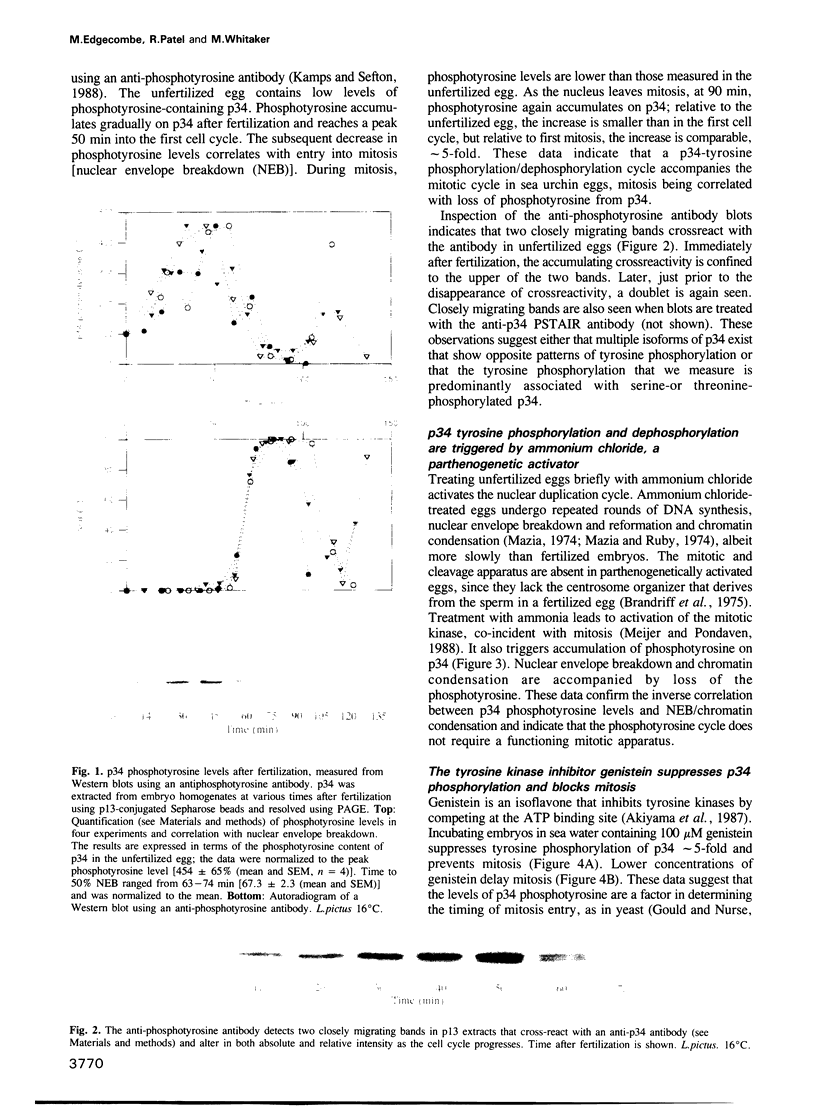
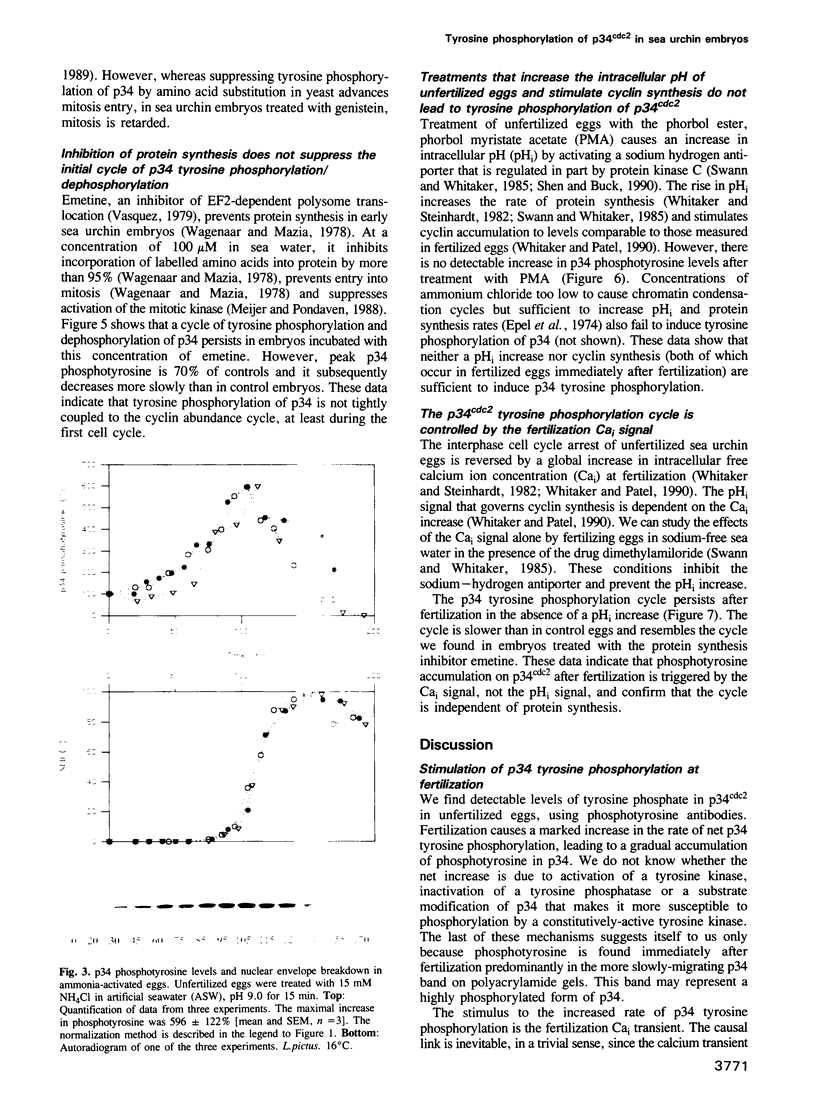
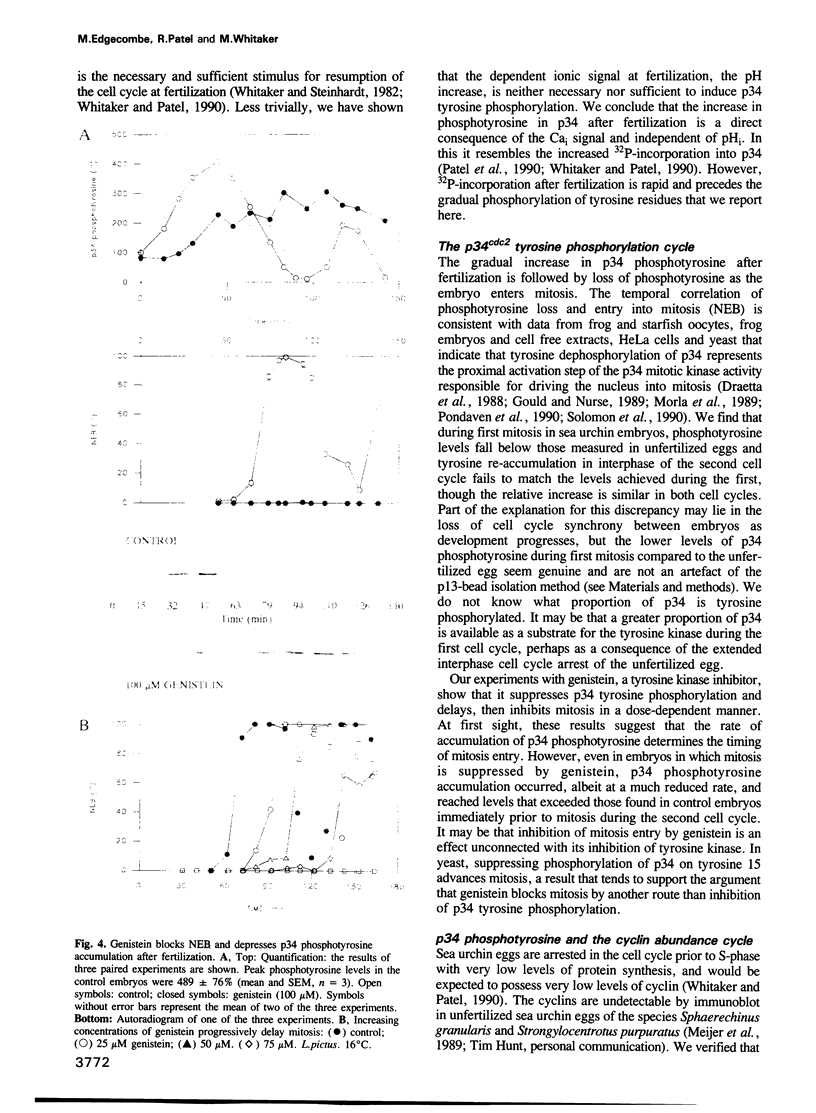
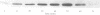Abstract
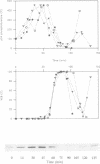
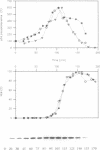
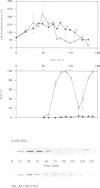
The activity of the cell cycle control protein p34cdc2 is post-translationally regulated in a variety of cell types. Using anti-phosphotyrosine antibodies, we find that p34cdc2-directed tyrosine kinase activity increases at fertilization in sea urchin eggs, leading to a gradual accumulation of phosphotyrosine on p34 during the early part of the cell cycle. Loss of phosphotyrosine from p34 accompanies entry into mitosis and phosphotyrosine reaccumulates as the embryo enters the next cell cycle. A similar pattern is seen when eggs are parthenogenetically activated with ammonium chloride. Tyrosine phosphorylation and phosphorylation/dephosphorylation cycles are suppressed when embryos are treated with the tyrosine kinase inhibitor genistein. On the other hand, a cycle persists when protein synthesis is inhibited with emetine, indicating that it is independent of the synthesis of another class of cell cycle control proteins, the cyclins. Additional experiments with the phorbol ester, phorbol myristate acetate, demonstrate that activating protein synthesis alone in unfertilized eggs does not result in stimulation of p34cdc2 tyrosine kinase activity. Our results indicate that p34 tyrosine phosphorylation cycles are triggered by the fertilization Cai transient. The first cycle is independent of the fertilization pHi signal, confirming that, in sea urchin embryos, the cycle is not tightly coupled to the cycle of cyclin abundance that is a prominent feature of the eukaryotic cell division cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Arion D., Meijer L. M-phase-specific protein kinase from mitotic sea urchin eggs: cyclic activation depends on protein synthesis and phosphorylation but does not require DNA or RNA synthesis. Exp Cell Res. 1989 Aug;183(2):361–375. doi: 10.1016/0014-4827(89)90397-2. [DOI] [PubMed] [Google Scholar]
