Abstract
Purpose
The goal of the study was to evaluate the repair of chondral lesions treated with combined autologous adult / allogenic juvenile cartilage fragments, compared with isolated adult and isolated juvenile cartilage fragments.
Methods
Fifty-eight adult (> 16 week old) and 5 juvenile (< 6 week old) New Zealand White female rabbits were used. A large osteochondral defect was created in the center of the femoral trochlea of adult rabbits. The rabbits were divided in 4 groups: Group 1= untreated defects (controls); Group 2= adult cartilage fragments; Group 3= juvenile cartilage fragments; Group 4= adult + juvenile cartilage fragments. Sacrifices were performed at 3 and 6 months. The defects were evaluated with ICRS macroscopic score, modified O’Driscoll score, and Collagen type II immunostaining.
Results
At 3 months, Group 4 performed better than Group 1, in terms of modified O’Driscoll score (p=0.001) and Collagen type II immunostaining (p=0.015). At 6 months, Group 4 showed higher modified O’Driscoll score (p=0.003) and Collagen type II immunostaining score (p<0.001)than Group 1. Histologically, also Group 3 performed better than Group 1 (p=0.03) and Group 4 performed better than Group 2 (p=0.004).
Conclusions
Mixing adult and juvenile cartilage fragments improved cartilage repair in a rabbit model. In the clinical setting, a new “one-stage” procedure combining the two cartilage sources can be hypothesized, with the advantages of improved chondral repair and large defect coverage, because of the use of an off-the-shelf juvenile allograft. Further studies on larger animals and clinical trials are required to confirm these results.
Keywords: Cartilage repair, in vivo, juvenile, adult, chondral fragments, rabbit
Introduction
Chondral and osteochondral lesions are common problems in orthopedics and their treatment still remains a challenge for orthopaedic surgeons. The prevalence of chondral lesions of any type has been estimated around 60–63% during knee arthroscopies and focal chondral or osteochondral defects around 19% of patients [15, 30].
Depending on the age of the patient, symptoms, previous surgeries, involvement of subchondral bone, size and chronicity of the defect, different options are available for the treatment of focal chondral defects, and these include: 1) Bone marrow stimulation techniques (i.e. microfractures), 2) Osteochondral auto/allograft transplantation, 3) Autologous chondrocyte implantation (ACI). All of these techniques have disadvantages, including: 1) fibrocartilage repair tissue with bone marrow stimulation techniques; 2) donor-site morbidity, technical difficulty in matching the lesion contour, defect-size limitations, and risk of cartilage and bone collapse, with osteochondral autograft transplantation; 3) graft availability, technical difficulties, costs, possible disease transmission, with osteochondral allograft transplantation. Autologous chondrocyte implantation showed to result in hyaline-like regenerative tissue with better histological and clinical outcomes than simple microfractures [10, 25, 26]. However, two surgical procedures and a costly cell expansion are required with this technique. To overcome these limitations, tissue engineering is prospecting new single-step solutions, combining scaffolds with different viable cell sources [16].
The use of freshly harvested autologous cartilage chips held in the defect by a scaffold has recently been described in small and large animal models [3, 12, 17, 18], reporting histological results similar to ACI [12, 17]. This technique has also recently proven to be safe, feasible and effective in a prospective clinical safety trial [6].
Juvenile chondrocytes have shown in vitro superior capabilities of producing cartilage extracellular matrix [1, 2, 9]. In addition, allogeneic juvenile cartilage fragments have recently been described for chondral repair and are currently on the US market (DeNovo NT Graft - “Natural Tissue Graft” Zimmer Inc, Warsaw, IN/ISTO Technologies Inc, St Louis, MO). Some preliminary clinical safety reports are available for the treatment of chondral lesions in the knee and ankle, but the literature is still sparse regarding this topic [12, 20, 21].
In the clinical setting, autologous cartilage fragments do not allow large defect coverage (due to the limited amount of autograft), and neocartilage derived from juvenile cells did not seem to show terminal chondrocyte differentiation (as demonstrated by the absence of Type X collagen and lack of cellular hypertrophy) [1]. To overcome these limitations and in the light of the recent findings that co-cultures of juvenile/adult human cartilage fragments showed superior matrix production compared with isolated adult cultures [5], the authors hypothesized a new “one-stage” procedure directly combining in situ autologous adult and allogenic juvenile cartilage fragments.
The goal of this study was to evaluate on a rabbit model the repair of chondral lesions treated with combined autologous adult/allogenic juvenile cartilage fragments, compared with isolated adult and isolated juvenile cartilage fragments.
The authors tested the null hypothesis that there was no difference between chondral lesions left untreated, treated with autologous chondral fragments, with juvenile chondral fragments, or with mixed autologous/juvenile fragments.
Material and methods
Animal committee approval was obtained (University of Iowa IACUC #0907156). Fifty eight adult (> 16 week old) and 5 juvenile (< 6 week old) New Zealand White female rabbits were used. The juvenile cartilage was freshly harvested every morning from the juvenile donors. These were humanely euthanized (Pentobarbital sodium injection 100 mg/Kg), and both knees were shaved and prepped in a sterile fashion, scrubbing three times with alcohol (70%) and three times with povidone-iodine. Through a 4 cm midline skin incision to the left and right knee, the cartilage from the trochlea, femoral condyles, patella and tibial plateau was harvested with a sharp scalpel (n° 15 blade) and minced in a wet environment (standard culture medium) in order to obtain fragments smaller than 1 mm3. The cartilage fragments were kept in the culture medium, before implantation in the adult rabbits the same day.
