Abstract
Meningoencephalitis caused by Cryptococcus neoformans (Cn) has become one of the leading causes of mortality in AIDS patients. Understanding the interactions between Cn and phagocytes is fundamental in exploring the pathogenicity of cryptococcal meningoencephalitis. Cn may be extracellular or contained in the monocytes, macrophages, neutrophils, dendritic cells and even endothelial cells. The internalized Cn may proliferate inside the host cells, or cause the lysis of host cells, or leave the host cells via non-lytic exocytosis, or even hijack the host cells (Trojan horse) for the brain dissemination, which are regulated by microbe factors and also immune molecules. Coexistence of protective and deleterious roles of phagocytes in the progression of cryptococcosis warrant further investigation.
Keywords: Cryptococcus neoformans, Macrophage, Neutrophil, Dendritic cell, Endothelial cell
Background
Cn has been co-evolved with the phagocyte predators, e.g., amoebas (Chrisman et al. 2010), paramecium (Frager et al. 2010), or nematodes (Casadevall et al. 2003), for a long history. As mammalian phagocytes may originate from the common ancestors, it is plausible to speculate that roles of host phagocytes against Cn manifest the complex interactions between fungi and phagocyte predators (Chrisman et al. 2010). Ideally, predators and their prey fight with each other and maintain the fine balance of nature, implying that neither the prey (Cn) nor the predators (e.g., amoeba, or host phagocytes) would be totally extinguished. Therefore, in the host both Cn and phagocytes would survive, leading to a latent infection, as evidenced by the recent research (Alanio et al. 2015).
Cryptococcal meningoencephalitis occurs only when Cn leaves the infected lung, transmigrates across the blood–brain-barrier (BBB) and proliferates in the brain parenchyma. As Cn is a facultative intracellular pathogen, it is speculated that the transmigrating Cn might be extracellular or within some phagocytes, thereby invading the central nervous system (CNS) via a trans-cellular pathway or Trojan horse pathway (Casadevall 2010). In the trans-cellular pathway, Cn is directly internalized by brain endothelial cells via endocytosis. In the Trojan horse pathway, some phagocytes carrying Cn enter the CNS. High-affinity Fcγ receptor 3A promotes the phagocytosis and significantly contributes to the cryptococcal meningoencephalitis (Rohatgi et al. 2013). Moreover, effective phagocytosis of Cn by macrophages counterintuitively predisposes to poor outcome (Sabiiti et al. 2014), confirming the link between phagocytosis of Cn and the high mortality in patients with cryptococcal meningoencephalitis (Alanio et al. 2011). Together, these data suggest that phagocytes may help Cn invade the CNS. In this review, the interactions between Cn and phagocytes (monocytes, macrophages, neutrophils, dendritic cells, and endothelial cells) are discussed.
Monocytes and macrophages
Circulating Cn could be detected in monocytes collected from peripheral blood or located in monocytes in the leptomeningeal capillaries. Besides, Cn also could be observed in macrophages in the leptomeningeal space, implying that monocytes and macrophages may play crucial roles in the pathogenesis of cryptococcal meningoencephalitis (Chretien et al. 2002). The outcomes of Cn interacting with macrophages include at least phagocytosis, replication, and non-lytic exocytosis (Coelho et al. 2014; Garcia-Rodas and Zaragoza 2012; Leopold Wager and Wormley 2014; McQuiston and Williamson 2012), implicating the existence of Trojan horse pathway for the brain dissemination (Casadevall 2010; Charlier et al. 2009) (Fig. 1).
Fig. 1.
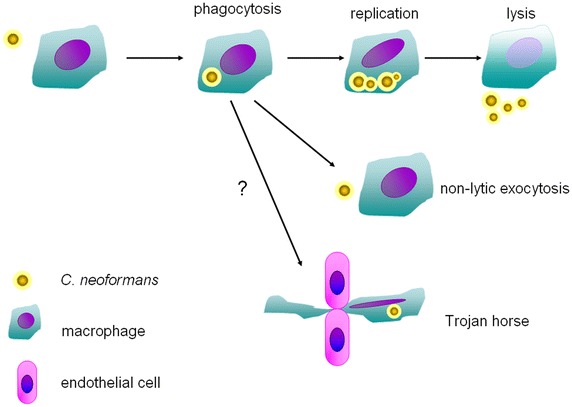
Roles of monocytes in the Cn pathogenesis. Upon infection, monocytes internalize and kill the Cn. However, Cn could also proliferate in the monocytes and escape the monocytes via the host cell lysis or non-lytic exocytosis. It is speculated that monocyte may also work as Trojan horse in the Cn brain dissemination.
Phagocytosis
Phagocytosis of Cn by macrophages is mediated by diverse factors, including complement proteins, specific antibodies, surfactant protein D (Geunes-Boyer et al. 2009, 2012) or the scavenger receptors SCARF1 and CD36 (Means et al. 2009). IgM and IgA specific to the capsular glucuronoxylomannan (GXM) promote complement-independent and CD18-dependent phagocytosis (Taborda and Casadevall 2002). Phagocytosis of Cn by lung macrophages is significantly impaired in the sIgM deficient mice (Subramaniam et al. 2010). Different from IgG1, IgM and IgA, IgG3-mediated phagocytosis is not associated with FcγR and CD18 (Saylor et al. 2010). In contrast, antiphagocytic protein 1 (App1) from Cn, binding with CR2/CR3, inhibits the phagocytosis of macrophages (Stano et al. 2009; Williams and Del Poeta 2011).
