., 2012; Johnstone et al., 2013; Natoli et al., 2009; O’Connell et al., 2012b), there has been a shift in attention toward translating these findings to developing engineered cartilage with human cells (Naumann et al., 2004). Adopting a suitable cell passage from the galvanotaxis response of the OA chondrocytes, as described above, we then cultured human cells to determine whether stimulation with growth factors that have been used to improve cartilage production in human articular chondrocyte pellet or self-aggregating constructs (Bian et al., 2011; Matsumoto et al., 2012; Yin et al., 2011) is translatable to their culture in agarose, a clinically-relevant hydrogel scaffold for cartilage repair strategies (Selmi et al., 2008). While incorporation of agarose hydrogel would affect the nature of cell–cell interactions indicative of cell-only constructs, fewer cells would be needed to generate an engineered tissue of a specified volume. Moreover, the ability to use human cells successfully with the agarose system would permit direct translation of strategies such as applied deformational loading (Bian et al., 2012; Mauck et al., 2002) and osmotic loading, which have been shown to promote in vitro functional cartilage tissue development of non-human mammalian cells encapsulated in agarose (Sampat et al., 2011). Thus, in this study, we examined matrix production and mechanical properties of 3D engineered cartilage in pellet and agarose culture under several different growth factor conditions.
2. Materials and methods
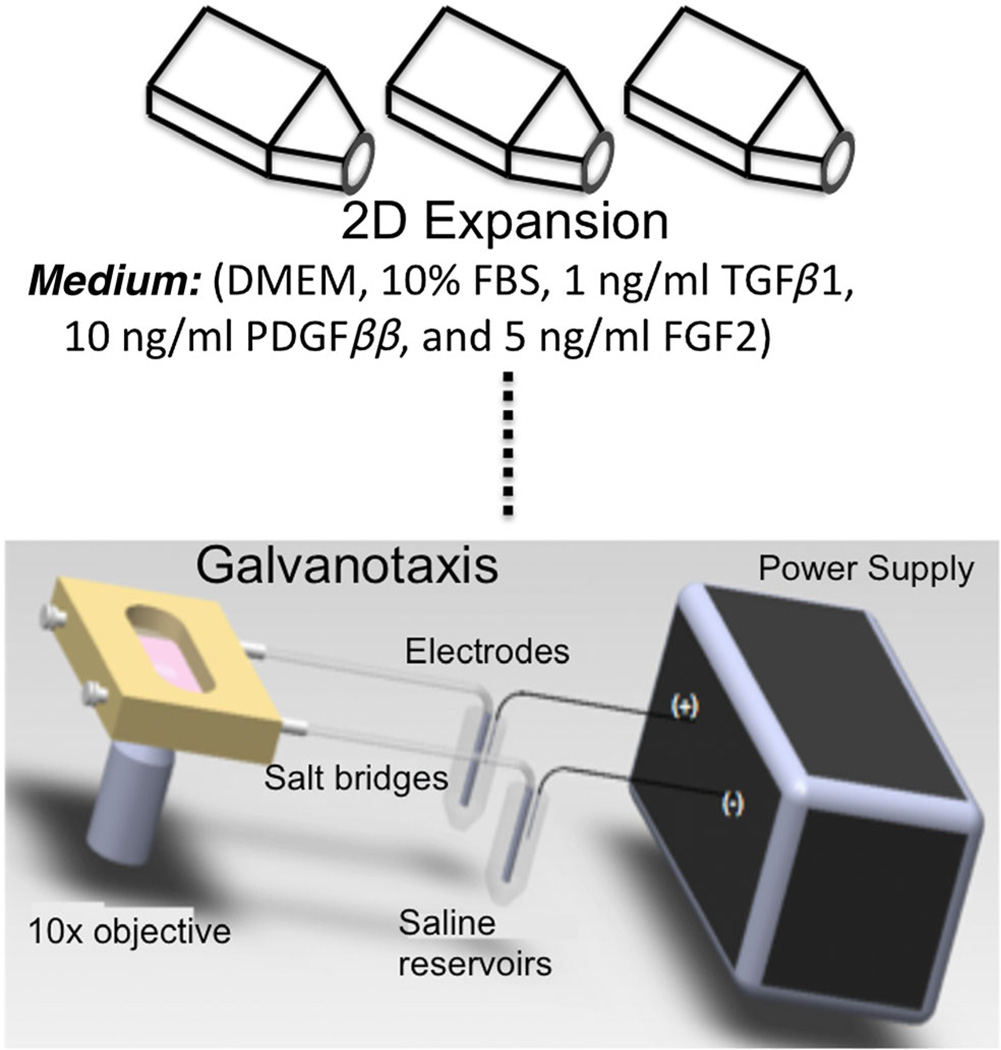
Articular cartilage was acquired according to an IRB-approved protocol from two patients, aged 45 and 51 years, receiving total knee replacement (New York University School of Medicine). Chondrocytes were acquired as described by Attur et al. (2012). Cells were expanded in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% serum, 1% 100 U/ml penicillin, 100 mg/ml streptomycin and amphotericin B (Invitrogen, Carlsbad, CA, USA), 1 ng/ml TGFβ1, 10 ng/ml PDGFββ and 5 ng/ml FGF2. Cells were passaged four times for galvanotaxis studies, and the results from the galvanotaxis study (study 1) informed which passage to use for 3D culture (study 2; Figure 1). The same donor cells were expanded for 3D cultures in pellets and agarose scaffolds.
Figure 1.