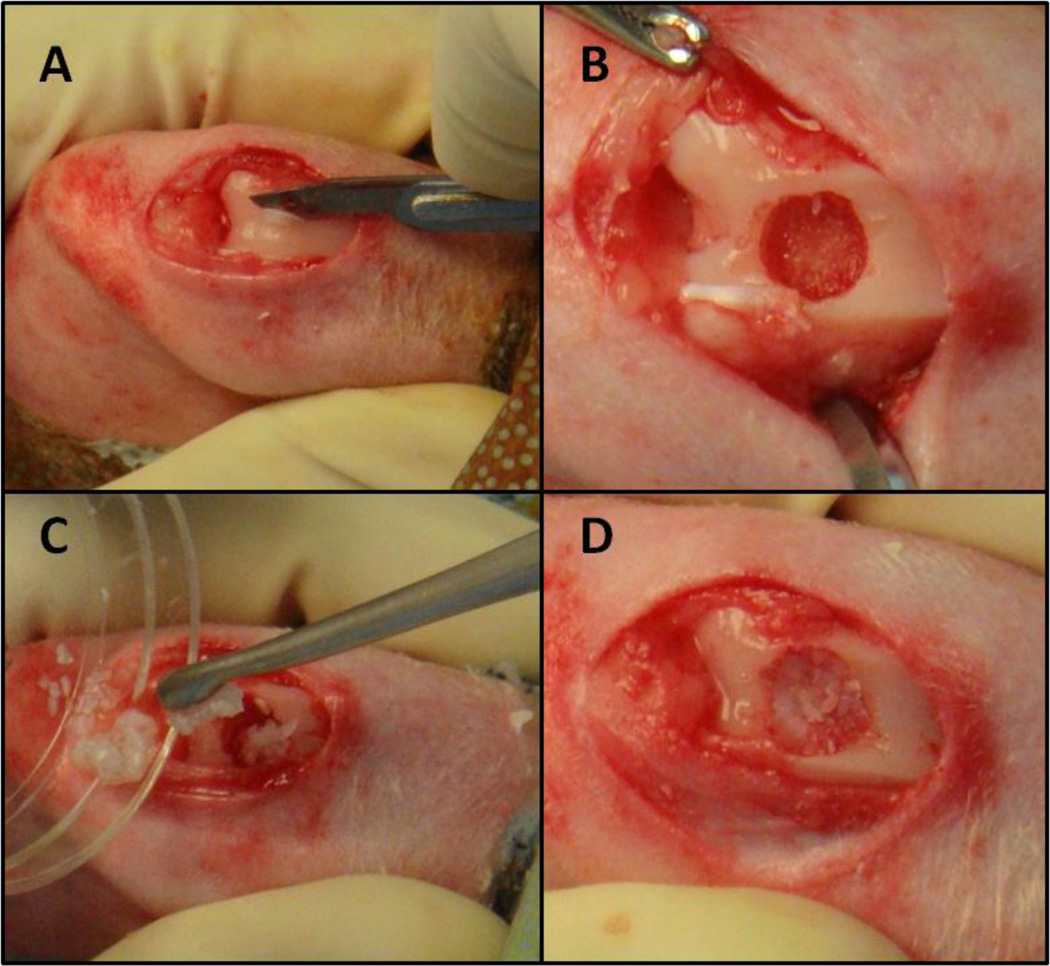
The adult rabbits received antibiotic prophylaxis with Enrofloxacin (10 mg/kg SQ) every 24h for 5 days, starting the day before surgery. Perioperative pain was controlled with Buprenorphine (0.03 mg/kg IM) pre-operatively. An intramuscular injection with Ketamine (20 mg/kg) and Medetomidine (0.4 mg/kg) was performed for the induction of the anesthesia. Tricotomy of the left knee was carried out with an over the counter shaving cream, in order to avoid injuries to the skin of the rabbit. General anesthesia was then administered using a facial mask with Isoflurane conveyed by pure oxygen. The left knee was prepped in a sterile fashion and scrubbed three times with alcohol (70%) and three more times with povidone-iodine. A sterile surgical field was then prepared around the left knee. Two surgeons and one anesthesiologist performed the procedure. An anterior midline skin incision (1.5–2 cm) with a medial parapatellar approach was carried out. The patella was gently retracted laterally and the trochlea exposed. A large osteochondral defect (diameter 4.5–5 mm, depth 2–2.5 mm) was manually created with a flat drill tip in the center of femoral trochlea (Figure 1). Care was taken to preserve the edges of the trochlear region (medial and lateral trochlear ridges), in order to have a contained defect. Before preparing the defect, the cartilage layer corresponding to the defect area was gently harvested with a sharp n° 15 blade and minced in a wet environment (standard culture medium), to obtain fragments smaller than 1 mm3 (Figure 1). Copious pulsatile lavages were performed with saline.