Replication
As a facultative intracellular pathogen, Cn replicates in and alkalifies the phagosome of macrophages, leading to phagosome breakage and macrophage lysis (Tucker and Casadevall 2002). Replication of Cn inside the macrophages requires F-box protein 1 and its substrate inositol phosphosphingo lipid-phospholipase C1 (Liu and Xue 2014). In addition, Cn phospholipase B1 (PLB1) promotes the survival of fungi in the macrophages by facilitating fungal eicosanoid production (Noverr et al. 2003). In addition, Cn proliferation may stimulate the abortive mitosis (Coelho et al. 2012) of some macrophages (Luo et al. 2005). Mechanisms behind the balance of Cn replication and macrophage lysis/mitosis, however, still remain elusive. Interestingly, a recent study on the dynamics of interactions between Cn and macrophages suggested that fungal background influences outcome during cryptococcal meningoencephalitis in humans (Alanio et al. 2011).
Non-lytic exocytosis
Besides breaking down the host macrophage, Cn could also escape from macrophages through non-lytic exocytosis or phagosome extrusion in vitro (Alvarez and Casadevall 2006; Ma et al. 2006) or in vivo (Nicola et al. 2011). Virulence factors from fungi, for example, secreted PLB1 and SEC14, are essential for non-lytic exocytosis (Chayakulkeeree et al. 2011). Host factors also regulate the non-lytic exocytosis. The addition of the weak bases ammonium chloride and chloroquine resulted in a significant increase of non-lytic exocytosis events, whereas the vacuolar ATPase inhibitor bafilomycin A1 had the opposite effect (Nicola et al. 2011). Interestingly, both phagosomal maturation dependent (Alvarez and Casadevall 2006) and independent (Ma et al. 2006) pathways have been reported for non-lytic exocytosis of Cn by macrophages. Arp2/3 complex-mediated actin polymerization has been show to inhibit non-lytic exocytosis (Johnston and May 2010). Antibody or complement, which mediates the phagocytosis, affects the outcome (Alvarez et al. 2008) but not the occurrence of non-lytic exocytosis (Alvarez and Casadevall 2006; Ma et al. 2006). Interestingly, autophagy knockdown increases the non-lytic exocytosis of Cn by macrophages (Nicola et al. 2012). Th1 and Th17 cytokines decrease the non-lytic exocytosis, while Th2 cytokines augment the extrusion of Cn out of macrophages (Voelz et al. 2009), which may contribute to the extravasation of Cn and the aggravation of the disease.
Trojan horse
It has been well documented that monocytes could transmigrate across the BBB and differentiate into perivascular macrophages. Thereby, it is tempting to hypothesize that monocyte harboring Cn might function as Trojan horse in the Cn brain dissemination. Phagocytosis of Cn inhibits the chemotaxis of macrophages stimulated by CX3CL1 and CSF-1 (Luo et al. 2009), which might slow down the crawling of macrophages containing Cn along the brain vasculature and therefore facilitate Cn transmigration. More compelling evidence for Trojan horse comes from the deliberate experiment showing that brain fungal burdens following injection with Cn internalized by macrophages, compared with free Cn inoculation, are significantly higher (Charlier et al. 2009). However, many issues for Trojan horse in Cn brain dissemination, including a direct observation rather the evaluation based on fungus quantification, and the mechanisms behind, still remain unresolved.
Neutrophils
It has been historically recognized that neutrophils have the ability to kill the Cn, participating in the first-line defenses before a cell-mediated immune response develops (Diamond et al. 1972; Lehrer and Ladra 1977). In vitro, neutrophil kills Cn effectively especially combined with granulocyte colony-stimulating factor (G-CSF) or granulocyte–macrophage colony stimulating factor (GM-CSF) (Chiller et al. 2002). In the murine model of cryptococcosis, G-CSF, if combined with fluconazole, is associated with the increased survival, suggesting that neutrophils contribute to host defenses in cryptococcal meningoencephalitis (Graybill et al. 1997). Administration of G-CSF into the AIDS patients increases the fungicidal activity and decreases the risk of infection (Vecchiarelli et al. 1995), which is associated with the enhanced leukotrienes from neutrophils upon G-CSF therapy (Coffey et al. 1998). In contrast, cryptococcosis is not usually associated with human neutropenia or with conditions characterized by defective neutrophil function (Casadevall and Perfect 1998), reflecting the complexity of roles of neutrophils against Cn. We hypothesize the blurring effects of neutrophils are due to the co-existing protective (positive) and deleterious (negative) roles from neutrophils in the Cn pathogenesis.
