Abstract
BACKGROUND
Invasive breast carcinoma has a more aggressive phenotype and a higher mortality rate in African American (AA) than in Caucasian American (CA) women. The characteristics of ductal carcinoma in situ (DCIS) in the AA population have not been extensively studied.
METHODS
The authors reviewed cases of DCIS diagnosed in AA and CA patients between 1996 and 2000 at their institution. Treatment and outcome were obtained from the clinical charts and the Surveillance, Epidemiology, and End Results database. They identified 217 AA (61%) and 141 CA (39%) patients.
RESULTS
AA women were significantly older at diagnosis (61 years vs 56 years, P = .001), and the size of the tumor was larger in AA patients (P = .001). The other pathological features examined were not statistically different between the 2 groups. Treatments with surgery and radiation were also similar. However, the CA patients were more likely to receive hormone therapy. Recurrence rate as DCIS or invasive carcinoma was similar in both patient groups, as was death due to disease. Time to recurrence with invasive carcinoma, however, was shorter for AA patients (32.8 ± 13 vs 58 ± 9; P = .02). Only overall survival (OS) rate was higher for CA patients (92% vs 71% at 10 years; P = .003).
CONCLUSIONS
Unlike invasive carcinoma, DCIS is diagnosed at a later age in AA patients. Except for larger size, DCIS does not have a more aggressive histology in AA patients. Treatment and recurrence rate were similar in both groups, as was death due to breast cancer. OS, however, was worse in AA women.
The incidence, morphology, and outcome of invasive breast carcinoma in African American (AA) women differ significantly from invasive breast carcinoma in Caucasian American (CA) women. Although the overall incidence of breast cancer is higher among CA women, AA women are more often diagnosed at a younger age; 20% of CA breast cancer patients are <50 years of age, compared with 30% to 40% of AA patients.1 In addition, AA women are more frequently diagnosed with higher-stage carcinomas, and the carcinomas are significantly more likely to be high grade, hormone receptor negative, aneuploid, and lymph node positive. These latter patterns persist even after controlling for stage and age.2 Mortality related to breast cancer in AA women is higher than in CA patients, particularly in younger age groups.3 These differences are most likely related to a combination of factors, including socioeconomic status, reproductive history, lifestyle experiences, and genetic factors.
A better understanding of the precursors to invasive breast cancer may help elucidate the mechanisms underlying the differences observed in invasive breast carcinoma in AA and CA women. The characteristics of ductal carcinoma in situ (DCIS) in the AA population are, however, poorly described, and there have been few comparisons of DCIS in AA patients with DCIS in CA patients. DCIS is a well-described precursor lesion of invasive carcinoma, currently representing 7% to 13% of the total cases of breast cancer according to data from 9 Surveillance, Epidemiology, and End Results (SEER) registries.4 This proportion has increased significantly in the United States with the widespread adoption of screening mammography in 1980.5 Few authors have previously reported differences in the incidence of DCIS among various racial/ethnic groups.6,7 However, possible differences in the pathological features of DCIS and the outcome of patients with this diagnosis among AA and CA women have not been addressed. We describe the clinical and pathologic features, treatment, and outcome of AA and CA patients diagnosed with DCIS at our institution, where half of the population seeking breast care is AA.
MATERIALS AND METHODS
After approval by the institutional review board at Wayne State University, we retrospectively identified women newly diagnosed with DCIS (not associated with invasive carcinoma) between the years 1996 and 2000 (inclusive) at the Detroit Medical Center/Wayne State University (DMC/WSU) and the Karmanos Cancer Institute (KCI). We excluded from the study patients who had a previous diagnosis of invasive breast carcinoma and patients who developed an invasive breast carcinoma within 6 months of the diagnosis of DCIS. We also excluded cases of DCIS with microinvasive carcinoma, defined as the largest focus of invasion measuring ≤1.0 mm.8 Cases of lobular carcinoma in situ were not included if they were not associated with DCIS.