Figure 1.
Surgical Technique. A) Harvest of the adult cartilage from the area where the defect was planned. B) A large osteochondral defect (diameter 4.5–5 mm, depth 2–2.5 mm) was manually created in the center of femoral trochlea. C) Filling of the defects with cartilage fragments, according to the assigned treatment group (Adult, Juvenile, or Juvenile + Adult cartilage fragments). D) Defect filled with cartilage fragments stabilized with 1.8%-2% low melt agarose.
The rabbits were randomly assigned to cases or controls. The control groups were left untreated. The cases were filled with: 1) autologous cartilage fragments (harvested from the defect area); 2) juvenile allogenic cartilage fragments (freshly harvested from the young rabbits); and 3) autologous + juvenile allogenic cartilage fragments in equal proportions (Figure 1). The defect was completely filled with cartilage fragments. Then the needle of a syringe filled with liquid agarose was put at the bottom of the defect and the agarose injected, while keeping the fragments in situ with a finger. The finger was then removed and a drop of agarose was put on top of the fragments. A 1.8%–2% low melt agarose was used. A higher concentration (2.5%) may have resulted in a more solid filling and quicker polymerization, but required higher temperature at the time of implantation, with the risk of damage to the surrounding cartilage. A 1.8%-2% concentration was preferred, in order to allow implantation at ~50°C and a good stability of the construct at body temperature. Lavages were performed at the time of agarose implantation, in order to reduce the heat damage to the surrounding cartilage.
The joint was then irrigated and the patella reduced into the trochlea. The joint capsule, the medial retinaculum, and the subcutaneous tissue were carefully sutured with 5-0 monofilament bio-absorbable sutures. The skin was closed with 3-0 braided bio-absorbable sutures. A spray liquid bandage was applied on top of the surgical wound. Post-operatively, the pain was controlled with Buprenorphine (0.03 mg/kg IM every 12h) for 48h. The rabbits were kept in an appropriate animal care facility and humanly sacrificed at 3 months and 6 months.
After euthanasia, the left knees were dissected and the defects macroscopically evaluated, considering: synovial fluid, synovial membrane, articular cartilage, menisci, bone, together with overall repair tissue fill, uniformity, color, and integration. The ICRS macroscopic score for cartilage repair was used [29]. Digital pictures of every defect were taken.
Osteochondral blocks encompassing the entire defect were dissected and fixed in 10% formalin. The samples were cut at 2 levels within the defect area (proximal and distal aspects of the defect) with a microtome. The decalcified paraffin sections (5 µm) were then stained both with Safranin-O fast green and with Collagen type II immunostaining. Histological evaluation was carried out using a modified O’Driscoll score [11, 12] and a 0–3 scale for Collagen type II immunostaining (0, no stain; 1, partial staining; 2, top to bottom or side to side; 3, top to bottom and side to side) [12]. The macroscopic and microscopic investigation was conducted by a blinded investigator, expert in cartilage repair.
Statistical analysis
Sample size calculation was performed “a priori” (G*Power version 3.0.10.) with α=0.05 and (1-β)=0.95, according to previous similar studies [19]. The calculated total sample size was 16 rabbits. Intra-observer reliability was evaluated with the Pearson’s product-moment correlation coefficient (r) and Cronbach’s alpha coefficient of internal consistency (ICRS macroscopic score r=0.99 and α=0.99; modified O’Driscoll score r=0.93 and α=0.97; Collagen type II score r=0.85 and α=0.91).
The results are presented with the mean and the standard deviation (SD). The different groups were tested for differences with one-way ANOVA in case of normal data distribution, or with Kruskal-Wallis in case of non-normal distribution (95% CI, p=0.05). The analysis was conducted with GraphPad version 6.05 for Windows.
Post-hoc power analysis was conducted with G*Power version 3.0.10. The power was more than 0.9 for both macroscopic and microscopic evaluations at 3 and 6 months. The power of Collagen type II scoring was 0.31 and 0.66 at 3 and 6 months, respectively.
Results
A few complications were recorded during the study. One rabbit died in the early post-operative period and 3 rabbits showed early signs of surgical site infection. Despite the antibiotic treatment, the infection did not resolve in two rabbits, which were euthanized. At post-mortem inspection of the knees, besides the signs of infection and one chronic lateral patellar dislocation, no other relevant findings were recorded and the osteochondral defect site showed a correct position of the agarose and cartilage fragments. These 3 cases with unresolved early complications were replaced with new ones. Late complications included: 1) one infection diagnosed at 3 month sacrifices; 2) two infections diagnosed at 6 month sacrifices; and 3) one death at 4 months and one at 5 months after surgery. These 5 cases were excluded from the study. A total of 50 rabbits (Table 1) were available for final evaluation at 3 months (26 rabbits) and at 6 months (24 rabbits).
Table 1.