Cn or the capsular polysaccharide glucuronoxylomannan (GXM) promotes the inflammatory cytokines (Retini et al. 1996) and chemokines (Lipovsky et al. 1998), thus displaying chemotactic activity on the neutrophils (Dong and Murphy 1993, 1995a). Paradoxically, GXM inhibits neutrophil migration or infiltration (Dong and Murphy 1995b), partially by reducing the L-selectin (Dong and Murphy 1996), E-selectin (Ellerbroek et al. 2002), IL-8 receptor (Lipovsky et al. 1998) of the neutrophils via cross-desensitization, or competitively binding with CD14 (Ellerbroek et al. 2004b), TLR4 (Ellerbroek et al. 2004b), CD18 (Dong and Murphy 1997) on the neutrophils. O-acetylation of GXM is a crucial motive for the inhibition of neutrophil recruitment (Ellerbroek et al. 2004a). Nevertheless, inoculation of Cn intravenously recruits neutrophils accumulated in pulmonary vessels, which is dependent on the complement 5a (C5a) (Lovchik and Lipscomb 1993). Neutrophils recruitment into the lung is also observed at the early phase (Abe et al. 2000; Feldmesser et al. 2000; Herring et al. 2005; Kawakami et al. 1999; Mednick et al. 2003) of Cn airway infection, although it might not be as evident as mononuclear cells in the mice infected with low-virulence strain (Feldmesser et al. 2000; Huffnagle et al. 1998). Recruitment of neutrophils into the lung is dependent on the chemokines including IL-8 (Guillot et al. 2008), MIP-2 and KC (Kawakami et al. 1999), which are elevated upon Cn infection. Cn could also negatively regulate the influx of neutrophils into the lung (O’Meara et al. 2013), deliberately reflecting the paradoxically dual roles in the interaction between neutrophils and Cn.
The recruited neutrophils in the lung internalize Cn after intratracheal inoculation (Feldmesser et al. 2000), which is mediated by complement 3 (C3) (Kozel et al. 1984). Neutrophils activation enhances the phagocytosis (Kozel et al. 1987); while capsule of Cn inhibits the phagocytosis by neutrophils (Richardson et al. 1993). Cn is a facultative intracellular pathogen in the macrophages (Feldmesser et al. 2000). It is largely unknown the definite fate of Cn ingested by neutrophils. Although speculatively Cn might be protected in the neutrophils (Mednick et al. 2003), most research focused on the killing of Cn (Miller and Mitchell 1991), which might happen intracellularly or extracellularly (Qureshi et al. 2010, 2011), in a oxidative-dependent or oxidative-independent manner (Qu and Wang 1991). Myeloperoxidase (MPO) is a neutrophil-specific enzyme closely associated with reactive-oxygen species. MPO-deficient mice infected with Cn intranasally or intravenously survive significantly shorter, due to the impaired clearance of fungus in the lung and the spleen (Aratani et al. 2006). Inhibition of sphingomyelin synthase (SMS) also profoundly impairs the ability of neutrophils to kill Cn, which are independent of phagocytosis (Qureshi et al. 2010).
Neutrophils are not only phagocytes but also the modulators of immune responses. Depletion of neutrophils results in a Th2 response and renders mice susceptible to Candida albicans infection (Romani et al. 1997). However, roles of neutrophil depletion on the infection of Cn are more complicated. Mice infected with Cn intratracheally survive significantly longer if neutrophils are transiently depleted 24 h before the fungus inoculation, which is associated with the higher levels of IL-10, TNF-α, IL-4 and IL-12 in the lung (Mednick et al. 2003). In contrast to the protective role of neutrophil depletion, mice defective in neutrophil-specific enzyme MPO are hyper-susceptible to Cn, which might result from higher level of IL-4 and reduced production of IL-12, IFN-γ in the lung (Aratani et al. 2006). To add complexity more, neutrophil depletion in the mice infected with Cn expressing IFN-γ results in increased IL-17A production from γδT cells, but has no role on the fungus burden (Wozniak et al. 2012) (Fig. 2).
Fig. 2.
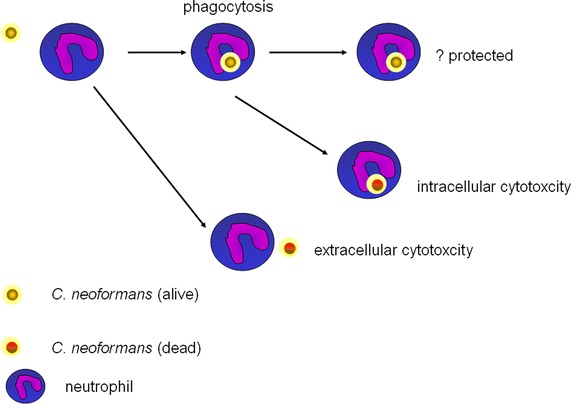
Roles of neutrophils in the Cn pathogenesis. Neutrophil could kill Cn extracellularly or intracellularly. Meanwhile, limited evidences argue that neutrophil may also protect the internalized Cn.
Dendritic cells
Upon Cn airway infection, CCR2 mediates the recruitment of Ly6Ghigh monocytes (Osterholzer et al. 2009a), which differentiate into dendritic cells (DCs) and contribute to the Th1 response (Osterholzer et al. 2008). As the most potent antigen presenting cells, DCs internalize Cn via mannose receptor and FcγR-II in vitro (Syme et al. 2002) and in vivo (Wozniak et al. 2006), which is partially inhibited by the capsule (Vecchiarelli et al. 2003). In contrast, mannoproteins, interacting with CD206 and CD209 (Mansour et al. 2006), promote the maturation of dendritic cells (Pietrella et al. 2005). In the CD206 deficient mice, maturation of dendritic cells upon mannoproteins, however, is not hampered (Dan et al. 2008). Complements and specific antibodies promote the phagocytosis of Cn by dendritic cells (Kelly et al. 2005). Following phagocytosis, DCs kill the intracellular Cn via the fusion of endosome and lysosome and present antigens to T cells (Wozniak and Levitz 2008). The direct cytotoxicity of DCs against Cn is further confirmed in a recent study showing that purified lysosomal enzymes, specifically cathepsin B, inhibit cryptococcal growth in vitro (Hole et al. 2012).