Schematic of galvanotaxis studies. Human chondrocytes were cultured in expansion medium containing 1 ng/ml TGFβ1, 10 ng/ml PDGFββ and 5 ng/ml FGF2 to prime the cells for 3D cultures. Galvanotaxis studies were performed for four passages
2.1. Galvanotaxis
At each passage, when confluence had been reached, cells were trypsinized and counted. One subset was replated for further expansion and another subset was resuspended at 50 × 103 cells/ml in DMEM containing 5% serum, amino acids (0.5× minimal essential amino acids, 1× non-essential amino acids), buffers (10 mm HEPES, 10 mm sodium bicarbonate, 10 mm TES, 10 mm BES) and antibiotics (100 U/ml penicillin, 100 mg/ml streptomycin) and allowed to equilibrate for 1 h. Then the cells were plated at 2.65 × 104 cells/cm2 onto sterile glass slides (Fisher Scientific, Pittsburgh, PA, USA), using removable silicone wells, and allowed to attach for 1 h in a 5% CO2 incubator at 37 °C. The slides were rinsed with medium to remove any non-adherent cells and placed into a custom galvanotaxis chamber under aseptic conditions (Figure 1). A modified parallel-plate flow chamber was used, as described previously (Chao et al., 2000). Briefly, two Plexiglas blocks with glass windows were separated by a uniform thickness rectangular Silastic spacer (McMaster-Carr, New Brunswick, NJ, USA) and held together with screws to create a sealed rectangular channel with defined geometry (5 cm length, 1.3 cm width, 250 µm height). A power supply (Keithley Instruments) delivered a current of 3.3 mA (6V/cm electric field strength) through the chamber, and experiments were performed at room temperature for 3 h. Control slides were treated similarly, except that no electric field was applied.
Cell migration patterns were captured using a digital camera connected to EOS Utility (Canon Rebel T3i, Canon, USA). Using a × 10 objective (1 pixel = 0.17 µm), approximately 30–40 cells/slide were observed; images were acquired at 10 min intervals. The incremental change in position for each cell was automatically tracked with a custom MATLAB algorithm. Overall cell migration speed was calculated as the net displacement divided by the 3 h time span. Migration direction was quantified as sin φ, where φ is the angle between the x coordinate axis and the migration vector, such that sin φ was defined as the value −1 when φ = 4.71 rad (270°), the direction of the cathode. The directional velocity, defined as the component of the speed directed toward the negative pole (Chao et al., 2000), was obtained by multiplying the cell’s speed by sin φ. Each data point represented the mean and standard error (SE; 60–80 samples). Two-way ANOVA was performed on cell speed, directed velocity and total displacement for factors of treatment (applied EFs vs control) and passage number. Tukey’s post hoc analysis was performed to assess significance between each treatment and passage group; significance was set at p ≤ 0.05.
To correlate chondrogenic phenotype with EF-induced migration direction, cells from passages that were directed to the cathode (P2) and anode (P3) were placed in pellet culture, and aggrecan expression was measured after 7 days (see section on 3D cultures, below, for culture details). Pellets were stored in Trizol reagent to perform RNA extraction and total sample cDNA generation. Real-time PCR was then run for human aggrecan and β-actin, a known housekeeping gene. Unpaired t-test was performed for aggrecan gene expression normalized to β-actin expression, to compare P2 and P3 OA chondrocytes.
2.2. 3D cultures
P2 human OA chondrocytes exhibited cathodal migration that yielded cell pellets with increased aggrecan expression over other passages examined (see Figure 3 and detailed results below). Previously, P2 bovine chondrocytes were found to display excellent capabilities in developing functional engineered cartilage in agarose cultures (O’Connell et al., 2014). Therefore, cells were passaged twice before using them in 3D culture studies for subsequent studies (Figure 2) (O’Connell et al., 2014).
Figure 3.
Cell migration parameters for passaged human chondrocytes: (A) overall speed for EF-treated cells increased with passage number; (B) directed velocity for those cells is orientated toward the cathode at P2 before changing direction toward the anode for later passages; (inset) speed and directed velocity of juvenile bovine chondrocytes at P2; *p < 0.05 vs control at each passage
Figure 2.
Schematic of tissue-engineering study for human chondrocytes acquired from osteoarthritic joints; four growth factor combinations were evaluated for pellet cultures and agarose constructs were cultured with either BMP2 or TGFβ3
Once confluence was reached during the second passage, cells were trypsinized and pellets were formed for each cell type by centrifugation. The pellets were cultured for 28 days in a chemically defined medium [CM; DMEM with 0.1 µm dexamethasone, 40 mg/ml l-proline, 50 mg/ml ascorbate 2-phosphate, 100 mg/ml sodium pyruvate, 1× ITS+ premix, 100 U/ml penicillin and 100 mg/ml streptomycin and amphotericin B (Invitrogen), supplemented with growth factors]. Based on previously reported values for growth factor concentrations and uses in 3D tissue-culture studies, pellets were cultured with CM supplemented with the following growth factor combinations: 50 ng/ml BMP2 with 10 ng/ml FGF2; and 50 ng/ml BMP2 with 10 ng/ml PDGFββ (Balakrishnan et al., 2014; Barbero et al., 2004; Chuang et al., 2012; de Mara et al., 2013; Handorf and Li, 2011; Johns and Athanasiou, 2008; Mayer-Wagner et al., 2011; Perrier et al., 2011). Pellets cultured with 10 ng/ml TGFβ3 only or 50 ng/ml BMP2 only served as single growth factor controls (Figure 2). TGFβ3 only was chosen as a study group, as it is directly comparable to our previous work with animal cell sources and one of the most commonly used growth factors in cartilage tissue-engineering studies (Huang et al., 2009; O’Connell et al., 2012b).
Pellets were cultured for 28 days and extracellular matrix production was assessed bi-weekly (i.e. days 0, 14 and 28; n = 5/group at each time point). Media aliquots were saved to measure GAG release into the medium and pellets were prepared for biochemical analysis. The pellet culture was repeated using chondrocytes from a second donor to demonstrate robustness of the results. DNA content was measured using the PicoGreen Kit (Invitrogen). GAGs were measured using DMMB assay, and collagen content was measured using hydoxyproline assay. Two-way ANOVA was performed on the biochemical properties, with the treatment group and time as factors. Bonferroni post hoc analysis was used when significance was found (p ≤ 0.05).