Macroscopic (ICRS macroscopic score), microscopic (modified O’Driscoll score), and immunohistochemistry results at 3 and 6 months.
| Groups | Sacrifices at 3 months | ||||||
|---|---|---|---|---|---|---|---|
| N of cases (total 26) |
Macro (0–12) |
Micro (0–28) |
Immuno (0–3) |
||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Controls | 9 | 3.3 | 3.7 | 11.8 | 4.4 | 1.1 | 0.6 |
| Adult | 6 | 5.5 | 3.6 | 14 | 2.1 | 1.4 | 0.5 |
| Juvenile | 6 | 5.7 | 2.7 | 14.3 | 6.5 | 1.7 | 0.8 |
| Adult + Juvenile | 5 | 8.2 | 0.8 | 20.2 | 3.7 | 2.2 | 0.4 |
| Sacrifices at 6 months | ||||||
|---|---|---|---|---|---|---|
| N of cases (total 24) |
Macro (0–12) |
Micro (0–28) |
Immuno (0–3) |
|||
| Mean | SD | Mean | SD | Mean | SD | |
| 7 | 5 | 1.9 | 13.4 | 3.3 | 0.8 | 0.7 |
| 7 | 6.4 | 1.9 | 14.3 | 2.9 | 1.7 | 0.8 |
| 4 | 7.2 | 1.7 | 18 | 2.2 | 2 | 0.8 |
| 6 | 8.2 | 2.3 | 18.8 | 1.2 | 2.5 | 0.4 |
3 months
Macroscopic evaluation
The defects were still evident in all samples, with different coverage of repair tissue. The groups treated with mixed adult-juvenile and only juvenile cartilage fragments showed the larger coverage, compared to the other groups. The repair tissue of mixed adult-juvenile and only juvenile cartilage fragments was white, non-continuous and with some areas of roughness. Most of the control groups showed minimal coverage with amorphous tissue. In all groups minimal arthritic and inflammatory changes involving the surrounding cartilage and the synovium were noticed. In terms of ICRS macroscopic evaluation scores, no significant differences were found between the groups (Table 1).
Microscopic evaluation
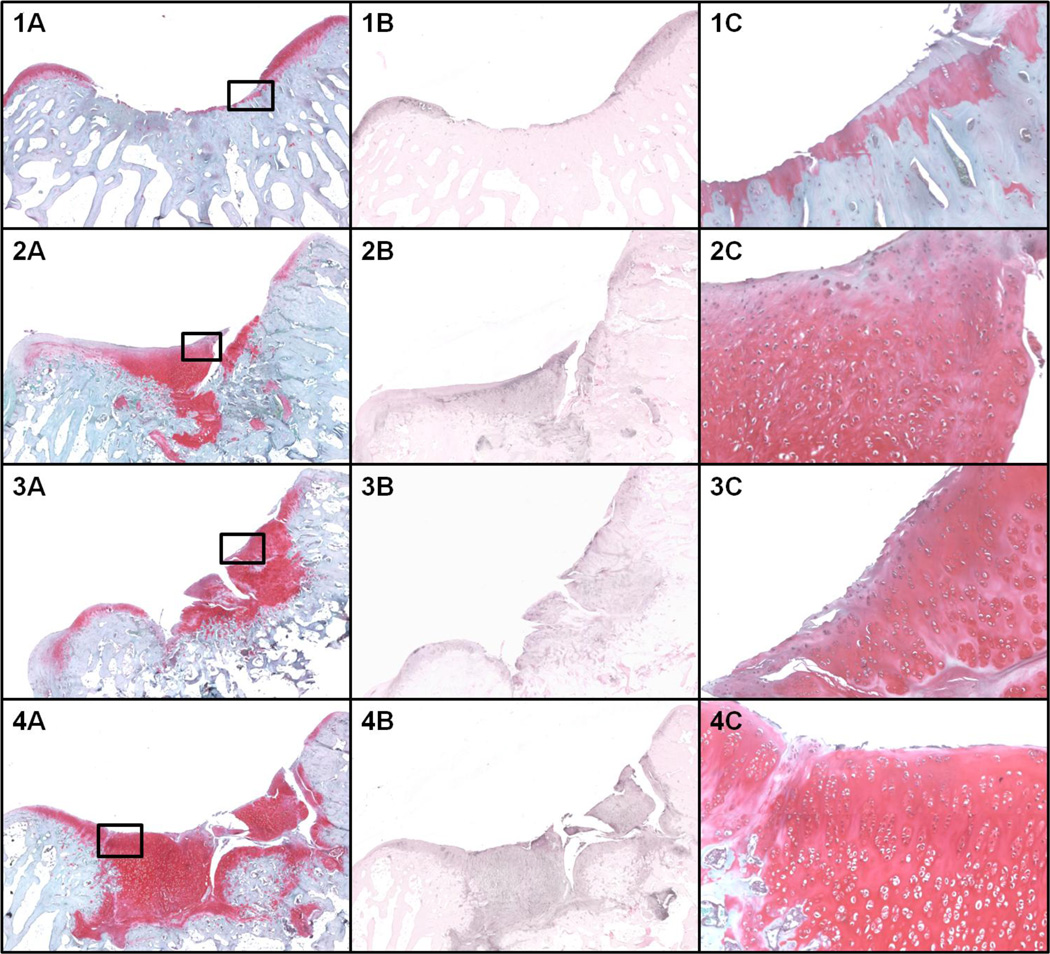
The modified O’Driscoll score reflected the macroscopic results with midrange scores for all groups, suggesting immature repair tissue (Table 1). In terms of modified O’Driscoll score, the mixed fragment group performed better than the controls (p= 0.001). The subchondral bone was minimally restored in all groups (Figure 2). No noticeable inflammatory reactions were evident in all groups.