In the lymphnodes, Langerhans cells and myeloid DC induce protective CD4+ T cell responses against Cn (Bauman et al. 2000), which is augmented by TNF-α (Bauman et al. 2003). Accordingly, TNF-α deficiency decreases mature dendritic cell trafficking and produces a chronic Cn infection (Herring et al. 2005). Compared with myeloid DCs, plasmacytoid DCs induce non-protective immune response against Cn (Bauman et al. 2000; Siegemund and Alber 2008). Besides, non-protective Th2 responses could also be induced by immature dendritic cells in the lung, which are promoted by Cn urease (Osterholzer et al. 2009b) (Fig. 3).
Fig. 3.
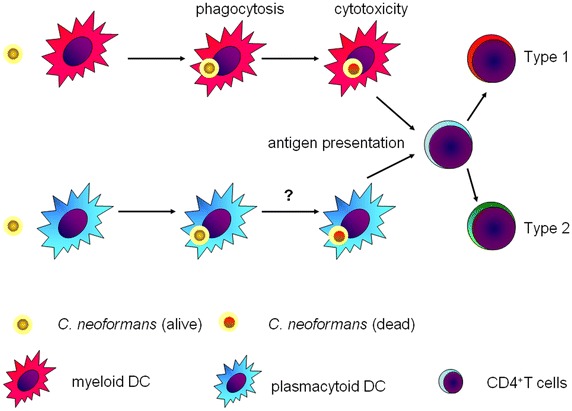
Roles of dendritic cells in the Cn pathogenesis. As most powerful antigen presenting cells, myeloid dendritic cells process and present Cn antigen to CD4+ T cells for the differentiation of cytotoxic Th1 cells. In contrast, plasmacytoid dendritic cells induce the non-protective Th2 cells. No evidence for the survival or death of Cn inside the plasmacytoid dendritic cells has been yet provided in the literature.
Endothelial cells
Different from monocytes/macrophages, neutrophils and dendritic cells, endothelial cells are not professional phagocytes. Yet, Cn is observed in the brain endothelial cells of infected mice (Chretien et al. 2002). In vitro, free Cn could be surrounded by microvillus-like membrane protrusions and subsequently internalized by brain endothelial cells (Chang et al. 2004). Multiple molecules are engaged in the interactions between endothelial cells and extracellular Cn. Hyaluronic acid (HA) from Cn is the ligand of CD44 on the endothelial cells (Jong et al. 2008). In the process of transcellular migration, CD44 is co-localized with phosphorylated caveolin-1, forming thread-like structure (Long et al. 2012) and promoting the lipid raft-dependent endocytosis (Huang et al. 2011). Fungal burden in the brain is significantly decreased in the CD44 deficient mice intravascularly infected with Cn (Jong et al. 2012). Besides HA-CD44 pathway, urease (Shi et al. 2010), plasmin (Stie and Fox 2012), or metalloprotease Mpr1 (Vu et al. 2014) promotes migration of Cn across the brain endothelium by facilitating attachment of cryptococci to the endothelial cells, which induces the cytoskeleton remodeling and internalization (Vu et al. 2013). Of note, some other fungi, for example, Candida albicans also invades brain endothelial cells via endocytosis (Filler and Sheppard 2006). Thus, it would be interesting to explore whether or not brain endothelial cells express some unique receptors for Cn. Although there is no evidence, it is hypothesized that the internalized Cn would be expulsed from endothelial cells into the brain neuropil. Mechanisms for the Cn exocytosis from the endothelial cells are largely unknown. Moreover, human brain endothelial cells (Filler and Sheppard 2006) but not human umbilical vein endothelial cells (Roseff and Levitz 1993) or mouse brain endothelial cells (Sabiiti and May 2012) may also have the capability to kill the internalized Cn (Fig. 4).
Fig. 4.
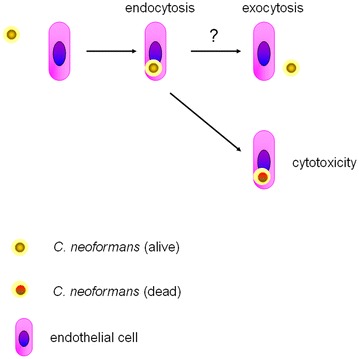
Roles of endothelial cells in the Cn pathogenesis. The major route for the BBB crossing by free Cn is transcellular pathway. As ferrymen, human brain microvasculature endothelial cells also kill the intracellular Cn via unidentified mechanisms.