Following the pellet study, cells were passaged for 3D culture in 2% w/v low-gelling agarose slab (Type VII, Sigma-Aldrich) at a final concentration of 30 × 106 cells/ml (Mauck et al., 2002). Constructs were punched from the slab (2.34 mm thickness) using a 4 mm diameter biopsy punch and cultured in CM supplemented with growth factors, based on the results from the pellet culture. Due to the number of cells required for agarose culture, chondrocyte-laden constructs were cultured with either 10 ng/ml TGFβ3 (control) or 50 ng/ml BMP2.
At day 28, cell viability in agarose constructs was assessed with the Live/Dead Kit to determine cell distribution throughout the construct (n = 1/group; Invitrogen). At day 56, mechanical, biochemical and histological analyses were performed (n = 6–8/group/donor). The equilibrium Young’s modulus was determined from a stress relaxation test in unconfined compression at 10% strain. A sinusoidal compressive strain (± 1%, 0.5 Hz) was then applied to determine the dynamic modulus at that frequency. Wet weights (ww) were measured following mechanical testing and the samples were dried to obtain dry weights (dw). DNA content was determined using the PicoGreen Kit (Invitrogen). The GAG and collagen content were determined using 1,9-dimethylmethylene blue (DMMB) and hydroxyproline assay, respectively. The ratio of hydroxyproline to collagen was assumed to be 7.64, based on the molecular weights provided in Hollander et al. (1994). The GAG and collagen contents were normalized by wet weight, dry weight and DNA content. Student’s t-test was performed to compare mechanical and biochemical properties assessed from 3D agarose cultures. Biochemical composition was normalized by wet and dry weight as a measure of matrix composition in the de novo cartilage, and for comparison with previously reported values of native cartilage. Normalization by DNA content was used as a measurement of cell activity in matrix production.
At day 56, extra constructs were preserved for histology to evaluate the distribution of cells, collagen and GAGs. Briefly, histological samples were embedded in paraffin wax and slides were prepared (8 µm sections). The sections were stained with safranin-O for GAG distribution and picrosirius red for collagen distribution. Sections were also stained for collagen types I, II and VI. Briefly, the sections were digested in 0.5 mg/ml testicular hyaluronidase, swollen in 0.5 m acetic acid, blocked in 10% normal goat serum (NGS) and labelled with 10% NGS containing monoclonal primary antibody for collagen types II and VI (Abcam). AlexaFluor 488-conjugated goat anti-mouse secondary antibody labelling (Invitrogen) and TOTO-3 nuclear counterstaining (Invitrogen) were performed to visualize the collagen networks and cells, respectively. After staining, slides were coverslipped with Fluoroshield (Sigma) and the sections were analysed using an inverted microscope with an Olympus Fluoview confocal system, with dual wavelength excitation at 488 and 640 nm.
3. Results
At all passages, chondrocytes exposed to an applied direct current electric field travelled faster and farther than cells not exposed to a field (p < 0.0001; Figure 3A). By late passage (P4), EF-exposed cells were more responsive to the applied field, showing increased migration speeds compared to early passage (P2) cells (11.6 ± 0.84 vs 8.34 ± 0.76 µm/h; p < 0.05; Figure 3A). This change in overall speed through passage number was mirrored with a change in migration direction travelled by EF-exposed cells. Control cells exhibited no preferential direction (i.e. directed velocity = 0 µm/h; p > 0.05); however, EF-exposed cells travelled significantly further toward a specific pole, depending on the passage. P2 cells expressed preferential migration toward the cathode (negative pole, −6.21 ± 0.95 µm/h; p < 0.0001); later passage cells began changing their direction of migration, turning notably toward the anode (positive pole) by P3 (Figure 3B; 1.21 ± 0.93 µm/h; p < 0.05) and increasingly continuing for P4 (Figure 3B; 2.39 ± 1.08 µm/h; p < 0.05). Therefore, P2 cells were used for 3D pellet and agarose culture studies.
Subsequently, a relationship between galvanotaxis response and chondrogenic phenotype was investigated for pellet cultures established from P2 and P3 OA chondrocytes (pooled donors). Cathodal-directed P2 cells exhibited a nearly five-fold increase in aggrecan expression compared to those derived from anodal-directed P3 cells (P2, 4.72 ± 2.52 × 10–5 vs P3, 0.97 ± 0.77 × 10–5; p < 0.05; n = 5–6 samples/group). Together, these findings led us to adopt P2 OA chondrocytes for our subsequent studies comparing the influence of growth factors on pellet and agarose cultures.