Figure 2.
Microscopic evaluation at 3 months. 1) control; 2) Adult cartilage fragment group; 3) Juvenile cartilage fragment group; 4) Juvenile + Adult cartilage fragment group. A) Safranin-O staining at 4X magnification. B) Collagen type II immune-staining at 4X magnification. C) Safranin-O staining at 10X magnification of the area selected in panel A.
Immunohistochemistry
The Collagen type II immunohistochemistry showed higher scores in the mixed fragment group compared with the controls (p= 0.015) (Table 1).
6 months
Macroscopic evaluation
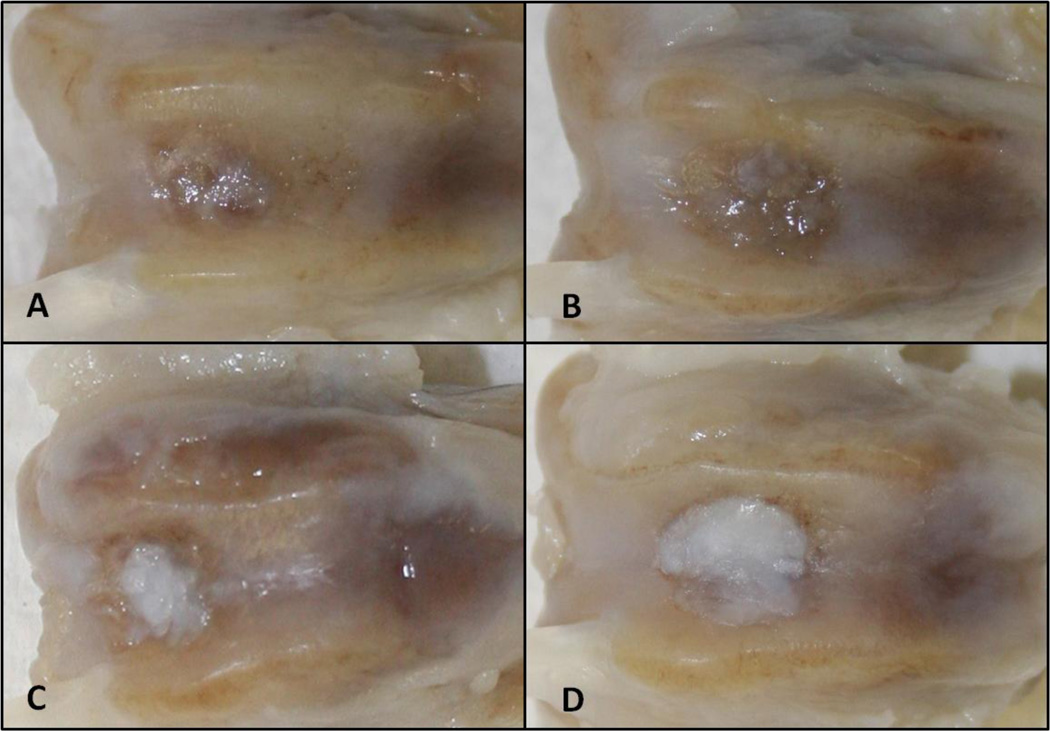
The defects were still evident in all samples, with different coverage of repair tissue (Figure 3). In terms of ICRS macroscopic evaluation scores no other significant differences were observed between the groups (Table 1). Mild degree of arthritic changes was evident in the controls and groups treated with adult cartilage fragments only.
Figure 3.
Macroscopic evaluation at 6 months. A) control; B) Adult cartilage fragment group; C) Juvenile cartilage fragment group; D) Juvenile + Adult cartilage fragment group.