Conclusions
Serological evidences suggest that people may have been infected with environmental Cn in early childhood (Goldman et al. 2001). Most of Cn infection might be asymptomatic unless the immune defense is significantly suppressed (e.g., organ transplant patients) or defective (e.g., AIDS patients). Cn is overwhelmingly distributed in the environment and may hide in the people with weakened immune system (Saha et al. 2007). However, only a fraction of organ transplant patients or AIDS patients would develop fatal cryptococcosis. Are there any unidentified factors breaking the fine balance between Cn and phagocytes in the lung or even in the brain? How do phagocytes dynamically interact with Cn in the brain vasculature? How do phagocytes containing Cn transmigrate to the brain parenchyma? How does the free Cn escape from the BBB endothelial cells? Obviously, roles of phagocytes in cryptococcosis deserve further investigation.
Authors’ contributions
MZ carried out the study and drafted the manuscript. DS participated in the design of the study. MS conceived of the study and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the start-up funds from the University of Maryland (to MS) and key project of Nanjing Medical University (2014NJMUZD010, to MZ).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Ethical statement All of above research was approved by the ethics committee of the University of Maryland.
Abbreviations
- Cn
Cryptococcus neoformans
- BBB
blood–brain-barrier
- CNS
central nervous system
- App1
antiphagocytic protein 1
- PLB1
phospholipase B1
- G-CSF
granulocyte colony-stimulating factor
- GXM
glucuronoxylomannan
- C3
complement 3
- MPO
myeloperoxidase
- SMS
sphingomyelin synthase
- DCs
dendritic cells
- HA
hyaluronic acid
Contributor Information
Mingshun Zhang, Email: mingshunzhang@njmu.edu.cn.
Donglei Sun, Email: sdlhust@gmail.com.
Meiqing Shi, Email: mshi@umd.edu.
References
- Abe K, Kadota J, Ishimatsu Y, Iwashita T, Tomono K, Kawakami K, et al. Th1–Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol Immunol. 2000;44:849–855. doi: 10.1111/j.1348-0421.2000.tb02573.x. [DOI] [PubMed] [Google Scholar]
- Alanio A, Desnos-Ollivier M, Dromer F (2011) Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. MBio 2(4). doi:10.1128/mBio.00158-11 [DOI] [PMC free article] [PubMed]
- Alanio A, Vernel-Pauillac F, Sturny-Leclere A, Dromer F (2015) Cryptococcus neoformans host adaptation: toward biological evidence of dormancy. MBio 6(2). doi:10.1128/mBio.02580-14 [DOI] [PMC free article] [PubMed]
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Saylor C, Casadevall A. Antibody action after phagocytosis promotes Cryptococcus neoformans and Cryptococcus gattii macrophage exocytosis with biofilm-like microcolony formation. Cell Microbiol. 2008;10:1622–1633. doi: 10.1111/j.1462-5822.2008.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Ishida-Okawara A, et al. Contribution of the myeloperoxidase-dependent oxidative system to host defence against Cryptococcus neoformans. J Med Microbiol. 2006;55:1291–1299. doi: 10.1099/jmm.0.46620-0. [DOI] [PubMed] [Google Scholar]
- Bauman SK, Nichols KL, Murphy JW. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J Immunol. 2000;165:158–167. doi: 10.4049/jimmunol.165.1.158. [DOI] [PubMed] [Google Scholar]
- Bauman SK, Huffnagle GB, Murphy JW. Effects of tumor necrosis factor alpha on dendritic cell accumulation in lymph nodes draining the immunization site and the impact on the anticryptococcal cell-mediated immune response. Infect Immun. 2003;71:68–74. doi: 10.1128/IAI.71.1.68-74.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. Cryptococci at the brain gate: break and enter or use a Trojan horse? J Clin Invest. 2010;120:1389–1392. doi: 10.1172/JCI42949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington DC: American Society for Microbiology; 1998. [Google Scholar]
- Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi—the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 2003;6:332–337. doi: 10.1016/S1369-5274(03)00082-1. [DOI] [PubMed] [Google Scholar]
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood–brain barrier. Infect Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, et al. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80:1088–1101. doi: 10.1111/j.1365-2958.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller T, Farrokhshad K, Brummer E, Stevens DA. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Med Mycol. 2002;40:21–26. doi: 10.1080/714031074. [DOI] [PubMed] [Google Scholar]
- Chretien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186:522–530. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Chrisman CJ, Alvarez M, Casadevall A. Phagocytosis of Cryptococcus neoformans by, and nonlytic exocytosis from, Acanthamoeba castellanii. Appl Environ Microbiol. 2010;76:6056–6062. doi: 10.1128/AEM.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Tesfa L, Zhang J, Rivera J, Goncalves T, Casadevall A. Analysis of cell cycle and replication of mouse macrophages after in vivo and in vitro Cryptococcus neoformans infection using laser scanning cytometry. Infect Immun. 2012;80:1467–1478. doi: 10.1128/IAI.06332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C, Bocca AL, Casadevall A. The intracellular life of Cryptococcus neoformans. Annu Rev Pathol. 2014;9:219–238. doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey MJ, Phare SM, George S, Peters-Golden M, Kazanjian PH. Granulocyte colony-stimulating factor administration to HIV-infected subjects augments reduced leukotriene synthesis and anticryptococcal activity in neutrophils. J Clin Invest. 