- Brandriff B., Hinegardner R. I., Steinhardt R. Development and life cycle of the parthenogenetically activated sea urchin embryo. J Exp Zool. 1975 Apr;192(1):13–24. doi: 10.1002/jez.1401920103. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 1987 Nov;6(11):3507–3514. doi: 10.1002/j.1460-2075.1987.tb02676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Epel D., Steinhardt R., Humphreys T., Mazia D. An analysis of the partial metabolic derepression of sea urchin eggs by ammonia: the existence of independent pathways. Dev Biol. 1974 Oct;40(2):245–255. doi: 10.1016/0012-1606(74)90127-4. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Labbé J. C., Dorée M., Hunt T., Karsenti E. Triggering of cyclin degradation in interphase extracts of amphibian eggs by cdc2 kinase. Nature. 1990 Jul 26;346(6282):379–382. doi: 10.1038/346379a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Labbé J. C., Capony J. P., Caput D., Cavadore J. C., Derancourt J., Kaghad M., Lelias J. M., Picard A., Dorée M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989 Oct;8(10):3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé J. C., Picard A., Karsenti E., Dorée M. An M-phase-specific protein kinase of Xenopus oocytes: partial purification and possible mechanism of its periodic activation. Dev Biol. 1988 May;127(1):157–169. doi: 10.1016/0012-1606(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Ruderman J. V. Control of programmed cyclin destruction in a cell-free system. J Cell Biol. 1989 Nov;109(5):1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D. Chromosome cycles turned on in unfertilized sea urchin eggs exposed to NH4OH. Proc Natl Acad Sci U S A. 1974 Mar;71(3):690–693. doi: 10.1073/pnas.71.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D., Ruby A. DNA synthesis turned on in unfertilized sea urchin eggs by treatment with NH4OH. Exp Cell Res. 1974 Mar 30;85(1):167–172. doi: 10.1016/0014-4827(74)90227-4. