Abstract
Background
The increasing prevalence of atrial fibrillation (AF) represents a public health issue. Identifying new predictors of AF is therefore necessary to plan preventive strategies. We investigated whether left ventricular (LV) systolic dysfunction by global longitudinal strain (GLS), a predictor of cardiovascular events, may predict new-onset AF in a population setting.
Methods and Results
Participants (n=675, mean age 71±9 years, 60% women) in sinus rhythm from the population-based Northern Manhattan Study underwent two- and three-dimensional echocardiography as part of the Cardiac Abnormalities and Brain Lesions (CABL) study. LV systolic function was assessed by LV ejection fraction (LVEF) and speckle-tracking GLS. Over a mean follow-up of 63.6±18.7 months, 32 (4.7%) new confirmed cases of AF occurred. Lower GLS [adjusted hazard ratio (aHR)/unit decrease=1.22, 95% confidence interval (CI)=1.04-1.43, p=0.015] and increased left atrial volume index (LAVi) (aHR/unit increase=1.12, 95% CI=1.07-1.17, p<0.001) were significantly associated with incident AF, whereas LVEF was not (p=0.176). GLS>-14.7% was associated with risk of new-onset AF with an aHR=3.2 (95% CI=1.4-7.5, p=0.007). The coexistence of abnormal GLS/abnormal LAVi was associated with a 28.6% incidence of AF (aHR=12.1, 95% CI=3.3-44.8, p<0.001) compared to participants with normal GLS/normal LAVi (AF incidence=2.0%). AF incidence was intermediate in those with either abnormal GLS or abnormal LAVi (9.3% and 11.1%, respectively). GLS prognostic value for incident AF was incremental over risk factors and LAVi.
Conclusions
LV systolic dysfunction by GLS was a powerful and independent predictor of incident AF. GLS assessment may improve AF risk stratification in addition to established parameters.
Keywords: atrial fibrillation, longitudinal strain, echocardiography, speckle tracking, risk prediction
In the United States, atrial fibrillation (AF) affects over 2 million persons. The prevalence of AF significantly increases in older age, with only 0.1% of the population affected before age 55, 3.8% affected over age 60, and 9.0% among those over 80 years old. Due to the aging of the population, AF prevalence is expected to rise significantly, with estimates ranging from 5.6 million to over 10 million by year 2050.1, 2 These figures make AF a public health issue, aggravated by its strong association with stroke, heart failure, and death.3, 4 It is therefore of critical importance to identify subjects at high-risk of developing AF, especially in the elderly subgroup of the general population.
Population studies identified age, hypertension, diabetes, heart failure, and myocardial infarction as major risk factors for development of AF.5 Among echocardiographic variables, increased left atrial volume is an established predictor of incident AF, independent of and incremental to clinical risk factors.6 Recently, echocardiographic speckle-tracking imaging provided new insights in cardiac function assessment, shifting the attention from traditional measures of LV cavity reduction such as LV ejection fraction (LVEF) to the analysis of myocardial tissue deformation. LV global longitudinal strain (GLS), a measure of the myocardial systolic deformation over the longitudinal axis, is emerging as a robust parameter able to detect early LV systolic dysfunction in a variety of conditions, even in subjects without overt cardiac disease.7, 8 A reduction of GLS is associated with unfavorable cardiovascular outcome and mortality, and its prognostic value is independent of LVEF.9, 10 Although coronary artery disease and congestive heart failure are established risk factors for AF, it is not known whether subclinical LV dysfunction assessed by GLS can predict incident AF in the general population and especially in the elderly, who are at greater risk of AF and therefore of ischemic stroke. Accordingly, we investigated the association between LV systolic dysfunction measured by speckle-tracking GLS and the development of new-onset AF in a community-based elderly cohort. We also assessed whether GLS provided additional prognostic information towards AF development over established AF predictors.
