Abstract
Melatonin is a ubiquitous signal molecule, playing crucial roles in plant growth and stress tolerance. Recently, toxic metal cadmium (Cd) has been reported to regulate melatonin content in rice; however, the function of melatonin under Cd stress, particularly in higher plants, still remains elusive. Here, we show that optimal dose of melatonin could effectively ameliorate Cd-induced phytotoxicity in tomato. The contents of Cd and melatonin were gradually increased over time under Cd stress. However, such increase in endogenous melatonin was incapable to reverse detrimental effects of Cd. Meanwhile, supplementation with melatonin conferred Cd tolerance as evident by plant biomass and photosynthesis. In addition to notable increase in antioxidant enzymes activity, melatonin-induced Cd stress mitigation was closely associated with enhanced H+-ATPase activity and the contents of glutathione and phytochelatins. Although exogenous melatonin had no effect on root Cd content, it significantly reduced leaf Cd content, indicating its role in Cd transport. Analysis of Cd in different subcellular compartments revealed that melatonin increased cell wall and vacuolar fractions of Cd. Our results suggest that melatonin-induced enhancements in antioxidant potential, phytochelatins biosynthesis and subsequent Cd sequestration might play a critical role in plant tolerance to Cd. Such a mechanism may have potential implication in safe food production.
Keywords: cadmium, food safety, melatonin, phytochelatins, tomato, vacuolar sequestration
Introduction
The rising concern on cadmium (Cd) as a toxic heavy metal, is not only due to its detrimental effects on crop production, but also for potential health hazards associated with food chain contamination (Hall, 2002; Mobin and Khan, 2007; Ajjimaporn et al., 2012). Given that Cd is not an essential nutrient, but easily assimilated in plants due to flexible specificity of ion channels and divalent metal transporters (Liu et al., 2003). Upon accumulation, Cd disrupts normal metabolism leading to diverse morphological, physiological, biochemical and cellular changes (Llamas et al., 2000; Pietrini et al., 2003). Inhibition of Calvin cycle enzymes, photosynthesis, and carbohydrate metabolism by Cd results in low biomass accumulation in plants (Mobin and Khan, 2007). Although Cd does not participate in Fenton-reaction, it induces reactive oxygen species (ROS) accumulation and eventually causes oxidative stress in plant cells (Romero-Puertas et al., 2002; Pietrini et al., 2003; Cui et al., 2012). Moreover, Cd stress alters plasma membrane permeability by inhibiting H+-ATPase, which plays important roles in ionic balance and substance transport (Janicka-Russak et al., 2012). At cellular level, Cd alters plant cell cycle and division, and increases chromosomal aberrations and malformed embryos (DalCorso et al., 2010). Cd contamination severely limits crop production particularly in marginal areas of developing countries, where cultivation is being injudiciously expanded to meet rising demand of food. On the other hand, Cd accumulation in edible plant parts ultimately poses threat to human health as Cd toxicity is closely associated with various health hazards including kidney damage, bladder and lung cancer (Ajjimaporn et al., 2012). Therefore, the enhancement of Cd tolerance in plant to boost crop yield in marginal area as well as reduction of Cd content in edible plant parts for food safety, are ongoing endeavors in plant science and food production.
Plants have intrinsically evolved sophisticated cellular mechanisms for Cd detoxification and tolerance such as immobilization, exclusion, chelation, compartmentalization of metal ions and the repair of cell structural alteration (di Toppi and Gabbrielli, 1999; Hall, 2002). The root cell wall, which is in direct contact with heavy metals in soil, can first release chelating compounds, such as phytosiderophores, nicotianamine and organic acids, to bind Cd and other heavy metals in the epidermis (Xiong et al., 2009). Plant cells can increase the leakage of solutes and reduce influx across plasma membranes to exclude Cd and other heavy metals (Meharg, 1993). Moreover, heat shock proteins and metallothioneins have been reported to repair and protect plasma membranes under heavy metal stress (Neumann et al., 1994; Hall, 2002). The chelation of heavy metals with high-affinity ligands in the cytoplasm and its subsequent compartmentalization in the vacuole are generally considered as the “first line” defense mechanisms in plant Cd tolerance (Hall, 2002; Park et al., 2012). Phytochelatins (PCs) comprise a family of peptides (γ-Glu-Cys)n-Gly (n = 2–11) that are synthesized from glutathione (GSH). PCs synthesis is rapidly induced by Cd treatment that chelate Cd and sequester the PC-Cd conjugates into the vacuoles (Rauser, 1995). Besides, GSH activates antioxidant enzymes such as superoxide dismutase (SOD), ascorbic acid peroxidase (APX), and catalase (CAT) that neutralize Cd-induced ROS for cellular redox homeostasis (Jozefczak et al., 2014).
In the past decade, the complex molecular mechanisms of Cd stress response have been extensively elucidated in plants (DalCorso et al., 2010). Involvement of several phytohormones such as abscisic acid (ABA), brassinosteroids (BRs), jasmonic acid (JA), ethylene (EHT), and salicylic acid (SA) was found to be associated with Cd stress response (Metwally et al., 2003; Maksymiec, 2007; Masood et al., 2012; Ahammed et al., 2013; Stroinski et al., 2013). Previously, we found that exogenous 24-epibrassinolide (EBR, a biologically active BR) could not only alleviate Cd-induced photosynthetic inhibition and oxidative stress, but also could reduce Cd content in tomato plants (Ahammed et al., 2013). Considering the hazardous effects of Cd on plants and animals, extensive researches are still underway to explore new natural compounds which can improve plant tolerance to Cd and/or minimize toxic Cd content in edible plant parts for food safety purpose.
Most recently, Cd has been reported to regulate melatonin content in rice (Oryza sativa), a ubiquitous signal molecule, well-accepted as a new plant growth regulator and/or biostimulator rather than plant hormone (Arnao and Hernández-Ruiz, 2014; Byeon et al., 2015). Melatonin (N-acetyl-5-methoxytryptamine) has long been known to be an important animal hormone involved in multiple biological processes (Escames et al., 2012; Calvo et al., 2013). Although its function as a hormone has been well-characterized in animals, our knowledge in plant kingdom is still fragmentary (Arnao and Hernández-Ruiz, 2014). In recent years, melatonin appeared as a research focus in plant biology. Melatonin is considered as a plant growth regulator because of its specific physiological functions such as regulation of the growth of roots, shoots and explants, seed germination, rhizogenesis and delay in leaf senescence (Arnao and Hernández-Ruiz, 2014). In addition, melatonin has been found to fortify plant tolerance to a range of abiotic stresses such as drought, cold, heat, salinity, chemical pollutants, herbicides, and UV radiation (Zhang et al., 2015). The mechanisms of melatonin-mediated stress tolerance involve the promotion of antioxidant biosynthesis, the activation of related enzymes and the direct scavenging of ROS following the exposure of plants to harsh environments (Li et al., 2012; Tan et al., 2012; Park et al., 2013; Zhang et al., 2013; Bajwa et al., 2014). Like any other abiotic stress, heavy metals such as Cd significantly induces melatonin content in lower as well as higher plant species. Strikingly, exogenous melatonin can ameliorate Cd-induced stress in green micro algae; however, its role in higher plants still remains elusive. Although PCs are well known to bind many toxic metals including Cd to promote metal detoxification and subsequent stress tolerance in plants, information relating to melatonin-induced PC biosynthesis and/or stress tolerance in higher plants is missing.
To better understand the mechanistic basis of melatonin in plant stress responses, we sought to identify and functionally analyze melatonin under Cd stress. We hypothesize that apart from its well-known antioxidative and chelating properties, melatonin might have a protective role in Cd tolerance involving other mechanisms. We consider PC biosynthesis as a novel target for melatonin-mediated Cd stress response. To testify this hypothesis, we carried out a set of experiments in tomato (Solanum lycopersicum L.) taking photosynthetic function and ROS generation as biomarkers. This study explored crucial mechanisms involved in melatonin-mediated Cd stress response which might have potential implication for ensuring food safety and food security in marginal agriculture.