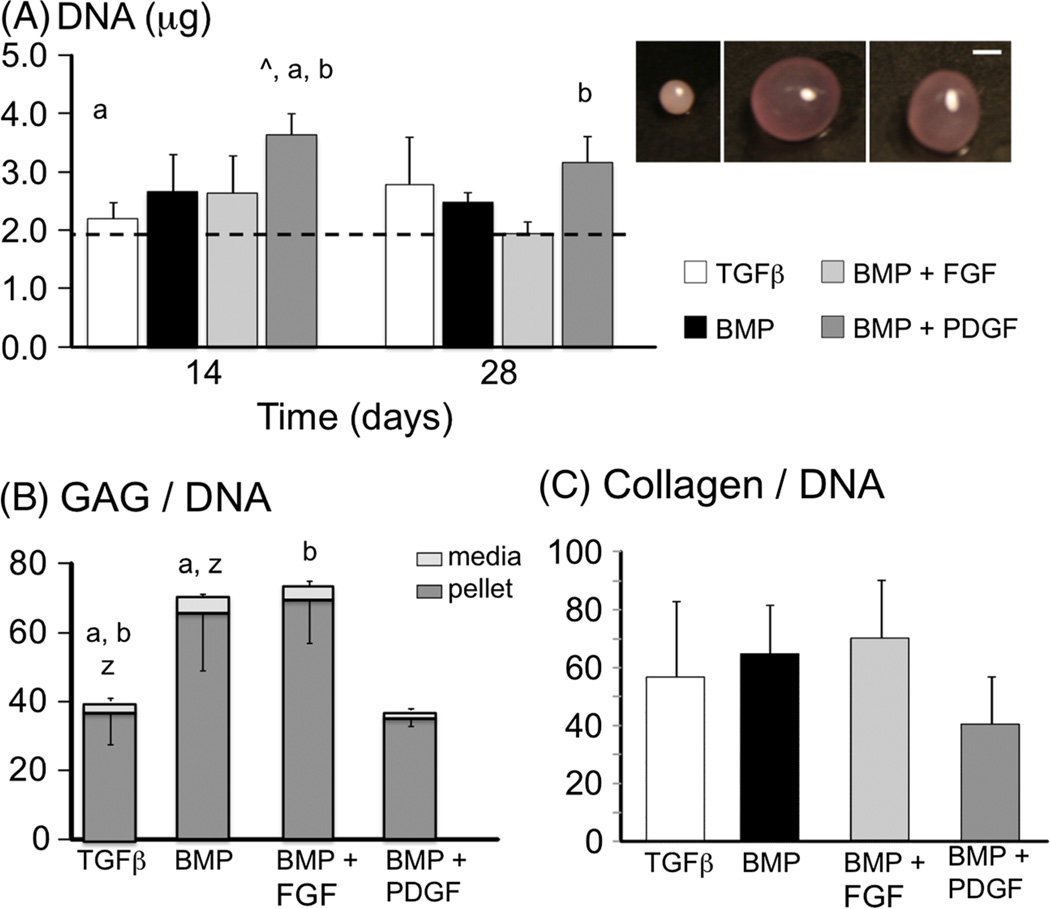
Pellets cultured in CM supplemented with BMP2 were significantly larger than the control (Figure 4, inset). Human ACs maintained cell viability throughout the culture period, as denoted by vital staining as well as DNA content (Figure 4A). Cell proliferation was observed for pellets cultured with BMP2 + PDGFββ, where the DNA content at day 14 was almost 2× the day 0 values (p < 0.0001; Figure 4A, ^). However, there was a decrease in DNA content from day 14 to day 28 (Figure 4A, b), resulting in a final DNA content that was not significantly different from day 0. By day 28, the GAG content in pellets cultured with BMP2 only and BMP2 + FGF2 was 80% greater than the control (p ≤ 0.015; Figure 4B). The GAGs measured in the media aliquots accounted for 5.0–7.4% of the total GAG content measured (i.e. pellet GAG + medium GAG). The GAG content in the medium normalized by DNA was greatest in the BMP2-only group and was 70% greater than the control (medium GAG/DNA for the BMP2 group = 4.9 ± 0.7 mg/mg; Figure 4B, z). At day 28, the collagen content normalized by DNA content was 56.6 ± 26.4 mg/mg for the control group, and there were no significant differences with growth factor treatment (p = 0.14; Figure 4C). The ratio of total GAG content: collagen content was not significantly different across the experimental groups (p = 0.12).
Figure 4.
(A) DNA content of pellets over the 28 day culture period; dotted line represents day 0 values; groups with the same letter were found to be significantly different from one another, i.e. p ≤ 0.05 a vs a); ^ represents p < 0.05 vs day 0 values. (B) GAG content normalized by DNA content at day 28; a and b represent differences in pellet GAG content, and z represents differences in medium GAG content. (C) Collagen content normalized by DNA content at day 28; no significant differences (p = 0.14): (inset) representative images of pellets at day 28; pellets were cultured with TGFβ3-only (left), BMP2 with FGF (middle) or BMP2 with PDGFββ; note that the BMP2-only group is not shown here; bar = 1 mm
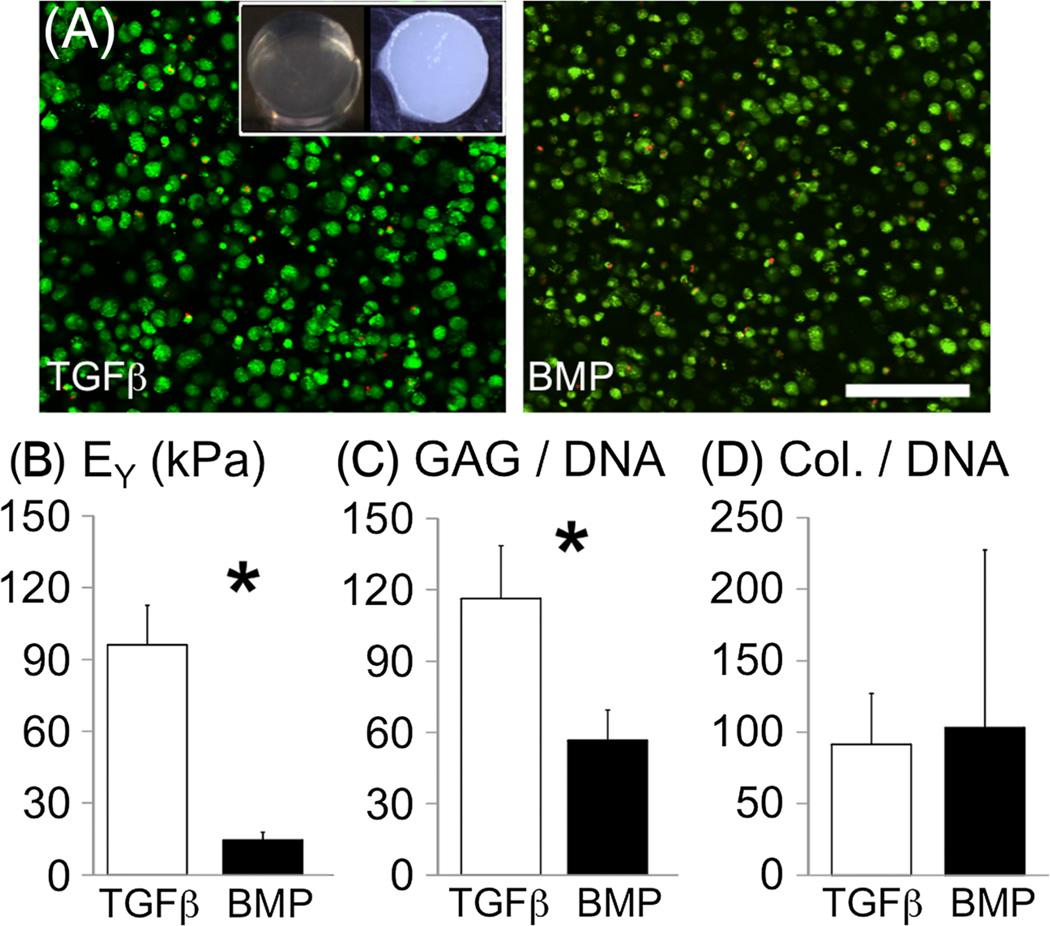
Cell viability in agarose was maintained throughout the culture period (Figure 5A, Figure 6A top row) and cell proliferation was observed in constructs cultured with TGFβ3 (DNA content at day 56, 6.85 ± 0.44 µg; vs 1.18 ± 0.16µg for the BMP2 group; p < 0.001; Figure 6A). Young’s modulus of the agarose constructs was 9.3 ± 1.2 kPa after casting (day 0). At day 56, Young’s modulus of the TGFβ3 group was 96.0 ± 16.5 kPa and was 6.5 times greater than in the BMP2 group (p = 0.0001; Figure 5B). Similarly, the dynamic modulus of the TGFβ3 group was 1120 ± 190 kPa and was six times greater than in the BMP2 group (175 ± 35 kPa; p < 0.0001). This finding was repeated independently using chondrocytes derived from another donor, where the Young’s modulus of engineered cartilage developed was approximately 2.5 times greater for the TGFβ group than the BMP2 group (data not shown).