For each patient, we retrieved the hematoxylin and eosin–stained sections from every breast biopsy/resection specimen performed for the diagnosis or treatment of the DCIS. The available sections were reviewed to assess the following pathological parameters related to DCIS: nuclear grade (l or low, 2 or intermediate, 3 or high), histologic type (cribriform, micropapillary, solid, comedo, papillary, and mixed), central necrosis, extension into lobules, presence of microinvasion, and presence and location of microcalcifications (in DCIS vs in benign breast tissue).9 Margin status on the final (or last) procedure performed for the treatment of DC IS was also noted; it was classified as positive if the DCIS was seen at the resection margin, close if the DCIS was <2.0 mm away from the margin, and negative if DCIS is seen >2.0 mm away from the margin. Estrogen receptor (ER) and progesterone receptor (PR) status, when available, was retrieved from the pathology reports. Immunohistochemistry was used to assess ER (6F11, Vector Laboratories Ltd, Burlingame, Calif) and PR (PgR636, DAKO Corporation, Calif) status; it was considered strongly positive if labeling was present in >10% of the DCIS nuclei, weakly positive if there was 1% to 10% nuclear labeling, and negative if 0% to 1% labeling was seen. The size of the DCIS was estimated from the main surgical procedure. In cases where DCIS formed a tumor seen on gross examination, the size of the DCIS was considered to be the gross tumor size. When DCIS was only seen microscopically, the size was estimated as the number of consecutive sections with DCIS × 4.0 mm (average section thickness) or the size of the largest focus on 1 slide (if the latter is estimated to be larger than that of consecutive sections). We also reviewed sections from lymph nodes when a sentinel lymph node biopsy or lymph node dissection was available.
Demographic, clinical, and follow-up information were obtained from the hospital medical records and the SEER database. The Detroit SEER Program includes all newly diagnosed cancer cases in residents of the Detroit Metropolitan Area, and provides active follow-up on all living patients; the latter is conducted annually to assess current vital status. The data collected by SEER for all cases of cancer include patient demographics, type of cancer, tumor characteristics, extent of disease at the time of diagnosis, and type of treatment received for the first course of therapy. The DMC/WSU and KCI hospital medical records (and pathology reports) were available electronically since 1996. Mammography studies, however, were available for patients diagnosed after 1998. The information collected included age at diagnosis, ethnicity, family history of breast cancer, clinical presentation, mammographic studies leading to the diagnosis of DCIS, treatment (hormone therapy, radiation, and chemotherapy), local recurrence data, and survival status. Family history of breast cancer was classified as strong if 1 first-degree relative <50 years of age had breast cancer or if 2 or more relatives had breast cancer with at least 1 being a first-degree relative. Any other positive family history of breast cancer was classified as weak. For local recurrence, we specified if it presented as DCIS or as invasive carcinoma, and if it occurred in the ipsilateral or contralateral breast.
Only women of AA and CA racial origins were included in the study. We studied the pathological and clinical parameters related to DCIS in relation to race (AA vs CA). Continuous data were analyzed using Student independent sample t test (ie, age at diagnosis), and Fisher exact and chi-square tests for categorical measures. Kaplan-Meier survival analyses were used to compare disease-free and overall survival times between groups. Significance values of P < .05 were considered statistically significant. All data were analyzed using SPSS v.15.0 for Windows (SPSS Inc., Chicago, Ill).