Materials and Methods
Plant Materials and Experimental Design
Tomato seeds (Solanum lycopersicum L. cv. Ailsa Craig) were germinated in a growth medium containing a mixture of vermiculite and perlite (3:1, v/v) in a greenhouse. When the first true leaves were fully expanded, a group of six uniform seedlings were transferred into a container (40 cm × 25 cm × 15 cm) filled with Hoagland’s nutrient solution for hydroponics culture. The growth conditions were as follows: a temperature of 25/17°C (day/night), a mean relative humidity of 80%, a photosynthetic photon flux density (PPFD) of 800 μmol m-2 s-1, and a photoperiod of 14/10 h (day/night).
To investigate the influence of Cd on endogenous melatonin content, the roots of tomato seedlings, at the four-leaf stage were treated with 25 and 100 μM Cd in hydroponics. Cd was supplied in the form of CdCl2 (analytical grade). Based on our preliminary experiments, 100 μM Cd was selected for studying effects of exogenous melatonin on Cd stress response. Foliar portions of tomato plants were sprayed with 0, 25, 50, 100, 250, and 500 μM melatonin. Hoagland’s nutrient solution with or without Cd, were changed at every 5 days followed by foliar spray of melatonin. The experiment was terminated 2 weeks after initial Cd treatment. Four replicates were used for each treatment, and each replicate consisted of 12 plants.
Gas Exchange and Chlorophyll Fluorescence Measurements
Net photosynthetic rate (Pn) and the maximum quantum efficiency of photosystem II photochemistry (Fv/Fm) were determined in the second fully expanded leaves from the plant tops. The Pn was determined using an infrared gas analyzer (IRGA) portable photosynthesis system (LI-COR 6400, Lincoln, NE, USA). The air temperature, air relative humidity, CO2 concentration, and PPFD for Pn measurement were 25°C, 85%, 400 μmol mol-1, and 1000 μmol m-2 s-1, respectively. The Fv/Fm was determined after 30 min of dark adaptation using an imaging pulse amplitude-modulated (PAM) fluorimeter (IMAG-MAXI; Heinz Walz, Effeltrich, Germany) as described (Zhou et al., 2014).
Determination of H2O2, Lipid Peroxidation and Electrolyte Leakage
The H2O2 and MDA concentrations in leaves and roots were assessed spectrophotometrically. To determine the H2O2 concentration, 0.3 g of fresh sample was homogenized in 3 mL of pre-cooled HClO4 (1.0 M) using a pre-chilled mortar and pestle (Willekens et al., 1997). The homogenates were then transferred to 10-mL plastic tubes and centrifuged at 6000 g for 5 min at 4°C. The pH of resulting supernatant’s was adjusted to 6.0 with 4 M KOH and centrifuged at 6000 g for 1 min at 4°C. The resulting supernatant was passed through an AG1.8 prepacked column (Bio-Rad, Hercules, CA, USA) and H2O2 was eluted with double-distilled H2O. The sample (800 μL) was mixed with 400 μL reaction buffer containing 4 mM 2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonic acid) and 100 mM potassium acetate at pH 4.4, 400 μL deionized water, and 0.25 U of horseradish peroxidase. Equal recovery from the different samples was checked by analyzing duplicate samples. The lipid peroxidation level was determined by quantifying the MDA equivalents using 2-thiobarbituric acid (TBA), as described by Hodges et al. (1999). In brief, samples (0.5 g) were homogenized in 5 mL 0.1% (w/v) trichloroacetic acid (TCA). The homogenates were centrifuged at 10,000 g for 10 min, and 4 mL 20% TCA containing 0.65% (w/v) TBA was added to 1 mL of the supernatants. The mixtures were heated in a hot water bath at 95°C for 25 min and immediately placed in an ice bath to stop the reaction. They were then centrifuged at 3000 g for 10 min, and the absorbance of the supernatants was recorded at 440, 532, and 600 nm. The MDA equivalents were calculated as described previously (Hodges et al., 1999). To the measurements of electrolyte leakage (EL), plant samples were cut into small pieces with razor blade, rinsed briefly with deionized water, and immediately placed in a tube with 5 mL of deionized water. EL was then measured before and after 4 h of rehydration and ultimately after autoclaving according to the method described by Bajji et al. (2002).
Histochemical Staining of H2O2 and in Leaves
H2O2 accumulation in leaves was visually detected by staining with 3, 3-diaminobenzidine (DAB) using the method as described (ThordalChristensen et al., 1997). Leaves were immediately removed from plants, submerged in DAB solution (1 mg mL-1, pH 3.8) and incubated under light for 6 h at 25°C. was detected with the nitroblue tetrazolium (NBT) staining procedure, which involved the incubation of the leaves with NBT (0.5 mg mL-1, pH 7.8) solution in the dark (Jabs et al., 1996).
Extraction and Assay of Antioxidant Enzymes
For enzyme extraction, 0.3 g of fresh sample was homogenized in 50 mM potassium phosphate buffer (pH 7.0) containing 0.1 mM EDTA and 1% polyvinylpyrrolidone (w/v). The homogenate was centrifuged at 12,000 g for 15 min at 4°C, and the supernatant was collected. This supernatant was used to measure the activities of SOD, guaiacol peroxidase (G-POD), APX, CAT, and glutathione reductase (GR) enzymes. SOD activity was assayed by determining its ability to inhibit the photochemical reduction of NBT as described (Beauchamp and Fridovic, 1971). G-POD activity was assessed using guaiacol as a substrate (Cakmak and Marschner, 1992). APX activity in the tomato leaves was determined as described (Nakano and Asada, 1981). CAT activity was measured according to the method as described (Cakmak and Marschner, 1992). GR activity was measured and calculated as described (Foyer and Halliwell, 1976). For the assessment of H+-ATPase activity, 0.3 g of fresh sample was homogenized in 50 mM Tris-HCl buffer. Then, the release of Pi was measured using an activity assay kit (Jiancheng Bio Co., Nanjing, Jiangsu, China) as described (Ahmed et al., 2013).
Extraction and Quantification of Endogenous Melatonin by HPLC
To measure the endogenous plant melatonin concentration, a direct sampling extraction procedure was used as described (Arnao and Hernandez-Ruiz, 2009a) with modifications. Fresh leaf or root samples (0.3 g) were cut into small sections and placed into a 15 mL tube containing 6 mL of chloroform, which was shaken overnight at 4°C in the dark. The sections were extracted again using 2 mL of chloroform. The supernatant was transferred to a C18 solid-phase extraction (SPE) cartridge (Waters, Milford, MA, USA) for the purification of melatonin. The extract was then concentrated to dryness under nitrogen. The residue was dissolved in 2 mL of the mobile phase for HPLC analysis as described (Korkmaz et al., 2014).
Extraction, Derivatization, and Separation of Thiol Compounds by HPLC
For thiol extraction, fresh leaf, or root samples (1 g) were homogenized with liquid nitrogen, and then 1 mL of extraction buffer containing 6.3 mM diethylene triamine pentaacetic acid (DTPA) and 0.1% (v/v) trifluoroacetic acid (TFA) was added. The supernatant was collected by centrifugation at 12,000 g for 10 min at 4°C. The derivatization of the thiol compounds GSH and PCs was analyzed with monobromobimane (mBBr) according to the method as described (Minocha et al., 2008). The derivative samples were filtered with 0.45 μm nylon syringe filters and stored at -20°C for up to 1 week for HPLC analyses. The thiol compounds were separated by HPLC using a fluorescence detector and a Phenomenex Synergi Hydro-RP C18 column (4 μm particle size, 100 mm × 4.6 mm) and a C18 SecurityGuardTM (5 μm, 4 mm × 3 mm) cartridge guard column. The excitation and emission wavelengths were set at 380 and 470 nm, respectively, as described (Minocha et al., 2008).
Histochemical Localization of Cd
The histochemical detection of Cd in both the leaf and root tissues was performed using a staining-based method (Seregin and Ivanov, 1997). This method involves staining with dithizone, a reagent that produces an insoluble red salt (Cd-dithizonate) in the presence of Cd. Plant samples were removed from the glutaraldehyde solution, and cross sections were taken from the root tips or the leaf veins. The sections were reacted with 2–3 drops of dithizone solution for 15 min, and then they were examined under a light microscope (BX61; Olympus Co., Tokyo, Japan).