Microscopic evaluation
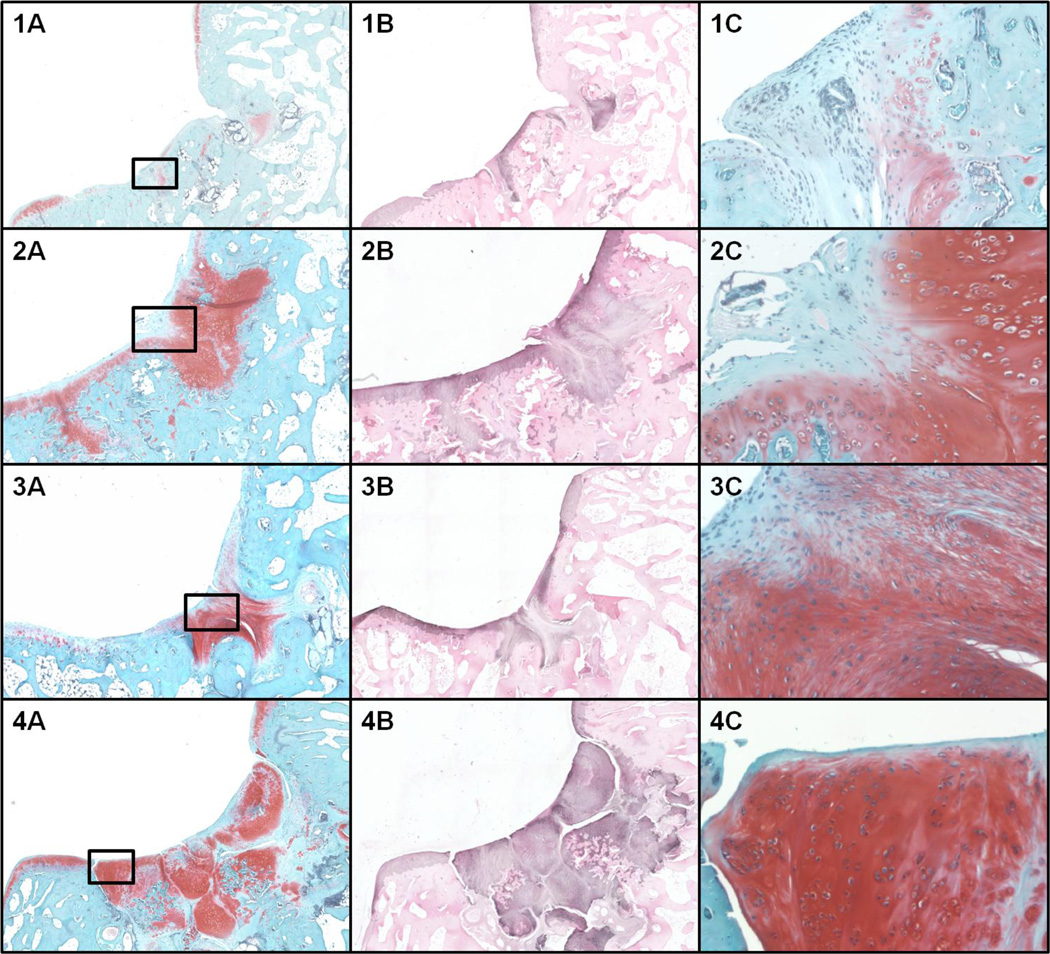
Subchondral bone was minimally restored in all groups (Figure 4). The repair tissue of control groups was mainly formed by fibrocartilage. The cases treated with isolated adult and isolated juvenile cartilage fragments presented some hyaline like features, but showed incomplete filling of the defects, surface fissures and some cysts. The cases treated with mixed adult- juvenile cartilage fragments presented hyaline like features (nearly normal Safranin O staining, complete filling of the defect), but also some chondrocyte clusters and surface lamination/fissures. No inflammatory reaction was evident in the groups. The modified O’Driscoll score showed that the cases treated with mixed adult- juvenile cartilage fragments performed better than the control groups (p= 0.003) and the groups treated with adult fragments only (p= 0.004). In addition, the juvenile fragment group showed higher modified O’Driscoll score than the controls (p=0.03).
Figure 4.
Microscopic evaluation at 6 months. 1) control; 2) Adult cartilage fragment group; 3) Juvenile cartilage fragment group; 4) Juvenile + Adult cartilage fragment group. A) Safranin-O staining at 4X magnification. B) Collagen type II immune-staining at 4X magnification. C) Safranin-O staining at 10X magnification of the area selected in panel A.
Immunohistochemistry
The Collagen type II Immunohistochemistry scoring system showed significantly higher scores in the groups treated with mixed adult- juvenile cartilage fragments compared to the control groups (p<0.001). No other significant differences were observed between the groups (Figure 4).
Discussion
The most important finding of the present study was that the null hypothesis was rejected, and the use of combined autologous adult / allogenic juvenile chondral fragments achieved good results, in terms of modified O’Driscoll score and Collagen type II production.
The concept of using autologous adult chondral fragments as a single-stage procedure for cell-based cartilage repair has been described several years ago [3]. More recently, some very well designed studies showed promising results with this technique in animal models and clinical trials. Lu et al. demonstrated hyaline-like repair tissue in the treatment with cartilage fragments of chondral defects in and large (goats) weight-bearing animals [18]. Frisbie et al. showed on a horse model that single-staged autologous cartilage fragments repair produced arthroscopic, histological, and immunohistochemistry results similar to ACI [12]. Cole et al. in a randomized controlled trial compared autologous cartilage fragment repair with microfracture in 29 patients with two year follow-up. The authors described significantly higher International Knee Documentation Committee (IKDC) score and Knee injury and Osteoarthritis Outcome Score (KOOS) for the autologous cartilage fragments group, compared to the microfracture group. The authors concluded that the new technique is a safe, feasible, and effective method that may improve long-term clinical outcomes for the treatment of focal chondral defects [6].