Figure 5.
(A) Cell viability of chondrocyte-seeded scaffolds: living cells shown in green and dead cells in red; bar = 150 µm; (inset) representative images of a construct at day 0 (left) and a construct cultured with TGFβ3 (right). (B) Equilibrium Young’s modulus, (C) GAG and (D) collagen contents normalized by dry weight for culture with TGFβ3 only or BMP2 only (day 56 values); *significant differences between groups
Figure 6.
Representative histology for agarose constructs cultured with TGFβ3 or BMP2. Samples were stained with haematoxylin and eosin (H&E) for cell distribution (top row), safranin-O for GAG distribution (middle row) and picrosirius red for collagen distribution (bottom row); (inset) representative image of collagen VI staining from immunohistochemistry; note that the darker region at the bottom of the safranin-O stain for the TGFβ3 group is due to a fold in the sample
Agarose constructs increased in opacity with culture, demonstrating increased matrix deposition throughout the construct (Figure 5, inset). GAG content of engineered constructs cultured with TGFβ3 was two to eight times greater than the BMP2 group for GAG normalized by dry weight (TGFβ3 group = 23.4 ± 5.4%/dw), wet weight (2.16 ± 0.51% ww) and DNA content (116.3 ± 22.3µg/µg; p < 0.003; Figure 5C). The intensity of safranin-O staining for GAGs was much greater for the TGFβ3 group and was more uniformly distributed throughout the construct than the BMP2 group (Figure 6, middle row). The collagen content of the TGFβ3 group was 18.03 ± 6.21% dw, or 91.3 ± 35.8µg/µg when normalized by DNA content, and was not significantly different from the collagen content of the BMP2 group (p > 0.3; Figure 5D). The collagen content normalized by wet weight was 1.66 ± 0.57% ww for the TGFβ3 group and was significantly greater than in the BMP2 group (0.48 ± 0.59 %/ww; p = 0.01). Collagen was uniformly distributed in both the TGFβ3 and BMP2 groups, but staining was greater for the TGFb3 group, which compared with the quantitative assay data (Figure 6, bottom row). Immunohistochemistry stained positive for collagen II and VI for the TGFβ3 and BMP2 groups. Collagen VI in the BMP2 group remained closer to the cell edge than in the TGFβ3 group (Figure 6, bottom row inset).
4. Discussion
Engineering of articular cartilage may provide a biological repair strategy for injured or arthritic cartilage. Extensive work has been performed using animal models and cells from animal donors (Hung et al., 2004; Johnstone et al., 2013; Kock et al., 2012; O’Connell et al., 2012b). However, direct translation of culture techniques and conditions appropriate for growing de novo animal cartilage tissue to human tissue has been challenging. Our laboratory, and others, have demonstrated that supplementation of expansion medium with a growth factor cocktail of FGF, PDGF and TGFβ1 is crucial for developing functional engineered cartilage in 3D cultures (Pei et al., 2008; Sampat et al., 2011). In this study, an applied DC EF was applied to human chondrocytes from OA cartilage to determine a passaging limit for 2D expansion, in order to maintain the chondrogenic phenotype. Galvanotaxis to the cathode is exhibited by normal chondrocytes and stem cells expanded with growth factors for defined passages (Chao et al., 2000; Tan et al., 2014). Our results suggest that using human chondrocytes at P2 may improve the matrix production and mechanical properties of 3D engineered functional cartilage. It was observed that matrix production by P2 human chondrocytes was dependent on the 3D culture environment (pellet vs agarose hydrogel) and growth factor supplementation. The findings from this study further support the notion that functional engineered cartilage can be developed from chondrocytes from OA tissues (Bian et al., 2011).
Autologous chondrocytes are the ideal cell source for clinically relevant engineered cartilage implants; however, these cells need to be expanded to obtain sufficient cell numbers for developing neocartilage. With each subsequent passage, the chondrocytes dedifferentiate towards a fibroblastic lineage, which is noted by a change in morphology and increased cell migration (Figure 3) (Chao et al., 2000, 2007).We observed that cell migration behaviour of OA P2 chondrocytes was similar to that of juvenile bovine chondrocytes, which is a robust cell source for developing neocartilage in vitro (O’Connell et al., 2012b). Although the current study was limited by the number of human donors (n = 2), we suggest that the high repeatability of our experiments across donors is indicative of a robust observation. As such, our findings reported here suggest that adult chondrocytes can be passaged twice for expansion, while maintaining the chondrogenic phenotype, for developing neocartilage.