Separation of Tissue Fractionations
To observe the subcellular compartmentalization of Cd, the tissues were separated into the following four fractions using differential centrifugation techniques: the cell wall (CW), vacuole (V), organelle (O), and soluble (S) fractions. All of the fractions except for the V fraction were separated as previously described (Wu et al., 2005). Cell vacuoles were separated according to the method as described (Marty and Branton, 1980). In brief, fresh (5 g) root and leaf samples were homogenized in pre-chilled extraction buffer containing 50 mM Tris-HCl (pH 7.5), 250 mM sucrose, 1.0 mM DTE (C4H10O2S2), 5.0 mM ascorbic acid and 1.0% w/v Polyclar AT PVPP, pH 7.5. The homogenate was sieved through a nylon cloth (80 μM), and the residue on the nylon cloth was washed twice with the same homogenization buffer and designated as the CW fraction. This first filtrate was centrifuged at 2,000 g for 10 min, and the precipitate was designated as the V fraction. The resulting supernatant solution was further centrifuged at 15,000 g for 30 min. The resulting pellet and supernatant were referred to as the O and S fractions, respectively. All steps were performed at 4°C.
Determination of Cd
The different separated cell fractions or 0.20 g of homogenized dry powdered sample were digested with an HClO4 and HNO3 mixture (v/v = 1:3) at 180°C. The digested colorless liquids were washed with 2 mL of diluted HNO3 (distilled water/concentrated HNO3 = 1:1) and were then washed three times with distilled water. The liquid was collected and transferred to 50 mL volumetric flasks and diluted to a constant volume. The Cd concentrations in the different fractions and the total Cd were analyzed using an atomic absorption spectrophotometer (AA-6300; Shimadzu Co., Kyoto, Japan; Wu et al., 2005).
Extraction of Total RNA and Quantitative Real-Time PCR (qRT-PCR) Analyses
Total RNA extraction from the tomato leaf and root tissues was performed with an RNA extraction kit (Tiangen, Shanghai, China). A 1 μg aliquot of total RNA was reverse-transcribed to generate cDNA using a ReverTra Ace qPCR RT Kit (Toyobo, Japan), following the manufacturer’s instructions. qRT-PCR was performed with SYBR Green PCR Master Mix (Takara, Tokyo, Japan) using an ABI Step One Plus real-time machine (Eppendorf, USA) under the default thermal cycling conditions with an added melting curve. The gene-specific primers used for qRT-PCR were designed based on the mRNA or expressed sequence tag (EST) sequences of the corresponding genes (Supplementary Table S1). The PCR conditions consisted of denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s. Relative transcript levels were calculated as described by Livak and Schmittgen (2001).
Statistical Analysis
Data were subjected to ANOVA and presented as the mean value for each treatment. Statistical analyses were performed using Data Processing System (DPS) statistical software package. A Tukey’s test (P < 0.05) was performed to evaluate the treatment effects. Each treatment value is the average of four to six replicates.
Results
Treatment with Cd Induces Melatonin Biosynthesis
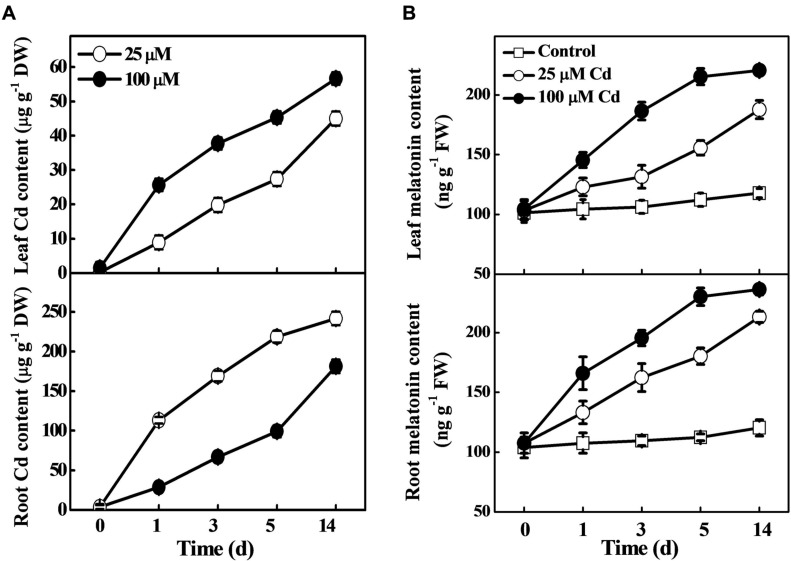
To examine the effect of ecotoxic Cd on melatonin biosynthesis, Cd uptake and translocation, we determined the concentrations of melatonin and Cd in roots and leaves of tomato plants at various time-points. As shown in Figure 1A, both the low (25 μM) and high (100 μM) concentration of Cd induced a substantial increase in Cd accumulation in tomato leaves and roots. In parallel with the Cd content, melatonin accumulation was gradually increased over time after Cd treatment. Notably, high Cd treatment induced high accumulation of melatonin as compared with low Cd treatment. Meanwhile, under optimum conditions, the melatonin concentration remained almost stable in both leaves and roots (Figure 1B). Eventually, the Cd-treated plants displayed approximately 59.6/77.2% and 87.4/96.3% increased melatonin concentration in leaves/roots than those in control under the low and high Cd treatments, respectively at 14 days (Figure 1B). These results confirmed that exogenous Cd could induce endogenous melatonin accumulation in leaves and roots of tomato plants.
FIGURE 1.
Accumulation of cadmium (Cd) and melatonin in tomato leaves and roots as influenced by exogenous Cd treatment. Tomato seedlings at the four-leaf stage were treated with 25 and 100 μM Cd in hydroponics. Endogenous contents of Cd (A) and melatonin (B) were determined at the indicated time points. The data are the means of four replicates, where vertical bars indicate standard errors. DW, dry weight; FW, fresh weight.
Effects of Exogenous Melatonin on Plant Growth, Photosynthesis, and Biomass Accumulation under Cd Stress
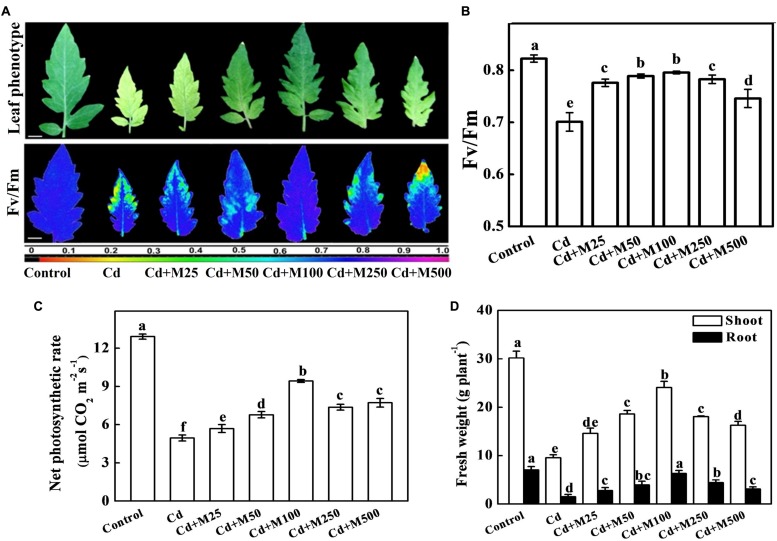
To investigate the role of exogenous melatonin in Cd tolerance, we first focused on the phenotypic changes that occurred in the tomato plants. As shown in Figure 2, plants treated with only Cd displayed severe leaf chlorosis, and decreased Fv/Fm (18.6%), Pn (62.1%), and fresh biomass (67.2%) when compared with that in control. In contrast, almost all doses of melatonin significantly reversed deleterious effects of Cd. Among the tested concentrations of melatonin, moderate concentration particularly 100 μM melatonin showed profound ameliorative effect as evident by 13.5 and 90.4% increased Fv/Fm and Pn, respectively compared with Cd alone treatment (Figures 2B,C). Similarly, Cd-induced reductions in the fresh weights of both shoot and root were significantly alleviated by the treatments with moderate concentrations of exogenous melatonin (Figure 2D). Considering the studied phenotypes, the order of efficiency for the melatonin concentrations in achieving positive effects against Cd was as follows: 100 > 50 > 250 > 500 > 25 μM.
FIGURE 2.