The chondrogenic potential of juvenile chondrocytes has been previously described. Adkisson et al. showed that juvenile chondrocytes, when maintained in static culture under defined serum-free conditions, deposited an extracellular matrix in the form of tissue disks, that showed collagenous matrices characteristic of articular cartilage from human infants [1]. It has also been shown that juvenile human chondrocytes have greater potential to restore articular cartilage than adult cells and that allogeneic juvenile chondrocytes do not stimulate immunologic responses in vivo [2]. In addition, unlike adult osteochondral grafts, juvenile grafts showed complete integration of cartilage at the graft/host junction in adult animals [27]. Comparable findings have been reported in animals with implantation of cell-based constructs prepared using juvenile articular chondrocytes [4, 23, 24]. Although juvenile chondral fragments are considered a minimally manipulated tissue (therefore exempted from FDA approval) and some preliminary clinical safety reports are available for the treatment of chondral lesions in the knee and ankle, the literature is still sparse regarding this topic [8, 13, 20–22, 28].
Co-culture has proven to be a powerful in vitro tool in unraveling the importance of cellular interactions during normal physiology, homeostasis, repair and regeneration [14]. In cartilage tissue engineering chondrocytes have been co-cultured with different sources of cells (i.e. synovial cells, osteoblasts, mesenchymal stem cells) showing promising results [7, 14].
To our best knowledge, only one in vitro study showed the results of co-culturing adult and juvenile chondrocytes or chondral fragments. The authors mixed human adult chondrocytes with human juvenile chondrocytes at different proportions (100, 50, 25, 12.5 and 0 %) in co-cultures for 6 weeks. The proteoglican content, safranin-O positive cells, and lactate production declined logarithmically with increasing proportions of adult cells, indicating a negative interaction between the two sources. The authors concluded that extracellular matrix production of juvenile chondrocytes was inhibited by adult chondrocytes. In the same study, opposite results were described with co-cultures (1:1) of human adult cartilage fragments and human juvenile cartilage fragments compared with juvenile and adult isolated cultures. The authors found that, using cartilage fragments, the matrix production of adult chondrocytes was stimulated by juvenile chondrocytes [5].
To our knowledge, the present study is the only one in the English literature describing in vivo the results of repairing chondral lesion with isolated juvenile cartilage fragments and combined autologous adult / allogeneic juvenile cartilage fragments. The mechanisms of the positive interaction between adult and juvenile cartilage fragments are still unknown. It has been speculated that manual fragmentation of the cartilage provokes an injury response, with secretion of chemokines and other factors. While adult chondrocytes are more involved in chemokine secretion, juvenile chondrocytes are more responsive to chemotactic factors than their adult counterparts [5]. As an effect, in the presence of injured adult cartilage, large numbers of juvenile cells migrate out of the original minced tissue, proliferate, and differentiate to form neocartilage. Theoretically, less neocartilage is produced in the groups treated only with juvenile fragments because of the relative absence of chemotactic factors from adult fragments [5].
The main limitation of this study is related to the rabbit model. The reparative potential of the rabbit is high, compared to humans, and focal chondral injuries are likely to undergo spontaneous repair, even in the control groups. To overcome this drawback, osteochondral lesions were performed in all samples. In this fashion, we could assess the in vivo behavior and repair tissue quality of mixed juvenile and adult chondral fragments. Therefore, these results need to be confirmed in large animal models and clinical trials. However, minimal restoration of the subchondral bone lowered the histological scores in all groups. As a second limitation, the Collagen type II immunostaining scoring is underpowered. However, as readers know, this means only that possible significant differences between the groups might have been unrecognized by the tests and not that the significant differences found have to be taken with caution.
Although the efficacy of this “one-stage” technique has yet to be verified in large animal models and clinical trials, mixing autologous adult and allogenic juvenile cartilage fragments seem to improve the quality of cartilage repair. In addition, in the clinical setting, covering large defects with autologous fragments can be challenging, due to the limited amount of autograft. Adding an off-the-shelf, juvenile, low manipulated, cartilage fragment source can overcome this problem without raising ethical issues. Repairing cartilage defects only with allogenic juvenile fragments is an alternative option with no donor site morbidity, but more costly and with the risk of immature cartilage repair [1].
Conclusions
In conclusion, mixing adult and juvenile cartilage fragments improved cartilage repair in a rabbit model, compared with the control and isolated adult fragment groups. The promising results of the present study proved the concept of a new “one-stage” surgical procedure for cartilage repair, directly combining “in situ” autologous and allogenic juvenile cartilage fragments, as a viable source of cells. Further studies on larger animals are required to confirm the effectiveness of this technique, before clinical trials.