RESULTS
Clinical and Demographic Features
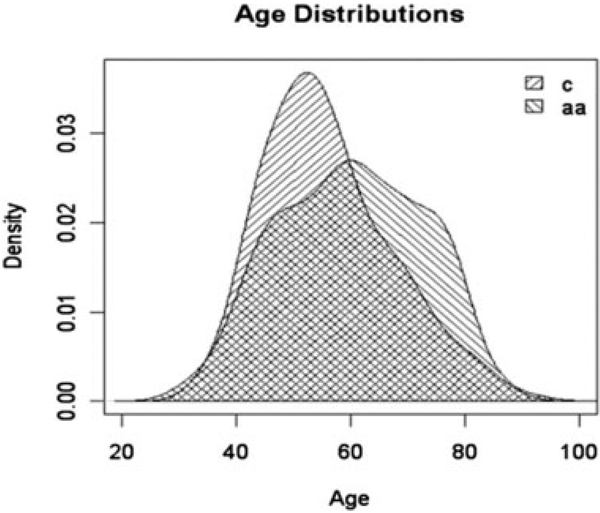
We identified 370 patients newly diagnosed with DCIS at our institution between 1996 and 2000. Twelve (3.2%) patients were either of unknown ethnicity, Hispanic, or Asian and were excluded from the study. Of the other 358 patients who were included, 217 were AA (61%) and 141 were CA (39%); their clinical and demographic features are summarized in Table 1. The AA patients were significantly older at the time of diagnosis than CA patients (mean 61 ± 13 vs 56 ± 11; P = .001). The age distributions were different between the 2 ethnic groups (Fig. 1). Among AA women, 41% were >64 years of age at diagnosis, compared with only 21% of the CA patients (P = .001).
Table 1.
Clinical and Demographic Features of the Patient Population
| AA | CA | P | |
|---|---|---|---|
| No. of patients (%) | 217 (61) | 141 (39) | — |
| Age at diagnosis, y | 60 ± 12 | 56 ± 11 | .001 |
| Clinical presentation, No. available | 82 | 54 | NS |
| Symptoms | 13 | 5 | |
| No symptoms | 69 | 49 | |
| Screening mammography, No. available | 69 | 49 | NS |
| Calcifications | 61 | 44 | |
| Mass/density | 8 | 5 | |
| Family history, No. available | 72 | 47 | NS |
| Strong | 21 | 11 | |
| Weak | 16 | 16 | |
| Negative | 35 | 20 |
AA indicates African American; CA, Caucasian American, NS, not significant.
FIGURE 1.
Age distribution in years for Caucasian American (c) and African American (aa) women with ductal carcinoma in situ in the current study.
Information on the clinical presentation of DCIS and mammographic data were available for 136 patients, 82 AA and 54 CA, representing 38% of the AA patients and 38% of the CA patients. Thirteen (16%) AA patients presented either with a breast mass (n = 6), nipple discharge (n = 4), or breast pain (n = 3). Five (9%) CA patients were symptomatic (2 had a breast mass, 1 had nipple discharge, and 2 presented with breast pain). In the remaining patients (69 AA and 49 CA), DCIS was diagnosed after a routine screening mammography. Mammography showed most often microcalcifications (88% of the cases overall) or a density/mass (12% of the cases). The frequency of clinical symptoms leading to the diagnosis of DCIS did not differ significantly between the 2 racial groups. The AA patients who presented with symptoms were younger than the AA patients who did not (mean age of 53 ± 13 years vs mean age of 61.7 ± 12 years; P = .02), whereas CA patients who were symptomatic had a mean age of 55 ± 5 years, similar to non-symptomatic patients (56.8 ± 11); however, the numbers are small for statistical comparison (only 5 symptomatic CA patients). DCIS was bilateral in 4 (1.8%) AA patients and in 3 (2.1%) CA patients.
The presence or absence of family history of breast cancer was noted in the charts of 119 patients, 72 AA and 47 CA (representing 33% of patients in each group). In the remaining cases, family history was not mentioned, and those patients were not counted in the analysis. Overall, 32 patients (21 AA and 11 CA) had a strong family history and 32 patients (16 AA and 16 CA) had a weak family history of breast cancer. For 65 patients (35 AA and 20 CA), family history of breast cancer was negative. No significant association was found between family history of breast cancer and race (P = not significant [NS]). The AA patients and the CA patients with family history of breast cancer (weak and strong) were younger (57.6 ± 10 years vs 61.6 ± 13 years for AA, and 50.4 ± 9 years vs 56 ± 9 years for CA) than the patients with no family history of breast cancer (P = .06 and .01, respectively).