Effects of melatonin on photochemical activity, photosynthetic capacity and biomass accumulation in tomato under Cd stress. (A) Images of leaf phenotype(upper panel) and the maximal photochemical efficiency of PSII (Fv/Fm, lower panel), (B) Fv/Fm values, (C) net photosynthetic rate, and (D) Shoot and root fresh weights in tomato plants after 14 days long Cd (100 μM) stress. The data shown here are the averages of four replicates, with the standard errors indicated by the vertical bars. Means denoted by the same letters did not significantly differ at a P < 0.05, according to Tukey’s test. M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin. False color code depicts at the bottom of (A) ranges vertically from 0.0 (black) to 1.0 (purple). Horizontal bars = 1.0 cm.
Melatonin Protects Plants from Cd-Induced Oxidative Damage
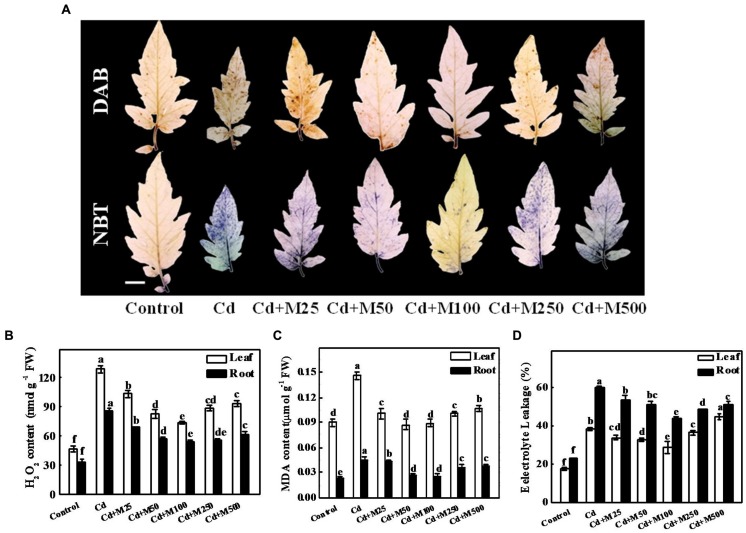
Results from histochemical study showed that the ROS (H2O2 and ) concentrations remained almost constant in leaves under control conditions; however, Cd treatment caused substantial increases in ROS accumulation (Figures 3A,B). Strikingly, melatonin application decreased the excess ROS accumulation under Cd stress (Figures 3A,B). The 100 μM melatonin treatment caused the greatest reduction in ROS accumulation resulting in 43.1/35.9% decreased H2O2 in the tomato leaves/roots under Cd stress (Figure 3B). The MDA concentrations and EL in leaves/roots were increased by approximately 112/139.2% and 118.5/159.3% in the leaf/root of Cd-treated plants compared with the control plants (Figures 3C,D). In line with the effect of melatonin on ROS accumulation, melatonin treatments prevented the Cd-induced increases in EL and MDA to a varying degree (Figures 3C,D). We also quantified the endogenous content of melatonin under different treatments (Supplementary Figure S1). It appears that exogenous melatonin has strong effect on endogenous melatonin accumulation under Cd stress, while greatest effect accounts for 100 μM melatonin. These results suggest that the application of melatonin induces endogenous melatonin content to mitigate Cd phytotoxicity through minimizing Cd-induced oxidative stress in the tomato plants.
FIGURE 3.
Effects of melatonin on ROS accumulation, lipid peroxidation, and membrane integrity after 14 days long Cd stress. (A) The in situ detection of H2O2 (upper panel) and (lower panel) in tomato leaves. Bar = 1.0 cm, (B) H2O2 concentrations in tomato leaves and roots, (C) MDA concentrations in tomato leaves and roots, and (D) Electrolyte leakage from tomato leaves and roots. Accumulation of H2O2 and in leaves was visually detected by staining with 3, 3-diaminobenzidine (DAB) and nitroblue tetrazolium (NBT), respectively. The data shown are the averages of four replicates, with the standard errors indicated by the vertical bars. The means denoted by the same letter within the same color histograms did not significantly differ at a P < 0.05, according to Tukey’s test. Cd, 100 μM cadmium; M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin; FW, fresh weight.
Melatonin Stimulates Activities of Antioxidant Enzyme and H+-ATPase to Confer Cd Tolerance
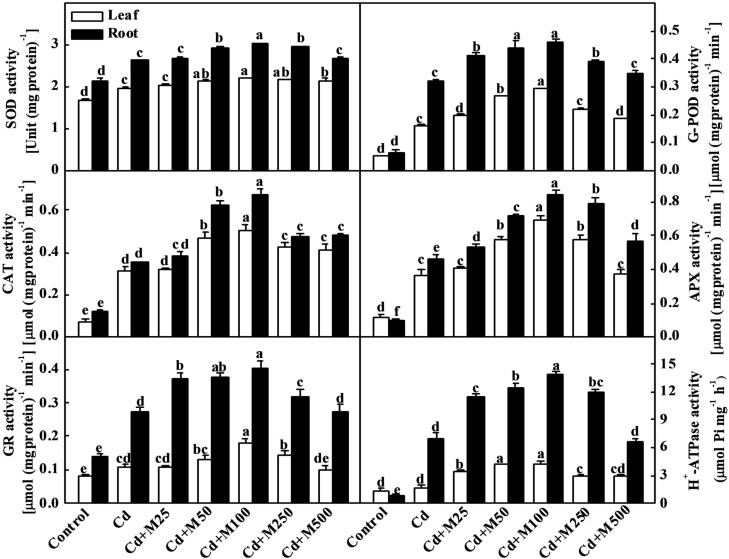
To investigate the role of melatonin in the regulation of the plant antioxidative system under Cd stress, we assessed the activities of SOD, G-POD, CAT, and APX. Cd treatment induced the activities of antioxidant enzymes both in leaves and roots (Figure 4). Interestingly, the exogenous application of different concentrations of melatonin induced further increases in the activities of these enzymes at various degrees. Melatonin treatment at 100 μM concentration showed the best efficiency in this regard. The activities of SOD, G-POD, CAT, APX, and GR were increased by approximately 19.5/15.5%, 84.5/42.8%, 66.6/91.4%, 86.5/82.6%, and 80.2/52.3%, respectively, in leaves/roots under Cd+M100 treatment compared with Cd alone (Figure 4). Likewise, melatonin treatment induced the activity of plasma membrane H+-ATPase which acts as a primary transporter for pumping protons out of the cell (Morsomme and Boutry, 2000). Although Cd induced a 7.1-fold increase in H+-ATPase activity in roots, there was no significant difference in H+-ATPase activity in leaves between the Cd-treated and control plants (Figure 4). Meanwhile, compared with Cd alone treatment, H+-ATPase activity was further induced by combined treatment of melatonin and Cd (Figure 4). These results provide evidence that melatonin-induced alleviation of oxidative stress is associated with the up-regulation of the activities of antioxidant enzymes and H+-ATPase in the leaves and roots of tomato plants.
FIGURE 4.
Activities of antioxidant enzymes and H+-ATPase in tomato leaves and roots as influenced by melatonin and Cd treatments. Tomato seedlings at the four-leaf stage were subjected to 100 μM Cd in hydroponics for 14 days. Meanwhile, foliar portion was sprayed with various concentrations of melatonin at every 5 days. The data shown here are the averages of four replicates, with the standard errors indicated by the vertical bars. The means denoted by the same letter did not significantly differ at a P < 0.05, according to Tukey’s test. Cd, 100 μM cadmium; M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin.