Acknowledgements
The study was funded with US DHHS, National Institutes of Health/NIAMS grant #1 P50 AR055533
Footnotes
The study was performed at the University of Iowa Hospitals and Clinics, Iowa City, IA, USA
References
- 1.Adkisson HD, Gillis MP, Davis EC, Maloney W, Hruska KA. In vitro generation of scaffold independent neocartilage. Clin Orthop Relat Res. 2001;391:S280–S294. doi: 10.1097/00003086-200110001-00026. [DOI] [PubMed] [Google Scholar]
- 2.Adkisson HD, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38:1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht F, Roessner A, Zimmermann E. Closure of osteochondral lesions using chondral fragments and fibrin adhesive. Arch Orthop Trauma Surg. 1983;101:213–217. doi: 10.1007/BF00436773. [DOI] [PubMed] [Google Scholar]
- 4.Aston JE, Bentley G. Repair of articular surfaces by allografts of articular and growth-plate cartilage. J Bone Joint Surg Br. 1986;68:29–35. doi: 10.1302/0301-620X.68B1.3941138. [DOI] [PubMed] [Google Scholar]
- 5.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39:2355–2361. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole BJ, Farr J, Winalski CS, Hosea T, Richmond J, Mandelbaum B, et al. Outcomes after a single-stage procedure for cell-based cartilage repair: a prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 7.Cooke ME, Allon AA, Cheng T, Kuo AC, Kim HT, Vail TP, et al. Structured three-dimensional co-culture of mesenchymal stem cells with chondrocytes promotes chondrogenic differentiation without hypertrophy. Osteoarthritis Cartilage. 2011;19:1210–1218. doi: 10.1016/j.joca.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farr J, Yao JQ. Chondral defect repair with particulated juvenile cartilage allograft. Cartilage. 2011;2:346–353. doi: 10.1177/1947603511405838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feder J, Adkisson HD, Kizer N, Hruska KA, Cheung R, Grodzinsky AJ, et al. The promise of chondral repair using neocartilage. In: Sandell LJ, Grodzinsky AJ, editors. Tissue Engineering in Musculoskeletal Clinical Practice. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2004. pp. 219–226. [Google Scholar]
- 10.Foldager CB. Advances in autologous chondrocyte implantation and related techniques for cartilage repair. Dan Med J. 2013;60:B4600. 2013. [PubMed] [Google Scholar]
- 11.Frisbie DD, Bowman S, Colhoun HA, DiCarlo EF, Kawcak CE, McIlwraith CW. Evaluation of autologous chondrocyte transplantation via a collagen membrane in equine articular defects: results at 12 and 18 months. Osteoarthritis Cartilage. 2008;16:667–679. doi: 10.1016/j.joca.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Frisbie DD, Lu Y, Kawcak CE, DiCarlo EF, Binette F, McIlwraith CW. In vivo evaluation of autologous cartilage fragment-loaded scaffolds implanted into equine articular defects and compared with autologous chondrocyte implantation. Am J Sports Med 37 Suppl. 2009;1:71S–80S. doi: 10.1177/0363546509348478. [DOI] [PubMed] [Google Scholar]
- 13.Hatic SO, 2nd, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3:361–364. doi: 10.1177/1938640010388602. [DOI] [PubMed] [Google Scholar]
- 14.Hendriks J, Riesle J, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med. 2007;1:170–178. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- 15.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 16.Jang KM, Lee JH, Park CM, Song HR, Wang JH. Xenotransplantation of human mesenchymal stem cells for repair of osteochondral defects in rabbits using osteochondral biphasic composite constructs. Knee Surg Sports Traumatol Arthrosc. 2014;22:1434–1444. doi: 10.1007/s00167-013-2426-y. [DOI] [PubMed] [Google Scholar]
- 17.Lind M, Larsen A. Equal cartilage repair response between autologous chondrocytes in a collagen scaffold and minced cartilage under a collagen scaffold: an in vivo study in goats. Connect Tissue Res. 2008;49:437–442. doi: 10.1080/03008200802325037. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, et al. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 19.Marmotti A, Bruzzone M, Bonasia DE, Castoldi F, Rossi R, Piras L, Maiello A, Realmuto C, Peretti GM. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20:2590–2601. doi: 10.1007/s00167-012-1920-y. [DOI] [PubMed] [Google Scholar]
- 20.McCormick F, Yanke A, Provencher MT, Cole BJ. Minced articular cartilage—basic science, surgical technique, and clinical application. Sports Med Arthrosc. 2008;16:217–220. doi: 10.1097/JSA.0b013e31818e0e4a. [DOI] [PubMed] [Google Scholar]
- 21.McNickle AG, Provencher MT, Cole BJ. Overview of existing cartilage repair technology. Sports Med Arthrosc. 2008;16:196–201. doi: 10.1097/JSA.0b013e31818cdb82. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Garrido C, Gold SL, Snikeris J, Burge A, Nguyen J, Potter HG, Warren RF, Williams RJ, Rodeo SA. Magnetic Resonance Imaging and Clinical Evaluation of Chondral lesions treated with Allografts Juvenile Cells. Orthop J Sports Med. 2013;1(4) suppl 1 [Google Scholar]
- 23.Perka C, Schultz O, Lindenhayn K, et al. Joint cartilage repair with transplantation of embryonic chondrocytes embedded in collagenfibrin matrices. Clin Exp Rheumatol. 2000;18:13–22. [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10S–19S. doi: 10.1177/0363546509350694. [DOI] [PubMed] [Google Scholar]
- 26.Saris DBF, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 27.Specchia N, Gigante A, Falciglia F, Greco F. Fetal chondral homografts in the repair of articular cartilage defects. Bull Hosp Jt Dis. 1996;54:230–235. [PubMed] [Google Scholar]
- 28.Tompkins M, Adkisson HD, Bonner KF. DeNovo NT Allograft. Oper Tech Sports Med. 2013;21:82–89. [Google Scholar]
- 29.van den Borne MP, Raijmakers NJ, Vanlauwe J, Victor J, de Jong SN, Bellemans J, et al. International Cartilage Repair Society. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage. 2007;15:1397–1402. doi: 10.1016/j.joca.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]