Pathological Features
The DCIS was low grade in 121 (34.7%) patients, intermediate grade in 127 (36.4%), and high grade in 101 (28.9%). In 190 (58.8%) cases, central necrosis was present. The architectural type was mixed in 123 (34.9%) tumors, solid in 111 (31.5%), cribriform in 78 (22.2%), micropapillary in 29 (8.2%), and papillary in 9 (2.6%). Cancerization of the lobules by DCIS was present in 69 (23.5%) cases. ER status was available for 18 DCIS lesions, and PR status was available for 16; ER was positive in 67% of these tumors (n = 12) and PR in 43.8% (n = 7). The mean tumor size was 1.56 cm (0.1–15.0 cm). Three (0.8%) patients had lymph node metastasis, 2 AA and 1 CA. Two cases were micrometastases (<2.0 mm) and 1 case was a macrometastasis. Microcalcifications were identified in the DCIS in 210 (64.2%) cases and only in benign breast tissue in 82 (25.1%) cases.
Overall, patients who presented with clinical symptoms (n = 18) compared with patients who were diagnosed after screening mammography (n = 118), had larger tumors (2.9 cm vs 1.3 cm, P = .0002), and had fewer microcalcifications (50% vs 92%, P = .0001). We did not compare the different parameters between AA and CA patients who were symptomatic because of the small number of patients in each group (13 and 5 patients, respectively).
Overall, there was no difference in the pathological features of the DCIS between patients with family history and patients without known family history of breast cancer (T size 1.67 vs 1.86 cm; 30% vs 27% grade III; 61% vs 57% with central necrosis; 52% vs 67% with microcalcifications in DCIS).
The pathological features of DCIS in AA and CA patients are summarized in Table 2. A comparison of the pathological features of DCIS in the AA and CA populations revealed that the tumors were larger in size in the AA patients as compared with the tumors from CA patients, with a mean size of 1.83 cm (0.1–15.0 cm) and 1.15 cm (0.1–5.5 cm), respectively (P = .001). There was no difference in the grade, architectural pattern, central necrosis, and cancerization of lobules between the 2 patient populations (Table 2). Microcalcifications were seen at the same frequency in AA (n = 177; 89.8%) and CA patients (n = 115; 88.5%); however, more DCIS-related calcifications were seen in AA (n = 138; 70%) than CA (n = 72; 55%) patients, and more benign breast tissue–related calcifications were seen in CA (n = 43; 19.8%) than AA (n = 39; 33.1%) patients (P = .001).
Table 2.
Pathological Features of DCIS in AA and CA Patients
| AA | CA | P | |
|---|---|---|---|
| Tumor Size, mean, cm | 1.83 | 1.15 | .001 |
| Grade (%) | NS | ||
| I | 78 (37) | 43 (31) | |
| II | 80 (38) | 47 (34) | |
| III | 53 (25) | 48 (35) | |
| Architectural pattern (%) | NS | ||
| Mixed | 79 (37) | 44 (32) | |
| Solid | 62 (29) | 49 (35) | |
| Cribriform | 45 (21) | 33 (24) | |
| Micropapillary | 20 (9) | 9 (7) | |
| Papillary | 7 (3) | 2 (1) | |
| Extension into lobules (% cases) | 43 (24) | 26 (23) | NS |
| Central necrosis (% cases) | 107 (55) | 83 (63) | NS |
| Microcalcifications (%) | .001 | ||
| In DCIS | 138 (70) | 72 (55) | |
| In benign tissue | 39 (20) | 43 (33) | |
| Absent | 20 (10) | 15 (12) | |
| Lymph node metastases, No. of cases | 2 | 1 | — |
AA indicates African American; CA, Caucasian American; NS, not significant; DCIS, ductal carcinoma in situ.