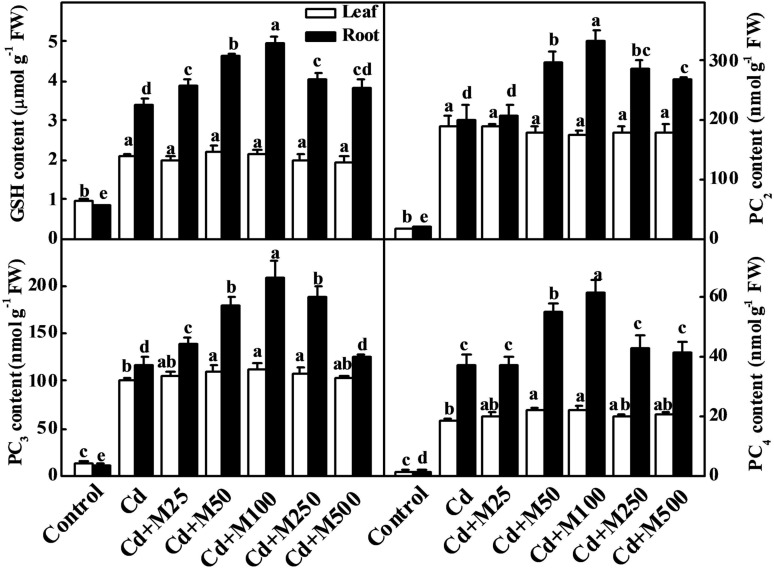
Melatonin Promotes Cd Sequestration in Tomato Plants
To determine the role of melatonin in the biosynthesis of intracellular chelating compounds, we examined the concentrations of various PCs (PC2, PC3, and PC4) and GSH (precursor of PCs synthesis) under Cd stress (Figure 5). Cd treatment significantly increased the endogenous concentrations of PC2, PC3, PC4, and GSH by 9.4/8.9-fold, 7.9/10.0-fold, 14.8/28.7-fold, and 1.2/3.0-fold in leaves/roots, respectively (Figure 5). Interestingly, the treatments with the different concentrations of exogenous melatonin further increased the levels of these thiol compounds in roots under Cd stress (Figure 5). The levels of GSH, PC2, PC3, and PC4 were increased by 46.0, 67.8, 80.7, and 66.5%, preferentially in roots following the 100 μM melatonin treatment under Cd stress (Figure 5).
FIGURE 5.
Effects of melatonin on GSH and PCs concentrations in tomato leaves and roots after 14 days long cadmium stress. The data shown here are the averages of four replicates, with the standard errors indicated by the vertical bars. The means denoted by the same letter within the same color histograms did not significantly differ at a P < 0.05, according to Tukey’s test. Cd, 100 μM cadmium; M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin; FW, fresh weight.
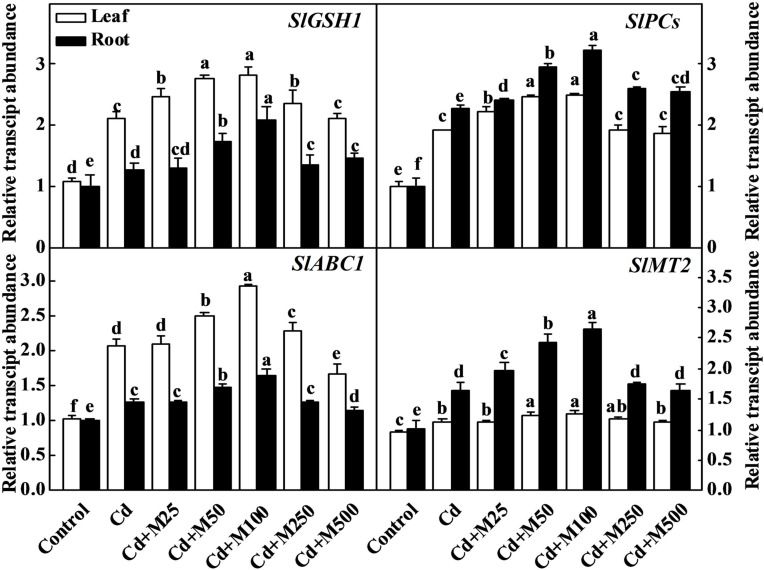
To further understand the molecular mechanism of melatonin-induced Cd detoxification, we analyzed the expression of the SlGSH1, SlPCs, SlMT2, and SlABC1 genes, which are responsible for the synthesis of GSH, PCs, and metallothionein (Whitelaw et al., 1997; Wang et al., 2010; Ahammed et al., 2013; Patade et al., 2013), and ABC transporter that transports Cd-PC complex to vacuole (Hall, 2002; Park et al., 2012). The transcript levels of SlGSH1, SlPCS, SlMT2, and SlABC1 were all induced by Cd stress (Figure 6). Importantly, exogenous melatonin further increased the transcript levels of those genes, and the 100 μM melatonin treatment was the most effective in this regard. The expression levels of SlGSH1, SlPCS, SlMT2, and SlABC1 were increased by approximately 1.4/1.7-fold, 1.3/1.5-fold, 1.2/1.7-fold, and 1.5/1.4-fold in the leaves/roots of the plants treated with 100 μM melatonin compared with those in the leaves/roots of the non-melatonin-treated plants under Cd stress (Figure 6). These observations suggest that melatonin might be involved in the biosynthesis of metal chelating compounds and transport of Cd during Cd stress.
FIGURE 6.
Expression genes related to thiol compound biosynthesis in tomato leaves and roots following 14 days cadmium stress as influenced by melatonin treatments. The data shown here are the averages of four replicates, with the standard errors indicated by the vertical bars. The means denoted by the same letter within the same color histograms did not significantly differ at a P < 0.05, according to Tukey’s test. Cd, 100 μM cadmium; M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin; FW, fresh weight.
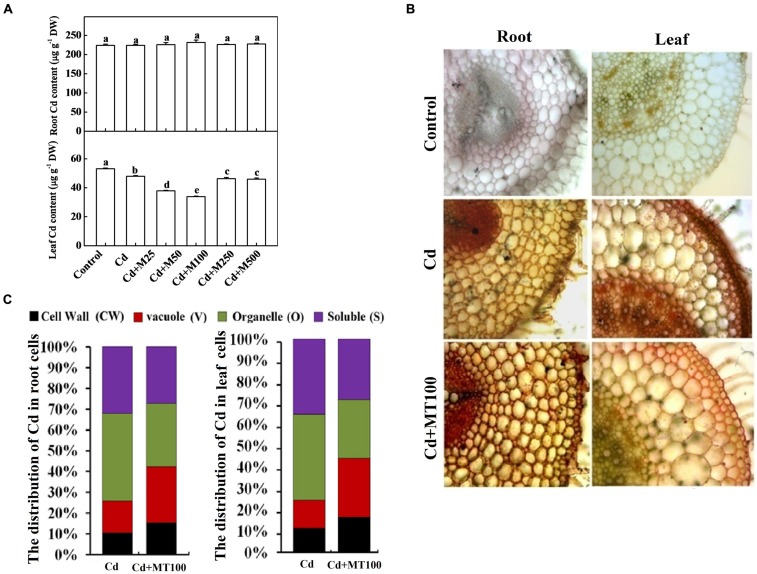
Role of Melatonin in the Uptake, Subcellular Distribution, and Localization of Cd in Plant Cells
To understand the effects of melatonin on Cd uptake and its cellular distribution in tomato cells, we assessed the total Cd content, subcellular distribution and localization in leaf and root tissues. The Cd concentrations were approximately 224.19 and 54.16 μg (g DW)-1 in roots and leaves, respectively, after 14-days long Cd treatment (Figure 7A). Interestingly, Cd accumulation was not altered in roots but was significantly reduced in leaves with the melatonin treatments (Figure 7A). Treatment with 100 μM melatonin resulted in the greatest reduction in the leaf Cd concentration which was about 39.15% below the Cd alone treatment (Figure 7A). We also visualized the localization of Cd in root and leaf tissues using a dithizone staining-based histochemical method. As shown in Figure 7B, compared with leaf tissue, intensive Cd accumulation was observed in root steles regardless of the melatonin treatment. Similarly, the less intensive brown color deposits in leaf tissues indicated lower Cd content in leaf as compared with root. In line with quantitative data, visualization of Cd in Cd + 100 μM melatonin treatment showed less Cd accumulation in leaf tissues when compared with Cd alone treatment (Figure 7B).
FIGURE 7.
The accumulation of cadmium in tomato plants and its subcellular distribution following 14 days long Cd stress as influenced by melatonin treatments. (A) Cd concentrations in tomato roots and leaves. (B) The in situ detection of Cd in tomato roots and leaves using a dithizone staining-based histochemical method. (C) The distribution of Cd in different subcellular compartments. The data shown in (A), are the averages of four replicates, with the standard errors indicated by the vertical bars. The means denoted by the same letter did not significantly differ at a P < 0.05, according to Tukey’s test. Cd, 100 μM cadmium; M25, 25 μM melatonin; M50, 50 μM melatonin; M100, 100 μM melatonin; M250, 250 μM melatonin; M500, 500 μM melatonin; DW, dry weight.