For the conditions of the present study, large differences were observed in matrix production between pellet and agarose culture conditions, which agrees with previous studies reporting gene expression of 3D cultures (Dehne et al., 2009). In pellet culture, BMP2 increased the swelling and physical size of the de novo tissue with human chondrocytes (Figure 4, inset). Furthermore, BMP2 demonstrated increased GAG production under specific conditions. However, matrix production and mechanical properties were higher for chondrocyte-seeded agarose constructs cultured with TGFβ3. It is possible that these differences in cell behaviour and matrix production are due to differences in cell–cell interactions, or in the diffusion of nutrients or growth factors (Bian et al., 2013; Pei et al., 2008). Although the sizes of TGFβ3 (13 kDa) and BMP2 (26 kDa) differ only by a factor of two; this difference may be sufficient to restrict diffusion of nutrients, such as growth factors, through the construct (Albro et al., 2009; Chahine et al., 2009).
Gene expression and, in turn, matrix production potential decreases significantly with age (Liu et al., 2013). Therefore, the results from this study, which used chondrocytes from OA knee joints, demonstrates promising results towards developing functional engineered cartilage from patient tissues. The GAG deposition observed in the agarose constructs was comparable to native values (2.2% ww at day 56; native cartilage 2–3% ww) (Treppo et al., 2000). Collagen deposition was significantly lower than native values (native cartilage = 10–15% ww) (O’Connell et al., 2012b; Treppo et al., 2000), which represents a limitation of the hydrogel scaffold system despite the fact that it maintains the round chondrocyte morphology.
The compressive Young’s modulus of engineered cartilage constructs cultured with TGFβ3 was approximately 100 kPa (native cartilage = 400–600 kPa) (Jurvelin et al., 2003; Treppo et al., 2000). These results are encouraging, as they suggest that human chondrocytes from OA tissues can be used to develop larger engineered cartilage tissues (i.e. millimeter-scale). Moreover, recent work has demonstrated better integration of biological repair tissues when the mechanical properties of the repair tissue are lower than those of the surrounding native tissue (Miot et al., 2012). Miot et al. (2012) demonstrated that a shorter in vitro culture for engineered cartilage provided a balance between integration with the native cartilage and matrix deposition. Therefore, these results suggest that engineered cartilage constructs may be developed in vitro with mechanical integrity that may be sufficient for initial repair and integration into native tissue.
While we demonstrate that neocartilage produced by chondrocytes from OA cartilage resembles native cartilage, it remains to be seen whether these tissues function as normal cartilage after implantation. Further assessment of the tissue response to applied stimuli, including proinflammatory cytokines, will help to determine whether the cells maintain an osteoarthritic phenotype. In this context, these engineered tissues could alternatively serve as a platform to study pathological cartilage (Musumeci et al., 2011).
There are many studies on human cells that have evaluated gene expression in monolayer culture in media supplemented with serum. Our preliminary findings in pellet culture with serum demonstrated an increase in cell proliferation; however, matrix production of serum-supplemented pellets was significantly lower than that in pellets cultured without serum (data not shown). These findings are comparable to the findings of Stewart et al. (2000), in which serum supplementation decreased gene expression of aggrecan and collagen in pellet culture when compared to a serum-free culture condition. Similarly, our pellets cultured with BMP2 + PDGF demonstrated that increases in cell proliferation resulted in a relative decrease in GAG content when normalized by DNA (Figure 4). These results demonstrate that culture conditions need to balance cell proliferation and matrix production, as some media components may cause cells to focus more energy on proliferation at the expense of synthesizing cartilage with mechanical and biochemical properties comparable to native values.
5. Conclusion
The duration of the studies performed here was significantly longer than most studies that evaluate gene expression or matrix production of monolayer and pellet cultures. Our findings suggest that culture periods > 8 weeks will be necessary to develop mature engineered cartilage in free swelling conditions prior to implantation (chondrocyte-seeded scaffolds cultured with TGFβ3 was approximately 100 kPa at day 56; Figure 5). While these findings are encouraging, long culture times are a significant limitation for clinical applications, due to the increased time between initial tissue harvest and final implantation surgeries. Future work will focus on improving the rate of matrix deposition by applying dynamic loading in culture, which have been shown to upregulate BMP2 and TGFβ3 expression (Nam et al., 2013; Tenney and Discher, 2009) and increase extracellular matrix deposition rates of agarose constructs seeded with bovine cells (Kelly et al., 2013; Sampat et al., 2011). Additionally, these efforts would likely benefit from incorporation of strategies that can provide further refinement of the cell population to those geared to produce tissue in 3D.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH; Grant Nos AR061988 to C.T.H., AR060361 to G.A.A. and C.T.H., AR054817 to S.B.A. and 1S10RR027943-01 to C.T.H.), the Coulter Foundation (to C.T.H.) and Columbia Technology Ventures (to C.T.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. In addition, G.D.O.C. was supported in part by a NIH diversity supplement and A.R.T. was supported in part by a graduate fellowship from the National Science Foundation (NSF).