Treatment
The different types of treatments received by our patient population are summarized in Table 3. There was no difference in the number of mastectomies between AA (n = 53; 25%) and CA (n = 45; 32%) patients. Among AA patients, 119 (49%) had excisional biopsy (1 or more) followed by radiation therapy, compared with 67 (55%) CA patients (P = NS). The rest of the patients (44 AA and 26 CA) had excisional biopsy without radiation (P = NS). Within the AA group, the final margin status was positive in 18 (8.4%) patients, close in 49 (22.8%), and negative in 148 (68.8%). Within the CA group, the final margin status was positive, close, or negative in 5 (3.6%), 24 (17.4%), and 109 (79%), respectively. The difference in the final margin status was not significantly different among the 2 ethnic groups. More CA patients received hormonal therapy (44 [31%] CA vs 38 [18%] AA; P = .04). Chemotherapy was given to 2 AA patients who had positive lymph nodes.
Table 3.
Treatment of AA and CA Patients With DCIS
| AA (%) | CA (%) | P | |
|---|---|---|---|
| Mastectomy | 53 (25) | 45 (32) | NS |
| Excisional biopsy + radiation | 119 (55) | 67 (49) | NS |
| Excisional biopsy without radiation | 44 (20) | 26 (19) | NS |
| Hormone therapy | 38 (18) | 44 (31) | .04 |
| Chemotherapy | 2 | 0 | — |
AA indicates African American; CA, Caucasian American; DCIS, ductal carcinoma in situ; NS, not significant.
Follow-up
Follow-up time ranged from 3 to 138 months, with a mean follow-up of 85 ± 24 months. AA and CA patients had similar rates of tumor recurrence with DCIS or with invasive carcinoma (Table 4). Recurrence with DCIS occurred in 5.1% (n = 11) of the AA patients and 4.2% (n = 6) of the CA patients (P = NS), with a mean time to recurrence of 37.1 ± 25 months and 31.8 ± 20 months, respectively (P = NS). Recurrence with invasive carcinoma occurred in 6.0% (n = 13) of AA patients and in 3.5% (n = 5) of CA patients (P = NS), with a mean time to recurrence of 33 ± 13 months and 58 ± 9 months, respectively (P = .02). Recurrence occurred in the contralateral breast in more than half of the cases (57%; 16 of 28 patients with known side of recurrence). There was no correlation between the side of recurrence and race. Recurrence with invasive carcinoma correlated with the size of the DCIS in AA patients but not in CA patients (P = .001). There was no correlation between recurrence with DCIS (without invasion) and the various pathological parameters in AA and CA patients.
Table 4.
Follow-up Data of AA and CA Patients With DCIS
| AA | CA | P | |
|---|---|---|---|
| Recurrence (%) | |||
| DCIS | 11 (5.1) | 6 (4.2) | NS |
| Invasive carcinoma | 13 (6.0) | 5 (3.5) | NS |
| Time to recurrence, mo | |||
| DCIS | 37.1 ± 25 | 31.8 ± 20 | NS |
| Invasive carcinoma | 32.8 ± 13 | 58 ± 9 | .02 |
| DOD | |||
| 5-y follow-up | 4% | 1% | |
| 10-y follow-up | 6% | 2% | NS |
| OS | |||
| 5-y follow-up | 90% | 95% | |
| 10-y follow-up | 71% | 92% | .003 |
AA indicates African American; CA, Caucasian American; DCIS, ductal carcinoma in situ; NS, not significant; DOD, dead of disease; OS, overall survival.
Death because of breast cancer was similar between the 2 ethnic groups. Among AA patients, 10 (6%) died of disease, whereas 1 (2%) CA patient had died of breast cancer at 10 years follow-up. The overall survival rates, however, were significantly worse for AA than for CA patients (71% and 92% at 10 years, respectively; P = .003). In the AA population, 34 patients died for reasons unrelated to breast disease, mostly from cardiovascular diseases (n = 20), whereas 9 CA patients died from various causes unrelated to their breast disease, including heart diseases, cirrhosis, motor vehicle accident, and diabetes.