To examine the subcellular distribution of Cd as influenced by exogenous melatonin treatments, we quantified the Cd content in various cellular compartments of leaf and root tissues. We found that under Cd alone treatment, the Cd levels in the cell wall (CW), vacuole (V), organelle (O), and soluble (S) fractions were approximately 10.6/11.3%, 15.3/13.1%, 42.1/40.2%, 32.0/35.4% of total Cd content in the root/leaf cells, respectively (Figure 7C). Importantly, treatment with 100 μM melatonin altered the subcellular distributions of Cd in both root and leaf cells. The Cd levels in the CW and V fractions, where Cd is preferentially sequestered to minimize cellular toxicity, were increased by 25.9/24.3% and 42.1/44.1% in the root/leaf cells, respectively after melatonin treatment (Figure 7C). Meanwhile, the Cd levels in the O and S fractions, where Cd is mostly free and highly toxic, were decreased by 74.1/75.6% and 57.8/55.9% in the root/leaf cells, respectively after melatonin treatment (Figure 7C). These findings indicate a critical role of melatonin in promoting Cd immobilization for minimizing cellular toxicity.
Discussion
In the present work, we elucidated some unique functions of melatonin in response to Cd stress in tomato, an important horticultural crop and model plant system as well. We noticed that endogenous melatonin could be induced by both low and high concentrations of Cd (Figure 1). Notably, exogenous application of melatonin significantly improved plant tolerance to Cd by enhancing the antioxidant capacity, PCs biosynthesis, and compartmentalization of Cd in cell walls and vacuoles (Figures 2–7). Besides, melatonin restricted Cd transport from root to shoot in order to protect photosynthetic apparatus from Cd-induced damages. We propose that, in addition to direct function of melatonin as antioxidant and metal chelator, melatonin-induced biosynthesis of phytochelatins and subsequent compartmentalization of Cd in cell wall and vacuole might play a critical role to confer Cd tolerance in tomato.
Melatonin is a major animal hormone involved in regulating seasonal reproductive function and modulating circadian rhythms. In recent years, a growing body of evidence has suggested that it plays a significant role in plant responses to environmental stimuli, including biotic and abiotic stresses (Park et al., 2013; Zhang et al., 2013; Bajwa et al., 2014; Lee et al., 2014). Oxidative stress resulted from various forms of environmental extremes has been reported to affect the endogenous level of melatonin in a range of plant species (Arnao and Hernandez-Ruiz, 2009b). Additionally, exogenous application of melatonin was found effective to protect plant cell from oxidative damage (Zhang et al., 2015). In the present study, exposure of tomato seedlings to low and high concentrations of Cd induced substantial increases in endogenous melatonin concentrations gradually over time. However, such increase in endogenous melatonin was unable to ameliorate oxidative stress possibly due to insufficient ROS scavenging by melatonin in parallel with Cd-induced ROS generation. Nevertheless, induction of endogenous melatonin by Cd may indicate an adaptive response in plants as melatonin is well-recognized as a ubiquitous signal molecule (Arnao and Hernández-Ruiz, 2014; Byeon et al., 2015). In contrast, exogenous application of melatonin efficiently ameliorate Cd-induced oxidative stress as characterized by low ROS accumulation, reduced MDA content and decreased EL percentage. We also noticed that exogenous melatonin greatly induced endogenous melatonin content in a concentration-dependent manner. The highest level of melatonin was recorded in 100 μM melatonin + Cd treatment followed by a decreasing trend with the increase in exogenous melatonin concentration (Supplementary Figure S1). This observation confirmed that exogenous melatonin could stimulate endogenous melatonin level in a dose dependent manner to confer Cd tolerance. It is worth mentioning that low and high concentration of melatonin showed significantly different roles in regulating plant growth and development even in the same species, which support our current observation (Zhang et al., 2015). By virtue, melatonin can cross cell membrane easily and enter subcellular compartments. Given that melatonin is a ubiquitous antioxidant and thus it might be plausible that exogenous melatonin functions as an antioxidant at the initial stage, which is strictly supply dependent (Poeggeler et al., 1996; Tan et al., 2000). As the frequency of melatonin application was sparse in our current study, exogenous melatonin might not suffice proper neutralization of ROS generated from continuous Cd exposure, indicating some other mechanisms involved in melatonin-mediated Cd stress tolerance. It is quite possible that exogenous melatonin may trigger endogenous melatonin biosynthesis which then serves as antioxidant and also regulates other defense pathways for environmental adaptation.
Recent studies have revealed that pre-sowing seed treatment with melatonin protects red cabbage seedlings against toxic Cu2+ (Posmyk et al., 2008), and the supplementation with exogenous melatonin alleviates Cd-induced stress in algae (Tal et al., 2011). In cucumber seedlings, melatonin treatment decreased chlorophyll degradation and enhanced the photosynthetic rate and antioxidant potential, and thereby ameliorating the deleterious effects of water stress (Zhang et al., 2013). Consistent with these findings, we also found that melatonin increased the chlorophylls contents (data not shown), photosynthetic capacity and biomass accumulation in both non-stressed and Cd-stressed plants. (Figure 2; Supplementary Figure S2). It is speculated that melatonin increases photosynthetic efficiency through an unusual biostimulatory pathway by improving the efficiency of photosystem II under both dark and light conditions in plants (Wang et al., 2013). Serving as a signal, melatonin can activate the antioxidant system to promote the scavenging of stress-induced ROS (Tal et al., 2011; Park et al., 2013). Accordingly, melatonin activated SOD, G-POD, APX, CAT, and GR in the present study, which might partially contribute to ROS scavenging under Cd stress (Figure 4). Taken together, it is quite likely that melatonin acts as a signal molecule to trigger defense systems, such as the antioxidant system, resulting in the alleviation of oxidative stress.
Phytochelatins are synthesized from GSH through an enzymatic pathway, and they chelate metal ions with the -SH groups of cysteine residues (Hall, 2002). The current study showed that thiol compounds, including GSH and PCs, accumulated under Cd stress in tomato leaves and roots, were further induced in roots by exogenous melatonin treatment (Figure 5). Melatonin also increased GR activity required to maintain the GSH/GSSG (oxidized GSH) ratio in tomato plants under Cd stress (Figure 4). Recently, Wang et al. (2012) have found that melatonin increases the GSH concentration to delay the senescence of apple leaves by stimulating the activity of γ-glutamylcysteine. The expression levels of the SlGSH1 and SlPCS genes, which are responsible for the biosyntheses of GSH and PCs in tomato plants, respectively, were increased by Cd. Exogenous melatonin treatment further upregulated expression of SlGSH1 and SlPCS (Figure 6). Although expression of those genes and GR activity were increased in the melatonin-treated leaves under Cd stress (Figure 6), the concentration of GSH in leaves remained unaltered (Figure 5). Ben Ammar et al. (2008) reported that a high level of PC synthesis in roots was coupled to a decrease in the GSH concentration in leaves which might partially support our current observation. It seems that post-transcriptional events might be involved in maintaining GSH and PCs concentration in tomato leaves.
In plant cells, the toxicity of free metal ions can be decreased by their reactions with metal ligands, including proteins, polysaccharides, and organic acids (Hall, 2002). Nevertheless, the fraction of free metal ions that are not chelated can be transferred to metabolic organelles, such as chloroplasts, mitochondria, and nuclei. These free metal ions cause malfunctions of cellular organelles and cell damage (Zeng et al., 2011). Several studies have revealed that decreased translocation of Cd from roots to shoots is an effective barrier which promotes Cd sequestration in the cortex and endodermis of roots (Akhter et al., 2012, 2014). In our study, although melatonin did not affect the Cd content in root, it (50 and 100 μM) significantly reduced the leaf Cd content, suggesting that melatonin might induce barrier for preventing translocation of Cd from root to shoot (Figures 7A,B). We also noticed that exogenous melatonin treatment greatly improved immobilization of Cd in the cell wall and vacuoles that substantially minimized Cd toxicity (Figure 7C). Cd immobilization may be correlated with the melatonin-induced biosynthesis of thiol compounds (Figure 5; Ben Ammar et al., 2008). Given that melatonin restricted Cd translocation from root to shoot, it might facilitate healthy cellular atmosphere for normal functioning in leaves. As leaves accumulated less Cd following melatonin treatment, demand for GSH and PCs in leaves was comparatively low for metal detoxification, while roots appeared as major site for Cd detoxification staying in direct contact. It seemed logical that the higher thiol-peptide levels in roots of melatonin-treated plants might act as a sink for thiol-reactive metal and thus these plants could sequester higher levels of Cd. All of these results indicate that exogenous melatonin increases the proportion of immobilized Cd through cell wall binding and vacuole sequestration, thereby increasing the detoxification of this metal (Zeng et al., 2011).