References
- Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Research Therapy. 2012;3:9. doi: 10.1186/scrt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro MB, Rajan V, Li R, et al. Characterization of the concentration-dependence of solute diffusivity and partitioning in a model dextran–agarose transport system. Cell Mol Bioeng. 2009;2:295–305. doi: 10.1007/s12195-009-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves da Silva ML, Martins A, Costa-Pinto AR, et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in Chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5:722–732. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- Attur M, Ben-Artzi A, Yang Q, et al. Perturbation of nuclear lamin A causes cell death in chondrocytes. Arthritis Rheum. 2012;64:1940–1949. doi: 10.1002/art.34360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B, Joshi N, Jayakrishnan A, et al. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- Barbero A, Grogan S, Schafer D, et al. Age related changes in human articular chondrocyte yield, proliferation and postexpansion chondrogenic capacity. Osteoarthr Cartilage. 2004;12:476–484. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bian L, Guvendiren M, Mauck RL, et al. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc Natl Acad Sc USA. 2013;110:10117–10122. doi: 10.1073/pnas.1214100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Zhai DY, Mauck RL, et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng A. 2011;17:1137–1145. doi: 10.1089/ten.tea.2010.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian L, Zhai DY, Zhang EC, et al. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng A. 2012;18:715–724. doi: 10.1089/ten.tea.2011.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine NO, Albro MB, Lima EG, et al. Effect of dynamic loading on the transport of solutes into agarose hydrogels. Biophys J. 2009;97:968–975. doi: 10.1016/j.bpj.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao PH, Lu HH, Hung CT, et al. Effects of applied DC electric field on ligament fibroblast migration and wound healing. Conn Tissue Res. 2007;48:188–197. doi: 10.1080/03008200701424451. [DOI] [PubMed] [Google Scholar]
- Chao PH, Roy R, Mauck RL, et al. Chondrocyte translocation response to direct current electric fields. J Biomech Eng. 2000;122:261–267. doi: 10.1115/1.429661. [DOI] [PubMed] [Google Scholar]
- Chiang H, Hsieh CH, Lin YH, et al. Differences between chondrocytes and bone marrow-derived chondrogenic cells. Tissue Eng A. 2011;17:2919–2929. doi: 10.1089/ten.tea.2010.0732. [DOI] [PubMed] [Google Scholar]
- Chuang CY, Shahin K, Lord MS, et al. The cartilage matrix molecule components produced by human foetal cartilage rudiment cells within scaffolds and the role of exogenous growth factors. Biomaterials. 2012;33:4078–4088. doi: 10.1016/j.biomaterials.2012.02.032. [DOI] [PubMed] [Google Scholar]
- de Mara CS, Duarte AS, Sartori-Cintra AR, et al. Chondrogenesis from umbilical cord blood cells stimulated with BMP-2 and BMP-6. Rheumatol Int. 2013;33:121–128. doi: 10.1007/s00296-011-2328-6. [DOI] [PubMed] [Google Scholar]
- Dehne T, Karlsson C, Ringe J, et al. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res Therapy. 2009;11:R133. doi: 10.1186/ar2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Fisher MB, Huang AH, et al. Functional properties of bone marrow-derived MSC-based engineered cartilage are unstable with very long-term in vitro culture. J Biomech. 2013;47:2173–2182. doi: 10.1016/j.jbiomech.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier J, Ross SM, Kanehisa J, et al. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J Cell Physiol. 1986;129:283–288. doi: 10.1002/jcp.1041290303. [DOI] [PubMed] [Google Scholar]
- Ghone NV, Grayson WL. Recapitulation of mesenchymal condensation enhances in vitro chondrogenesis of human mesenchymal stem cells. J Cell Physiol. 2012;227:3701–3708. doi: 10.1002/jcp.24078. [DOI] [PubMed] [Google Scholar]
- Gunja NJ, Dujari D, Chen A, et al. Migration responses of outer and inner meniscus cells to applied direct current electric fields. J Orthop Res. 2012;30:103–111. doi: 10.1002/jor.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunja NJ, Hung CT, Bulinski JC. The Physiology of Bioelectricity in Development. Boca Raton, FL: Tissue Regeneration and Cancer Press; 2010. Effects of DC electric fields on migration of cells of the musculoskeletal system. [Google Scholar]
- Handorf AM, Li WJ. Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PloS One. 2011;6:e22887. doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander AP, Heathfield TF, Webber C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AH, Stein A, Tuan RS, et al. Transient exposure to transforming growth factor-β3 improves the mechanical properties of mesenchymal stem cell-laden cartilage constructs in a density-dependent manner. Tissue Eng A. 2009;15:3461–3472. doi: 10.1089/ten.tea.2009.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Johns DE, Athanasiou KA. Growth factor effects on costal chondrocytes for tissue engineering fibrocartilage. Cell Tissue Res. 2008;333:439–447. doi: 10.1007/s00441-008-0652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Alini M, Cucchiarini M, et al. Tissue engineering for articular cartilage repair – the state of the art. Eur Cells Mater. 2013;25:248–267. doi: 10.22203/ecm.v025a18. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Buschmann MD, Hunziker EB. Mechanical anisotropy of the human knee articular cartilage in compression. Proceedings of the Institution of Mechanical Engineers Part H. J Eng Med. 2003;217:215–219. doi: 10.1243/095441103765212712. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Roach BL, Weidner ZD, et al. Tissue-engineered articular cartilage exhibits tension-compression nonlinearity reminiscent of the native cartilage. J Biomech. 2013;46:1784–1791. doi: 10.1016/j.jbiomech.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock L, van Donkelaar CC, Ito K. Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res. 2012;347:613–627. doi: 10.1007/s00441-011-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlarz H, Gunnarsson CL, Fang H, et al. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60:3546–3553. doi: 10.1002/art.24984. [DOI] [PubMed] [Google Scholar]
- Liu H, Zhao Z, Clarke RB, et al. Enhanced tissue regeneration potential of juvenile articular cartilage. Am J Sports Med. 2013;41:2658–2667. doi: 10.1177/0363546513502945. [DOI] [PubMed] [Google Scholar]
- Luo X, Pietrobon R, Sun SX, et al. Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine. 2004;29:79–86. doi: 10.1097/01.BRS.0000105527.13866.0F. [DOI] [PubMed] [Google Scholar]