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Azzi L., Wang J. Y. Cyclin B targets p34cdc2 for tyrosine phosphorylation. EMBO J. 1991 Jun;10(6):1545–1554. doi: 10.1002/j.1460-2075.1991.tb07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Pondaven P. Cyclic activation of histone H1 kinase during sea urchin egg mitotic divisions. Exp Cell Res. 1988 Jan;174(1):116–129. doi: 10.1016/0014-4827(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell biology: the cell cycle as a cdc2 cycle. Nature. 1989 Nov 2;342(6245):14–15. doi: 10.1038/342014a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Paris J., Le Guellec R., Couturier A., Le Guellec K., Omilli F., Camonis J., MacNeill S., Philippe M. Cloning by differential screening of a Xenopus cDNA coding for a protein highly homologous to cdc2. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1039–1043. doi: 10.1073/pnas.88.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondaven P., Meijer L., Beach D. Activation of M-phase-specific histone H1 kinase by modification of the phosphorylation of its p34cdc2 and cyclin components. Genes Dev. 1990 Jan;4(1):9–17. doi: 10.1101/gad.4.1.9. [DOI] [PubMed] [Google Scholar]
- Shen S. S., Buck W. R. A synthetic peptide of the pseudosubstrate domain of protein kinase C blocks cytoplasmic alkalinization during activation of the sea urchin egg. Dev Biol. 1990 Aug;140(2):272–280. doi: 10.1016/0012-1606(90)90077-v. [DOI] [PubMed] [Google Scholar]
- Sluder G., Lewis K. Relationship between nuclear DNA synthesis and centrosome reproduction in sea urchin eggs. J Exp Zool. 1987 Oct;244(1):89–100. doi: 10.1002/jez.1402440111. [DOI] [PubMed] [Google Scholar]
- Sluder G., Miller F. J., Cole R., Rieder C. L. Protein synthesis and the cell cycle: centrosome reproduction in sea urchin eggs is not under translational control. J Cell Biol. 1990 Jun;110(6):2025–2032. doi: 10.1083/jcb.110.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. Stimulation of the Na/H exchanger of sea urchin eggs by phorbol ester. Nature. 1985 Mar 21;314(6008):274–277. doi: 10.1038/314274a0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]