Cadmium competes for the same transport systems with mineral nutrients, and it alters plasma membrane stability and the ionic balance (Llamas et al., 2000). Plasma membrane H+-ATPase transports ions and organic compounds across the plasma membrane (Morsomme and Boutry, 2000). Melatonin can be transformed into 5-methoxytryptamine and thus stimulating H+-ATPase activity in plants (Hardeland et al., 1995). Nevertheless, melatonin also protects the plasma membrane lipid environment by preventing Cd-induced ROS generation (Tan et al., 2012) and maintaining increased levels of antioxidants (Zhang et al., 2013). In line with previous reports, the current study showed that melatonin induced H+-ATPase activity under Cd stress (Figure 4), which might have significant role in maintaining plasma membrane stability and EL to confer Cd tolerance (Figure 3D).
Survival of plants under polluted environments largely depends on plants’ ability to sequester and/or detoxify toxic pollutants such as Cd. This study showed that both exogenous melatonin and Cd stress could induce endogenous melatonin accumulation. However, exogenous melatonin had complex and influential effects on plant growth, ROS scavenging, antioxidant potential, thiol compound biosynthesis and Cd immobilization in cell walls and vacuoles under Cd stress. Even though, exogenous melatonin had no effect on root Cd content, it significantly decreased leaf Cd content, indicating its potential role in regulating Cd translocation. Therefore, these findings can be implicated for developing new strategies to produce safe food in an eco-friendly manner particularly in marginal areas where Cd contamination is a limiting factor for crop production. Further studies are needed to provide genetic evidence in support of the involvement of melatonin in Cd detoxification and to elucidate the subsequent signaling cascades in plants.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31430076, 31401877), the Geological Exploration Foundation of Zhejiang Province, China (2014002-02).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00601
References
- Ahammed G. J., Choudhary S. P., Chen S., Xia X., Shi K., Zhou Y., et al. (2013). Role of brassinosteroids in alleviation of phenanthrene–cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J. Exp. Bot. 64 199–213. 10.1093/jxb/ers323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed I. M., Dai H. X., Zheng W. T., Cao F. B., Zhang G. P., Sun D. F., et al. (2013). Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 63 49–60. 10.1016/j.plaphy.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Ajjimaporn A., Botsford T., Garrett S. H., Sens M. A., Zhou X. D., Dunlevy J. R., et al. (2012). ZIP8 expression in human proximal tubule cells, human urothelial cells transformed by Cd+2 and As+3 and in specimens of normal human urothelium and urothelial cancer. Cancer Cell Int. 12 16 10.1186/1475-2867-12-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter M. F., McGarvey B., Macfie S. M. (2012). Reduced translocation of cadmium from roots is associated with increased production of phytochelatins and their precursors. J. Plant Physiol. 169 1821–1829. 10.1016/j.jplph.2012.07.011 [DOI] [PubMed] [Google Scholar]
- Akhter M. F., Omelon C. R., Gordon R. A., Moser D., Macfie S. M. (2014). Localization and chemical speciation of cadmium in the roots of barley and lettuce. Environ. Exp. Bot. 100 10–19. 10.1016/j.envexpbot.2013.12.005 [DOI] [Google Scholar]
- Arnao M. B., Hernandez-Ruiz J. (2009a). Assessment of different sample processing procedures applied to the determination of melatonin in plants. Phytochem. Anal. 20 14–18. 10.1002/Pca.1083 [DOI] [PubMed] [Google Scholar]
- Arnao M. B., Hernandez-Ruiz J. (2009b). Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 46 295–299. 10.1111/j.1600-079X.2008.00660.x [DOI] [PubMed] [Google Scholar]
- Arnao M. B., Hernández-Ruiz J. (2014). Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 19 789–797. 10.1016/j.tplants.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Bajji M., Kinet J. M., Lutts S. (2002). The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regul. 36 61–70. 10.1023/A:1014732714549 [DOI] [Google Scholar]
- Bajwa V. S., Shukla M. R., Sherif S. M., Murch S. J., Saxena P. K. (2014). Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 56 238–245. 10.1111/Jpi.12115 [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovic I. (1971). Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44 276–287. 10.1016/0003-2697(71)90370-8 [DOI] [PubMed] [Google Scholar]
- Ben Ammar W., Mediouni C., Tray B., Ghorbel M. H., Jemal F. (2008). Glutathione and phytochelatin contents in tomato plants exposed to cadmium. Biol. Plantarum 52 314–320. 10.1007/s10535-008-0065-9 [DOI] [Google Scholar]
- Byeon Y., Lee H. Y., Hwang O. J., Lee H. J., Lee K., Back K. (2015). Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 58 470–478. 10.1111/Jpi.12232 [DOI] [PubMed] [Google Scholar]
- Cakmak I., Marschner H. (1992). Magnesium-deficiency and high light-intensity enhance activities of superoxide-dismutase, ascorbate peroxidase, and glutathione-reductase in bean-leaves. Plant Physiol. 98 1222–1227. 10.1104/Pp.98.4.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. R., Gonzalez-Yanes C., Maldonado M. D. (2013). The role of melatonin in the cells of the innate immunity: a review. J. Pineal Res. 55 103–120. 10.1111/Jpi.12075 [DOI] [PubMed] [Google Scholar]
- Cui W. T., Li L., Gao Z. Z., Wu H. H., Xie Y. J., Shen W. B. (2012). Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 63 5521–5534. 10.1093/Jxb/Ers201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G., Farinati S., Furini A. (2010). Regulatory networks of cadmium stress in plants. Plant Signal. Behav. 5 663–667. 10.4161/psb.5.6.11425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Toppi L. S., Gabbrielli R. (1999). Response to cadmium in higher plants. Environ. Exp. Bot. 41 105–130. 10.1016/S0098-8472(98)00058-6 [DOI] [Google Scholar]
- Escames G., Ozturk G., Bano-Otalora B., Pozo M. J., Madrid J. A., Reiter R. J., et al. (2012). Exercise and melatonin in humans: reciprocal benefits. J. Pineal Res. 52 1–11. 10.1111/j.1600-079X.2011.00924.x [DOI] [PubMed] [Google Scholar]
- Foyer C. H., Halliwell B. (1976). Presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic-acid metabolism. Planta 133 21–25. 10.1007/Bf00386001 [DOI] [PubMed] [Google Scholar]
- Hall J. L. (2002). Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 53 1–11. 10.1093/jexbot/53.366.1 [DOI] [PubMed] [Google Scholar]
- Hardeland R., Balzer I., Poeggeler B., Fuhrberg B., Uria H., Behrmann G., et al. (1995). On the primary functions of melatonin in evolution: Mediation of photoperiodic signals in a unicell, photooxidation, and scavenging of free-radicals. J. Pineal Res. 18 104–111. 10.1111/j.1600-079X.1995.tb00147.x [DOI] [PubMed] [Google Scholar]
- Hodges D. M., DeLong J. M., Forney C. F., Prange R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207 604–611. 10.1007/s004250050524 [DOI] [PubMed] [Google Scholar]
- Jabs T., Dietrich R. A., Dangl J. L. (1996). Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Sci. 273 1853–1856. 10.1126/science.273.5283.1853 [DOI] [PubMed] [Google Scholar]
- Janicka-Russak M., Kabala K., Burzynski M. (2012). Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 63 4133–4142. 10.1093/Jxb/Ers097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozefczak M., Keunen E., Schat H., Bliek M., Hernandez L. E., Carleer R., et al. (2014). Differential response of Arabidopsis leaves and roots to cadmium: glutathione-related chelating capacity vs antioxidant capacity. Plant Physiol. Biochem. 83 1–9. 10.1016/j.plaphy.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Korkmaz A., Deger O., Cuci Y. (2014). Profiling the melatonin content in organs of the pepper plant during different growth stages. Sci. Hortic. 172 242–247. 10.1016/j.scienta.2014.04.018 [DOI] [Google Scholar]
- Lee H. Y., Byeon Y., Back K. (2014). Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res 57 262–268. 10.1111/Jpi.12165 [DOI] [PubMed] [Google Scholar]