1998;102:663–670. doi: 10.1172/JCI2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect Immun. 2008;76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond RD, Root RK, Bennett JE. Factors influencing killing of Cryptococcus neoformans by human leukocytes in vitro. J Infect Dis. 1972;125:367–376. doi: 10.1093/infdis/125.4.367. [DOI] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Mobility of human neutrophils in response to Cryptococcus neoformans cells, culture filtrate antigen, and individual components of the antigen. Infect Immun. 1993;61:5067–5077. doi: 10.1128/iai.61.12.5067-5077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Effects of the two varieties of Cryptococcus neoformans cells and culture filtrate antigens on neutrophil locomotion. Infect Immun. 1995;63:2632–2644. doi: 10.1128/iai.63.7.2632-2644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Intravascular cryptococcal culture filtrate (CneF) and its major component, glucuronoxylomannan, are potent inhibitors of leukocyte accumulation. Infect Immun. 1995;63:770–778. doi: 10.1128/iai.63.3.770-778.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Cryptococcal polysaccharides induce L-selectin shedding and tumor necrosis factor receptor loss from the surface of human neutrophils. J Clin Invest. 1996;97:689–698. doi: 10.1172/JCI118466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong ZM, Murphy JW. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek PM, Hoepelman AI, Wolbers F, Zwaginga JJ, Coenjaerts FE. Cryptococcal glucuronoxylomannan inhibits adhesion of neutrophils to stimulated endothelium in vitro by affecting both neutrophils and endothelial cells. Infect Immun. 2002;70:4762–4771. doi: 10.1128/IAI.70.9.4762-4771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbroek PM, Lefeber DJ, van Veghel R, Scharringa J, Brouwer E, Gerwig GJ, et al. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. J Immunol. 2004;173:7513–7520. doi: 10.4049/jimmunol.173.12.7513. [DOI] [PubMed] [Google Scholar]
- Ellerbroek PM, Ulfman LH, Hoepelman AI, Coenjaerts FE. Cryptococcal glucuronoxylomannan interferes with neutrophil rolling on the endothelium. Cell Microbiol. 2004;6:581–592. doi: 10.1111/j.1462-5822.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/IAI.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frager SZ, Chrisman CJ, Shakked R, Casadevall A. Paramecium species ingest and kill the cells of the human pathogenic fungus Cryptococcus neoformans. Med Mycol. 2010;48:775–779. doi: 10.3109/13693780903451810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodas R, Zaragoza O. Catch me if you can: phagocytosis and killing avoidance by Cryptococcus neoformans. Fems Immunol Med Microbiol. 2012;64:147–161. doi: 10.1111/j.1574-695X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- Geunes-Boyer S, Oliver TN, Janbon G, Lodge JK, Heitman J, Perfect JR, et al. Surfactant protein D increases phagocytosis of hypocapsular Cryptococcus neoformans by murine macrophages and enhances fungal survival. Infect Immun. 2009;77:2783–2794. doi: 10.1128/IAI.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geunes-Boyer S, Beers MF, Perfect JR, Heitman J, Wright JR. Surfactant protein D facilitates Cryptococcus neoformans infection. Infect Immun. 2012;80:2444–2453. doi: 10.1128/IAI.05613-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DL, Khine H, Abadi J, Lindenberg DJ, Pirofski L, Niang R, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- Graybill JR, Bocanegra R, Lambros C, Luther MF. Granulocyte colony stimulating factor therapy of experimental cryptococcal meningitis. J Med Vet Mycol. 1997;35:243–247. doi: 10.1080/02681219780001221. [DOI] [PubMed] [Google Scholar]
- Guillot L, Carroll SF, Badawy M, Qureshi ST. Cryptococcus neoformans induces IL-8 secretion and CXCL1 expression by human bronchial epithelial cells. Respir Res. 2008;9:9. doi: 10.1186/1465-9921-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring AC, Falkowski NR, Chen GH, McDonald RA, Toews GB, Huffnagle GB. Transient neutralization of tumor necrosis factor alpha can produce a chronic fungal infection in an immunocompetent host: potential role of immature dendritic cells. Infect Immun. 2005;73:39–49. doi: 10.1128/IAI.73.1.39-49.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hole CR, Bui H, Wormley FL, Jr, Wozniak KL. Mechanisms of dendritic cell lysosomal killing of Cryptococcus. Sci Rep. 2012;2:739. doi: 10.1038/srep00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Long M, Wu CH, Kwon-Chung KJ, Chang YC, Chi F, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine phosphorylation-regulated kinase 3 (DYRK3) J Biol Chem. 2011;286:34761–34769. doi: 10.1074/jbc.M111.219378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle GB, Boyd MB, Street NE, Lipscomb MF. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010;6:e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A, Wu CH, Shackleford GM, Kwon-Chung KJ, Chang YC, Chen HM, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol. 2008;10:1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- Jong A, Wu CH, Gonzales-Gomez I, Kwon-Chung KJ, Chang YC, Tseng HK, et al. Hyaluronic acid receptor CD44 deficiency is associated with decreased Cryptococcus neoformans brain infection. J Biol Chem. 2012;287:15298–15306. doi: 10.1074/jbc.M112.353375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Shibuya K, Qureshi MH, Zhang T, Koguchi Y, Tohyama M, et al. Chemokine responses and accumulation of inflammatory cells in the lungs of mice infected with highly virulent Cryptococcus neoformans: effects of interleukin-12. FEMS Immunol Med Microbiol. 