DISCUSSION
The results from our patient population show that AA women with ductal carcinoma in situ of the breast are significantly older at diagnosis than are CA women. In both racial groups, DCIS is mainly detected by screening mammography, and a small subset of patients are symptomatic at diagnosis. DCIS is also significantly larger in AA women than it is in CA women. The frequency of cases with high-grade cytology and central necrosis is similar between the 2 racial groups, as is the risk of recurrence, time to recurrence, and diseased free survival. Overall survival appears to be worse for AA patients with DCIS than it is for CA patients with DCIS.
Population-based data from different SEER programs show striking differences in invasive breast cancer in relation to race/ethnicity. AA women face a greater risk for being diagnosed with early onset invasive disease, their carcinomas show a more aggressive phenotype, and their mortality rate from breast cancer is significantly higher.10–12 Similar findings were reported in a study of women diagnosed with invasive breast cancer in the Detroit Metropolitan area—where our medical center is located—and identified through the Detroit SEER registry.13 In this study, AA women with invasive carcinoma were more likely than CA women to be younger at diagnosis, with 33% of AA women diagnosed at age 50 years or younger, compared with 24% of CA women. AA women were also more likely to present with larger-diameter invasive cancers, with a higher number of metastases to axillary lymph nodes, more poorly differentiated invasive carcinomas, and a greater proportion of ER- or PR-negative carcinomas. AA patients had a worse breast cancer–specific survival compared with CA women among women with regional breast cancer disease. These racial differences in invasive breast cancer are most likely related to a combination of factors, including biologic and genetic factors, socioeconomic status, and/or factors related to the quality of medical care received.
It is generally accepted that DCIS is a precursor lesion for invasive breast carcinoma; studies involving small numbers of patients as well as large clinical trials showed that if left untreated, DCIS progresses to invasive carcinoma in up to 50% of the cases, usually after years of diagnosis.14,15 Because DCIS progresses to invasive breast cancer, and because the diagnosis of DCIS is mainly dependent on access to screening mammography and proper medical care, we hypothesized that racial differences would be observed in patients with DCIS, just as differences are observed in patients with invasive breast cancer.
We found that in our patient population, AA women with DCIS were significantly older than CA women at diagnosis; 41% of the AA women with DCIS were diagnosed at the age of 65 years or older, whereas 21% of the CA patients were diagnosed in this age group. In keeping with our finding, other studies noted an increase in the diagnosis of DCIS in older AA women.7 A study examining the racial differences in the incidence and treatment of DCIS in women diagnosed in California between the years 1988 and 1999 noted a sudden increase in the age-specific rate of DCIS among AA women around the age of 65 years.7 These results could be explained by the better access to mammography screening for AA women older than 65 years, the age after which Medicare covers 80% of the cost of a mammogram every other year. A detailed analysis of the California registry data, however, did not entirely support the hypothesis of a delayed screening for older AA women. For example, the incidence of small invasive carcinomas measuring <2 cm, the type of invasive cancer most often detected by screening mammography, was not increased in black women older than 65 years of age in their cohort.