- Li C., Wang P., Wei Z. W., Liang D., Liu C. H., Yin L. H., et al. (2012). The mitigation effects of exogenous melatonin on salinity-induced stress in Malus hupehensis. J. Pineal Res. 53 298–306. 10.1111/j.1600-079X.2012.00999.x [DOI] [PubMed] [Google Scholar]
- Liu J. G., Liang J. S., Li K. Q., Zhang Z. J., Yu B. Y., Lu X. L., et al. (2003). Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere 52 1467–1473. 10.1016/S0045-6535(03)00484-3 [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Llamas A., Ullrich C. I., Sanz A. (2000). Cd2+ effects on transmembrane electrical potential difference, respiration and membrane permeability of rice (Oryza sativa L) roots. Plant Soil 219 21–28. 10.1023/A:1004753521646 [DOI] [Google Scholar]
- Maksymiec W. (2007). Signaling responses in plants to heavy metal stress. Acta Physiol. Plant. 29 177–187. 10.1007/s11738-007-0036-3 [DOI] [Google Scholar]
- Marty F., Branton D. (1980). Analytical characterization of beetroot vacuole membrane. J. Cell Biol. 87 72–83. 10.1083/Jcb.87.1.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood A., Iqbal N., Khan N. A. (2012). Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 35 524–533. 10.1111/j.1365-3040.2011.02432.x [DOI] [PubMed] [Google Scholar]
- Meharg A. A. (1993). The role of the plasmalemma in metal tolerance in angiosperms. Physiol. Plant 88 191–198. 10.1111/j.1399-3054.1993.tb01777.x [DOI] [Google Scholar]
- Metwally A., Finkemeier I., Georgi M., Dietz K. J. (2003). Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 132 272–281. 10.1104/pp.102.018457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minocha R., Thangavel P., Dhankher O. P., Long S. (2008). Separation and quantification of monothiols and phytochelatins from a wide variety of cell cultures and tissues of trees and other plants using high performance liquid chromatography. J. Chromatogr. A 1207 72–83. 10.1016/j.chroma.2008.08.023 [DOI] [PubMed] [Google Scholar]
- Mobin M., Khan N. A. (2007). Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J. Plant Physiol. 164 601–610. 10.1016/j.jplph.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Morsomme P., Boutry M. (2000). The plant plasma membrane H+-ATPase: structure, function and regulation. Biochim. Biophys. Acta 1465 1–16. 10.1016/S0005-2736(00)00128-0 [DOI] [PubMed] [Google Scholar]
- Nakano Y., Asada K. (1981). Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol. 22 867–880. [Google Scholar]
- Neumann D., Lichtenberger O., Gunther D., Tschiersch K., Nover L. (1994). Heat-shock proteins induce heavy-metal tolerance in higher-plants. Planta 194 360–367. 10.1007/BF00197536 [DOI] [Google Scholar]
- Park J., Song W. Y., Ko D., Eom Y., Hansen T. H., Schiller M., et al. (2012). The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 69 278–288. 10.1111/j.1365-313X.2011.04789.x [DOI] [PubMed] [Google Scholar]
- Park S., Lee D. E., Jang H., Byeon Y., Kim Y. S., Back K. (2013). Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 54 258–263. 10.1111/j.1600-079X.2012.01029.x [DOI] [PubMed] [Google Scholar]
- Patade V. Y., Khatri D., Kumari M., Grover A., Gupta S. M., Ahmed Z. (2013). Cold tolerance in Osmotin transgenic tomato (Solanum lycopersicum L.) is associated with modulation in transcript abundance of stress responsive genes. Springerplus 2 117 10.1186/2193-1801-2-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F., Iannelli M. A., Pasqualini S., Massacci A. (2003). Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex steudel. Plant Physiol. 133 829–837. 10.1104/pp.103.026518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeggeler B., Reiter R. J., Hardeland R., Tan D. X., BarlowWalden L. R. (1996). Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 2 179–184. [DOI] [PubMed] [Google Scholar]
- Posmyk M. M., Kuran H., Marciniak K., Janas K. M. (2008). Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 45 24–31. 10.1111/j.1600-079X.2008.00552.x [DOI] [PubMed] [Google Scholar]
- Rauser W. E. (1995). Phytochelatins and related peptides - structure, biosynthesis, and function. Plant Physiol. 109 1141–1149. 10.1104/pp.109.4.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas M. C., Palma J. M., Gomez M., Del Rio L. A., Sandalio L. M. (2002). Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell Environ. 25 677–686. 10.1046/j.1365-3040.2002.00850.x [DOI] [Google Scholar]
- Seregin I. V., Ivanov V. B. (1997). Histochemical investigation of cadmium and lead distribution in plants. Russ. J. Plant Physiol. 44 791–796. [Google Scholar]
- Stroinski A., Gizewska K., Zielezinska M. (2013). Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 57 121–127. 10.1007/s10535-012-0135-x [DOI] [Google Scholar]
- Tal O., Haim A., Harel O., Gerchman Y. (2011). Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 62 1903–1910. 10.1093/Jxb/Erq378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D. X., Hardeland R., Manchester L. C., Korkmaz A., Ma S. R., Rosales-Corral S., et al. (2012). Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63 577–597. 10.1093/Jxb/Err256 [DOI] [PubMed] [Google Scholar]
- Tan D. X., Manchester L. C., Reiter R. J., Qi W. B., Karbownik M., Calvo J. R. (2000). Significance of melatonin in antioxidative defense system: reactions and products. Biol. Signals Recept. 9 137–159. 10.1159/000014635 [DOI] [PubMed] [Google Scholar]
- ThordalChristensen H., Zhang Z. G., Wei Y. D., Collinge D. B. (1997). Subcellular localization of H2O2 in plants. Plant J. 11 1187–1194. 10.1046/j.1365-313X.1997.11061187.x [DOI] [Google Scholar]
- Wang L. N., Yang L. M., Yang F. J., Li X. G., Song Y. P., Wang X. F., et al. (2010). Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J. Plant Physiol. 167 1298–1306. 10.1016/j.jplph.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Wang P., Sun X., Li C., Wei Z., Liang D., Ma F. (2013). Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 54 292–302. 10.1111/jpi.12017 [DOI] [PubMed] [Google Scholar]
- Wang P., Yin L. H., Liang D., Li C., Ma F. W., Yue Z. Y. (2012). Delayed senescence of apple leaves by exogenous melatonin treatment: toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 53 11–20. 10.1111/j.1600-079X.2011.00966.x [DOI] [PubMed] [Google Scholar]
- Whitelaw C. A., LeHuquet J. A., Thurman D. A., Tomsett A. B. (1997). The isolation and characterisation of type II metallothionein-like genes from tomato (Lycopersicon esculentum L.). Plant Mol. Biol. 33 503–511. 10.1023/A:1005769121822 [DOI] [PubMed] [Google Scholar]
- Willekens H., Chamnongpol S., Davey M., Schraudner M., Langebartels C., VanMontagu M., et al. (1997). Catalase is a sink for H2O2 and is indispensable for stress defence in C-3 plants. EMBO J. 16 4806–4816. 10.1093/emboj/16.16.4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F. B., Dong J., Qian Q. Q., Zhang G. P. (2005). Subcellular distribution and chemical form of Cd and Cd-Zn interaction in different barley genotypes. Chemosphere 60 1437–1446. 10.1016/j.chemosphere.2005.01.071 [DOI] [PubMed] [Google Scholar]
- Xiong J., An L. Y., Lu H., Zhu C. (2009). Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230 755–765. 10.1007/s00425-009-0984-5 [DOI] [PubMed] [Google Scholar]
- Zeng F. R., Zhou W. H., Qiu B. Y., Ali S., Wu F. B., Zhang G. P. (2011). Subcellular distribution and chemical forms of chromium in rice plants suffering from different levels of chromium toxicity. J. Plant Nutr. Soil Sci. 174 249–256. 10.1002/jpln.200900309 [DOI] [Google Scholar]
- Zhang N., Sun Q. Q., Zhang H. J., Cao Y. Y., Weeda S., Ren S. X., et al. (2015). Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 66 647–656. 10.1093/Jxb/Eru336 [DOI] [PubMed] [Google Scholar]
- Zhang N., Zhao B., Zhang H. J., Weeda S., Yang C., Yang Z. C., et al. (2013). Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 54 15–23. 10.1111/j.1600-079X.2012.01015.x [DOI] [PubMed] [Google Scholar]
- Zhou J., Wang J., Li X., Xia X. J., Zhou Y. H., Shi K., et al. (2014). H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 65 4371–4383. 10.1093/Jxb/Eru217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.