1999;25:391–402. doi: 10.1111/j.1574-695X.1999.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Chen J, Yauch LE, Levitz SM. Opsonic requirements for dendritic cell-mediated responses to Cryptococcus neoformans. Infect Immun. 2005;73:592–598. doi: 10.1128/IAI.73.1.592-598.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Highison B, Stratton CJ. Localization on encapsulated Cryptococcus neoformans of serum components opsonic for phagocytosis by macrophages and neutrophils. Infect Immun. 1984;43:574–579. doi: 10.1128/iai.43.2.574-579.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Pfrommer GS, Redelman D. Activated neutrophils exhibit enhanced phagocytosis of Cryptococcus neoformans opsonized with normal human serum. Clin Exp Immunol. 1987;70:238–246. [PMC free article] [PubMed] [Google Scholar]
- Lehrer RI, Ladra KM. Fungicidal components of mammalian granulocytes active against Cryptococcus neoformans. J Infect Dis. 1977;136:96–99. doi: 10.1093/infdis/136.1.96. [DOI] [PubMed] [Google Scholar]
- Leopold Wager CM, Wormley FL., Jr Classical versus alternative macrophage activation: the Ying and the Yang in host defense against pulmonary fungal infections. Mucosal Immunol. 2014;7:1023–1035. doi: 10.1038/mi.2014.65. [DOI] [PubMed] [Google Scholar]
- Lipovsky MM, Gekker G, Hu S, Ehrlich LC, Hoepelman AI, Peterson PK. Cryptococcal glucuronoxylomannan induces interleukin (IL)-8 production by human microglia but inhibits neutrophil migration toward IL-8. J Infect Dis. 1998;177:260–263. doi: 10.1086/517368. [DOI] [PubMed] [Google Scholar]
- Liu TB, Xue C. Fbp1-mediated ubiquitin-proteasome pathway controls Cryptococcus neoformans virulence by regulating fungal intracellular growth in macrophages. Infect Immun. 2014;82:557–568. doi: 10.1128/IAI.00994-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Huang SH, Wu CH, Shackleford GM, Jong A (2012) Lipid raft/caveolae signaling is required for Cryptococcus neoformans invasion into human brain microvascular endothelial cells. J Biomed Sci 19:19. doi:10.1186/1423-0127-19-19 [DOI] [PMC free article] [PubMed]
- Lovchik JA, Lipscomb MF. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am J Respir Cell Mol Biol. 1993;9:617–627. doi: 10.1165/ajrcmb/9.6.617. [DOI] [PubMed] [Google Scholar]
- Luo Y, Tucker SC, Casadevall A. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J Immunol. 2005;174:7226–7233. doi: 10.4049/jimmunol.174.11.7226. [DOI] [PubMed] [Google Scholar]
- Luo Y, Isaac BM, Casadevall A, Cox D. Phagocytosis inhibits F-actin-enriched membrane protrusions stimulated by fractalkine (CX3CL1) and colony-stimulating factor 1. Infect Immun. 2009;77:4487–4495. doi: 10.1128/IAI.00530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16:2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol. 2006;176:3053–3061. doi: 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- McQuiston TJ, Williamson PR. Paradoxical roles of alveolar macrophages in the host response to Cryptococcus neoformans. J Infect Chemother. 2012;18:1–9. doi: 10.1007/s10156-011-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206:637–653. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick AJ, Feldmesser M, Rivera J, Casadevall A. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur J Immunol. 2003;33:1744–1753. doi: 10.1002/eji.200323626. [DOI] [PubMed] [Google Scholar]
- Miller MF, Mitchell TG. Killing of Cryptococcus neoformans strains by human neutrophils and monocytes. Infect Immun. 1991;59:24–28. doi: 10.1128/iai.59.1.24-28.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola AM, Robertson EJ, Albuquerque P, da Derengowski LS, Casadevall A (2011) Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. MBio 2(4). doi:10.1128/mBio.00167-11 [DOI] [PMC free article] [PubMed]
- Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, De Jesus M, et al. Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans. Infect Immun. 2012;80:3065–3076. doi: 10.1128/IAI.00358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–1547. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA (2013) Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. MBio 4(1). doi:10.1128/mBio.00522-12 [DOI] [PMC free article] [PubMed]
- Osterholzer JJ, Curtis JL, Polak T, Ames T, Chen GH, McDonald R, et al. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. J Immunol. 2008;181:610–620. doi: 10.4049/jimmunol.181.1.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB. Accumulation of CD11b + lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol. 2009;183:8044–8053. doi: 10.4049/jimmunol.0902823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Surana R, Milam JE, Montano GT, Chen GH, Sonstein J, et al. Cryptococcal urease promotes the accumulation of immature dendritic cells and a non-protective T2 immune response within the lung. Am J Pathol. 2009;174:932–943. doi: 10.2353/ajpath.2009.080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun. 2005;73:820–827. doi: 10.1128/IAI.73.2.820-827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Wang A (1991) Purification and antimicrobial activity of human neutrophil defensins. Zhonghua Yi Xue Za Zhi 71:616-619, 642 [PubMed]