Data published in 2006 by the American Cancer Society on the prevalence of mammography by age and state, obtained from the Centers for Disease Control’s 2004 Behavioral Risk Factor Surveillance System, shows that current overall usage of mammography at the national level and in the state of Michigan is similar among CA and AA women (68% of CA and 66% of AA in 1998 and 72% of CA and 68% of AA in 2000, for example, had a least 1 mammogram within the past 2 years).16 When divided by age category, data from the same sources shows no significant difference between AA and CA in regard to mammography utilization within each age category and no increase in mammography use for older AA women (in the year 2000, 67% of CA vs 61% AA in women aged 40–49 years, 80.5% CA vs 78% AA in women aged 50–64 years, and 68% CA vs 65.5% AA in women older than 64 years had a mammogram within the last 2 years). There are no data addressing this issue specifically in the Detroit area. Data from this area, however, show that AA women are not as adequately followed after an abnormal mammogram as are CA women; 34% of CA patients had inadequate follow-up after they had an abnormal mammogram, compared with 49% of AA patients.17 This finding might partially explain the higher frequency of DCIS in older AA women. Data on screening and previous mammograms are not available for our patients. Therefore, whether the difference in age at DCIS diagnosis that we found in our population is because of a delay of diagnosis in our AA patients or whether DCIS more often affects older AA patients, whereas invasive carcinoma is a disease of younger AA women, is a question that remains to be answered.
Two grades of DCIS are recognized. Low-grade DCIS progresses to low-grade invasive cancer, and high-grade DCIS progresses to high-grade invasive carcinoma. 18 Because invasive carcinoma diagnosed in AA women is more often high grade, one might speculate that DCIS would tend to be high grade in the AA population. In our study, the phenotype of the DCIS did not differ between AA and CA women, and aggressive features such as high grade, central necrosis, and the presence of microinvasion associated with DCIS (data not shown) were seen equally in both patient populations. Thus, differences in the grade of DCIS cannot explain the racial differences in the grade of invasive carcinoma.
The reported effect of race on the risk of recurrent disease in women diagnosed with DCIS varies according to different studies. In the study by Li et al., AA women had an increased risk of ipsilateral and contralateral invasive breast cancer when compared with CA patients, and the risk was mainly increased for higher-stage recurrence (stage II and III/IV).19 Gao et al. similarly showed that among women with localized invasive or in situ breast cancer, AA women had an increased risk of contralateral breast cancer compared with CA women.20 Consistent with our results, however, a study from the Connecticut registry found that AA women did not have an increased risk of a second in situ or invasive contralateral breast cancer as compared with other racial groups.21 The finding that in our population the rate of recurrence of DCIS is similar in both racial groups could be explained by the similar pathological features of the DCIS we observed in AA and CA women in addition to the similarity in the local treatment of DCIS in both groups. The rate of mastectomies, excisional biopsy, and radiation therapy was similar in AA and CA women. It is noteworthy that in our sample most women received their care in the same comprehensive cancer center.
We found in our study, however, that AA women recurred with invasive disease significantly earlier than CA women (58 ± 9 vs 32.8 ± 13 months). Moreover, the recurrence with invasive carcinoma in AA patients correlated with the original size of DCIS.
Death related to breast disease (because of recurrence with invasive carcinoma) is also similar in both groups within our population (6% AA vs 2% CA at 10 years). Overall survival, however, appears to be worse in AA patients (71% and 92% at 10 years, respectively; P = .003). Patients in the latter group died mainly of cardiovascular conditions. In fact, a previous study from the same medical center also showed that patients with invasive breast cancer treated at our medical center were more likely to have had a diagnosis of 1 or more comorbid conditions along with their breast cancer.22
Although the absolute number of patients in our study is small, it is the first to describe the features and outcome of DCIS in AA women and compare it with that of CA women. Further larger-scale studies are needed to confirm our findings and explain the higher age at presentation of AA women with DCIS. AA patients in our study recurred with invasive disease earlier than CA patients, and their recurrence was related to the original size of the DCIS. This finding raises the question of a more aggressive follow-up for AA patients after the diagnosis and treatment of DCIS, mainly in cases where the DCIS is large in size. Our study also shows that at least in our population, comorbidities (eg, cardiovascular diseases, diabetes, liver diseases) remain an important factor involved in the death of AA patients with breast diseases.
Footnotes
Conflict of Interest Disclosures
The authors made no disclosures.