- Luyten FP, Cunningham NS, Vukicevic S, et al. Advances in osteogenin and related bone morphogenetic proteins in bone induction and repair. Acta Orthop Belg. 1992;58(suppl 1):263–267. [PubMed] [Google Scholar]
- Matsumoto E, Furumatsu T, Kanazawa T, et al. ROCK inhibitor prevents the dedifferentiation of human articular chondrocytes. Biochem Biophys Res Commun. 2012;420:124–129. doi: 10.1016/j.bbrc.2012.02.127. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Seyhan SL, Ateshian GA, et al. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- Mayer-Wagner S, Passberger A, Sievers B, et al. Effects of low frequency electromagnetic fields on the chondrogenic differentiation of human mesenchymal stem cells. Bioelectromagnetics. 2011;32:283–290. doi: 10.1002/bem.20633. [DOI] [PubMed] [Google Scholar]
- Mendelson A, Frank E, Allred C, et al. Chondrogenesis by chemotactic homing of synovium, bone marrow, and adipose stem cells in vitro. FASEB J. 2011;25:3496–3504. doi: 10.1096/fj.10-176305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot S, Brehm W, Dickinson S, et al. Influence of in vitro maturation of engineered cartilage on the outcome of osteochondral repair in a goat model. Eur Cells Mater. 2012;23:222–236. doi: 10.22203/ecm.v023a17. [DOI] [PubMed] [Google Scholar]
- Musumeci G, Loreto C, Carnazza ML, et al. OA cartilage derived chondrocytes encapsulated in polyethylene glycol; diacrylate PEGDA; for the evaluation of cartilage restoration and apoptosis in an in vitro model. Histol Histopathol. 2011;26:1265–1278. doi: 10.14670/HH-26.1265. [DOI] [PubMed] [Google Scholar]
- Nam J, Perera P, Rath B, et al. Dynamic regulation of bone morphogenetic proteins in engineered osteochondral constructs by biomechanical stimulation. Tissue Eng A. 2013;19:783–792. doi: 10.1089/ten.tea.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng A. 2009;15:3119–3128. doi: 10.1089/ten.tea.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann A, Dennis JE, Aigner J, et al. Tissue engineering of autologous cartilage grafts in three-dimensional in vitro macro-aggregate culture system. Tissue Eng. 2004;10:1695–1706. doi: 10.1089/ten.2004.10.1695. [DOI] [PubMed] [Google Scholar]
- O’Connell GD, Fong JV, Joffe A, et al. Trimethylamine N-oxide as a media supplement for cartilage tissue engineering. J Orthop Res. 2012a;30(12):1898–1905. doi: 10.1002/jor.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell GD, Lima EG, Bian L, et al. Toward engineering a biological joint replacement. J Knee Surg. 2012b;25:187–196. doi: 10.1055/s-0032-1319783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell GD, Nims RJ, Green J, et al. Time and dose-dependent effects of chondroitinase ABC on growth of engineered cartilage. Eur Cell Mater. 2014;27:312–320. doi: 10.22203/ecm.v027a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Shim MS, Shim SH, et al. Chondrogenic potential of stem cells derived from amniotic fluid, adipose tissue, or bone marrow encapsulated in fibrin gels containing TGFβ3. Biomaterials. 2011;32:8139–8149. doi: 10.1016/j.biomaterials.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Pei M, He F, Boyce BM, et al. Repair of full-thickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthr Cartilage. 2009;17:714–722. doi: 10.1016/j.joca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation. 2008;76:1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier E, Ronziere MC, Bareille R, et al. Analysis of collagen expression during chondrogenic induction of human bone marrow mesenchymal stem cells. Biotechnol Lett. 2011;33:2091–2101. doi: 10.1007/s10529-011-0653-1. [DOI] [PubMed] [Google Scholar]
- Robinson KR. The responses of cells to electric fields: a review. J Cell Biol. 1985;101:2023–2027. doi: 10.1083/jcb.101.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MT, Gomes ME, Reis RL. Current strategies for osteochondral regeneration: from stem cells to pre-clinical approaches. Curr Opin Biotechnol. 2011;22(5):726–733. doi: 10.1016/j.copbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Sailor LZ, Hewick RM, Morris EA. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996;14:937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- Sampat SR, O’Connell GD, Fong JV, et al. Growth factor priming of synovium derived stem cells for cartilage tissue engineering. Tissue Eng A. 2011;17:2259–2265. doi: 10.1089/ten.tea.2011.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmi TA, Verdonk P, Chambat P, et al. Autologous chondrocyte implantation in a novel alginate–agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597–604. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- Sillman AL, Quang DM, Farboud B, et al. Human dermal fibroblasts do not exhibit directional migration on collagen I in direct-current electric fields of physiological strength. Exp Dermatol. 2003;12:396–402. doi: 10.1034/j.1600-0625.2002.120406.x. [DOI] [PubMed] [Google Scholar]
- Soong HK, Parkinson WC, Bafna S, et al. Movements of cultured corneal epithelial cells and stromal fibroblasts in electric fields. Invest Ophthalmol Vis Sci. 1990;31:2278–2282. [PubMed] [Google Scholar]
- Stewart MC, Saunders KM, Burton-Wurster N, et al. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. J Bone Miner Res. 2000;15:166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- Tan AR, Alegre-Aguaron E, O’Connell GD, et al. Passage-dependent relationship between mesenchymal stem cell mobilization and chondrogenic potential. Osteoarthr Cartilage. 2014 doi: 10.1016/j.joca.2014.10.001. In press – epub ahead of print: 2014 Oct 17. pii: S1063-4584(14) 01279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney RM, Discher DE. Stem cells, microenvironment mechanics, and growth factor activation. Curr Opin Cell Biol. 2009;21:630–635. doi: 10.1016/j.ceb.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos JS, De Croos JN, Park SS, et al. Integration of tissue-engineered cartilage with host cartilage: an in vitro model. Clin Orthop Relat Res. 2011;469:2785–2795. doi: 10.1007/s11999-011-1856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treppo S, Koepp H, Quan EC, et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- Wu L, Prins HJ, Helder MN, et al. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng A. 2012;18:1542–1551. doi: 10.1089/ten.TEA.2011.0715. [DOI] [PubMed] [Google Scholar]
- Yin J, Yang Z, Cao YP, et al. Characterization of human primary chondrocytes of osteoarthritic cartilage at varying severity. Chin Med J. 2011;124:4245–4253. [PubMed] [Google Scholar]
- Yoo HJ, Yoon SS, Park SY, et al. Gene expression profile during chondrogenesis in human bone marrow derived mesenchymal stem cells using a cDNA microarray. J Korean Med Sci. 2011;26:851–858. doi: 10.3346/jkms.2011.26.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]