- Qureshi A, Subathra M, Grey A, Schey K, Del Poeta M, Luberto C. Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans. PLoS ONE. 2010;5:e15587. doi: 10.1371/journal.pone.0015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi A, Grey A, Rose KL, Schey KL, Del Poeta M. Cryptococcus neoformans modulates extracellular killing by neutrophils. Front Microbiol. 2011;2:193. doi: 10.3389/fmicb.2011.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retini C, Vecchiarelli A, Monari C, Tascini C, Bistoni F, Kozel TR. Capsular polysaccharide of Cryptococcus neoformans induces proinflammatory cytokine release by human neutrophils. Infect Immun. 1996;64:2897–2903. doi: 10.1128/iai.64.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MD, White LJ, McKay IC, Shankland GS. Differential binding of acapsulate and encapsulated strains of Cryptococcus neoformans to human neutrophils. J Med Vet Mycol. 1993;31:189–199. doi: 10.1080/02681219380000241. [DOI] [PubMed] [Google Scholar]
- Rohatgi S, Gohil S, Kuniholm MH, Schultz H, Dufaud C, Armour KL, et al. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. MBio. 2013;4:e00573–e00613. doi: 10.1128/mBio.00573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Bistoni F, Puccetti P. Initiation of T-helper cell immunity to Candida albicans by IL-12: the role of neutrophils. Chem Immunol. 1997;68:110–135. doi: 10.1159/000058688. [DOI] [PubMed] [Google Scholar]
- Roseff SA, Levitz SM. Effect of endothelial cells on phagocyte-mediated anticryptococcal activity. Infect Immun. 1993;61:3818–3824. doi: 10.1128/iai.61.9.3818-3824.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiiti W, May RC. Capsule independent uptake of the fungal pathogen Cryptococcus neoformans into brain microvascular endothelial cells. PLoS One. 2012;7:e35455. doi: 10.1371/journal.pone.0035455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabiiti W, Robertson E, Beale MA, Johnston SA, Brouwer AE, Loyse A, et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest. 2014;124:2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha DC, Goldman DL, Shao X, Casadevall A, Husain S, Limaye AP, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin Vaccine Immunol. 2007;14:1550–1554. doi: 10.1128/CVI.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor CA, Dadachova E, Casadevall A. Murine IgG1 and IgG3 isotype switch variants promote phagocytosis of Cryptococcus neoformans through different receptors. J Immunol. 2010;184:336–343. doi: 10.4049/jimmunol.0902752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegemund S, Alber G. Cryptococcus neoformans activates bone marrow-derived conventional dendritic cells rather than plasmacytoid dendritic cells and down-regulates macrophages. FEMS Immunol Med Microbiol. 2008;52:417–427. doi: 10.1111/j.1574-695X.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- Stano P, Williams V, Villani M, Cymbalyuk ES, Qureshi A, Huang Y, et al. App1: an antiphagocytic protein that binds to complement receptors 3 and 2. J Immunol. 2009;182:84–91. doi: 10.4049/jimmunol.182.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stie J, Fox D. Blood–brain barrier invasion by Cryptococcus neoformans is enhanced by functional interactions with plasmin. Microbiology. 2012;158:240–258. doi: 10.1099/mic.0.051524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syme RM, Spurrell JC, Amankwah EK, Green FH, Mody CH. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcgamma receptor II for presentation to T lymphocytes. Infect Immun. 2002;70:5972–5981. doi: 10.1128/IAI.70.11.5972-5981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborda CP, Casadevall A. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity. 2002;16:791–802. doi: 10.1016/S1074-7613(02)00328-X. [DOI] [PubMed] [Google Scholar]
- Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci USA. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A, Monari C, Baldelli F, Pietrella D, Retini C, Tascini C, et al. Beneficial effect of recombinant human granulocyte colony-stimulating factor on fungicidal activity of polymorphonuclear leukocytes from patients with AIDS. J Infect Dis. 1995;171:1448–1454. doi: 10.1093/infdis/171.6.1448. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A, Pietrella D, Lupo P, Bistoni F, McFadden DC, Casadevall A. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. J Leukoc Biol. 2003;74:370–378. doi: 10.1189/jlb.1002476. [DOI] [PubMed] [Google Scholar]
- Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Eigenheer RA, Phinney BS, Gelli A. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect Immun. 2013;81:3139–3147. doi: 10.1128/IAI.00554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu K, Tham R, Uhrig JP, Thompson GR, 3rd, Na Pombejra S, Jamklang M, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. MBio. 2014;5:e01101–e01114. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V, Del Poeta M. Role of glucose in the expression of Cryptococcus neoformans antiphagocytic protein 1, App1. Eukaryot Cell. 2011;10:293–301. doi: 10.1128/EC.00252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Levitz SM. Cryptococcus neoformans enters the endolysosomal pathway of dendritic cells and is killed by lysosomal components. Infect Immun. 2008;76:4764–4771. doi: 10.1128/IAI.00660-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Vyas JM, Levitz SM. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infect Immun. 2006;74:3817–3824. doi: 10.1128/IAI.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Kolls JK, Wormley FL., Jr Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gammadelta T cells. BMC Immunol. 2012;13:65. doi: 10.1186/1471-2172-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]