References
- 1.Newman LA. Breast cancer in African-American women. Oncologist. 2005;10:1–14. doi: 10.1634/theoncologist.10-1-1. [DOI] [PubMed] [Google Scholar]
- 2.Newman LA, Bunner S, Carolin K, et al. Ethnicity related differences in the survival of young breast carcinoma patients. Cancer. 2002;95:21–27. doi: 10.1002/cncr.10639. [DOI] [PubMed] [Google Scholar]
- 3.Reis L, Eisner M, Kosary M, et al., editors. SEER cancer statistics review, 1975–2000. Bethesda, MD: National Cancer Institute; 2003. [Google Scholar]
- 4.Joslyn SA. Ductal carcinoma in situ: trends in geographic, temporal, and demographic patterns of care and survival. Breast J. 2006;12:20–27. doi: 10.1111/j.1075-122X.2006.00182.x. [DOI] [PubMed] [Google Scholar]
- 5.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. JAMA. 1996;275:913–918. [PubMed] [Google Scholar]
- 6.Simon MS, Lemanne D, Schwartz AG, Martino S, Swanson GM. Recent trends in the incidence of in situ and invasive breast cancer in the Detroit metropolitan area (1975–1988) Cancer. 1993;71:769–774. doi: 10.1002/1097-0142(19930201)71:3<769::aid-cncr2820710320>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Innos K, Horn-Ross PL. Recent trends and racial/ethnic differences in the incidence and treatment of ductal carcinoma in situ of the breast in California women. Cancer. 2003;97:1099–1106. doi: 10.1002/cncr.11104. [DOI] [PubMed] [Google Scholar]
- 8.Green FL, Page DL, Fleming ID, et al., editors. AJCC Staging Manual. 6th ed. New York, NY: Springer; 2002. [Google Scholar]
- 9.The Consensus Conference Committee. Consensus conference on the classification of ductal carcinoma in situ. Cancer. 1997;80:1798–1802. doi: 10.1002/(sici)1097-0142(19971101)80:9<1798::aid-cncr15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Li CI, Malone KE, Daling JR. Differences in breast cancer hormone receptor status and histology by race and ethnicity among women 50 years of age and older. Cancer Epidemiol Biomarkers Prev. 2002;11:601–607. [PubMed] [Google Scholar]
- 11.Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97:134–147. doi: 10.1002/cncr.11051. [DOI] [PubMed] [Google Scholar]
- 12.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Simon MS, Banerjee M, Crossley-May H, Vigneau FD, Noone AM, Schwartz K. Racial differences in breast cancer survival in the Detroit metropolitan area. Breast Cancer Res Treat. 2006;97:149–155. doi: 10.1007/s10549-005-9103-x. [DOI] [PubMed] [Google Scholar]
- 14.Warnberg F, Yuen J, Holmberg L. Risk of subsequent invasive breast cancer after breast carcinoma in situ. Lancet. 2000;355:724–725. doi: 10.1016/S0140-6736(99)03703-4. [DOI] [PubMed] [Google Scholar]
- 15.Sanders ME, Schuyler PA, Dupont WD, Page DL. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 16.Smigal C, Jemal A, Ward E, et al. Trends in breast cancer by race and ethnicity: update 2006. CA Cancer J Clin. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy BD, Yood MU, Boohaker EA, Ward RE, Rebner M, Johnson CC. Inadequate follow-up of abnormal mammograms. Am J Prev Med. 1996;12:282–288. [PubMed] [Google Scholar]
- 18.Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO. Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol. 2008;32:513–523. doi: 10.1097/PAS.0b013e318161d1a5. [DOI] [PubMed] [Google Scholar]
- 19.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 1988–2001. Cancer. 2006;106:2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: a population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 21.Claus EB, Stowe M, Carter D, Holford T. The risk of a contralateral breast cancer among women diagnosed with ductal and lobular breast carcinoma in situ: data from the Connecticut tumor registry. Breast. 2003;12:451–456. doi: 10.1016/s0960-9776(03)00152-8. [DOI] [PubMed] [Google Scholar]
- 22.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I–III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243–248. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]