METHODS
Study population
The Cardiovascular Abnormalities and Brain Lesion (CABL) study is a community-based epidemiologic study designed to investigate potential cardiovascular predictors of silent cerebrovascular disease in the community. CABL based its recruitment on the Northern Manhattan Study (NOMAS), a population prospective study investigating the epidemiology and risk factors for stroke and cardiovascular disease that enrolled 3,298 participants from the community living in northern Manhattan between 1993 and 2001. The study design and recruitment details of NOMAS have been described previously.11 Beginning in 2003, participants were invited to participate in a brain MRI sub-study if they: 1) were at least 50 years of age; 2) had no contraindications to MRI; and 3) did not have a prior diagnosis of stroke. From September 2005 to July 2010, NOMAS MRI participants that voluntarily agreed to undergo an extensive cardiovascular evaluation were included in CABL. Digital echocardiographic exams with native data for speckle-tracking assessment were available in 854 CABL participants. In 125, image quality was suboptimal for speckle-tracking analysis. Of the remaining 729 participants, 44 had either history of AF or AF at study enrollment documented by 12-lead ECG tracing; an additional 5 were excluded because of significant mitral valve disease, leading to a study sample of 680. Written informed consent was obtained from all study participants. The study protocol was approved by the Institutional Review Boards of Columbia University Medical Center and of the University of Miami.
Baseline assessment and follow-up
Cardiovascular risk factors were ascertained through direct examination and interview by trained research assistants as previously described.11 Hypertension was defined as systolic blood pressure (BP) ≥140 mmHg or diastolic BP ≥90 mmHg, or self-reported history of hypertension or use of anti-hypertensive medication. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or self-reported history of diabetes or use of diabetes medications. Hypercholesterolemia was defined as total serum cholesterol >240 mg/dL, self-report of hypercholesterolemia or use of lipid-lowering treatment. Obesity was defined as a body mass index ≥ 30 kg/m2. Coronary artery disease was defined as a history of myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention. All subjects were followed-up annually by telephone interviews. Development of new-onset AF was ascertained through a questionnaire by trained research personnel and then confirmed by analysis of ECG tracings or medical records. Only confirmed AF cases were included in the analysis.
Echocardiographic assessment
Transthoracic echocardiography was performed using a commercially available system (iE 33, Philips, Andover, MA) by a registered cardiac sonographer according to a standardized protocol. LV wall thickness and diameters were measured from a parasternal long-axis view according to the recommendations of the American Society of Echocardiography.12 LV volumes and LVEF were calculated using the biplane modified Simpson’s rule. LV end-diastolic diameter and LV volumes were indexed by body surface area. LV mass was calculated with a validated method13 and indexed by body surface area (LV mass index). LV relative wall thickness was calculated as: 2 × posterior wall thickness/LV end-diastolic diameter. Left atrial volume was measured by three-dimensional echocardiography as previously described14 and indexed by body surface area (left atrial volume index, LAVi). Abnormal LAVi was defined as a value greater than the 95th percentile of the LAVi distribution (31.0 ml/m2) in a healthy subgroup of participants free of cardiovascular risk factors. LV diastolic function assessment has been previously described.15 Briefly, in apical 4-chamber view, peak early velocity (E), its deceleration time (DT), and late velocity (A) of mitral inflow were measured by pulsed-wave Doppler with sample volume placed at mitral valve tips. Peak early diastolic velocity (e’) of the lateral and septal mitral annulus were evaluated by pulsed-wave tissue-Doppler and averaged. LV diastolic dysfunction was defined as: E/A ≤ 0.7 or DT>260 ms; or E/A between 0.7-1.5 and e’ < 7 cm/s; or E/A > 1.5 and e’ < 7 cm/s or DT<140 ms.16 GLS analysis was performed by two-dimensional speckle-tracking technique using commercially available software (Philips QLAB Advanced Quantification Software, version 8.1) from two-dimensional gray-scale loops as described previously.9, 17 GLS was calculated averaging the negative peak of longitudinal strain from 12 ventricular segments from the apical 4-chamber and 2-chamber views. Abnormal GLS was previously defined as GLS > -14.7% (GLS is a negative number, therefore less negative values correspond to smaller systolic longitudinal shortening), representing the cut-off identifying the lower 5% of the GLS distribution in a healthy subgroup of participants.9 Inter-observer reproducibility of GLS measurement was assessed in a random sample of 20 study participants. Intra-class correlation coefficient for GLS was 0.85. Mean difference between measurements was 0.08±2.4%, and the coefficient of variation (standard deviation/mean) was 0.09.
Statistical analysis
Data are presented as means ± standard deviation for continuous variables and as percentage for categorical variables. The t-test and Chi-square test were used to assess differences between groups. Cox proportional hazards models were used to assess the association of echocardiographic parameters with incident AF, and hazard ratios (HR) and 95% confidence intervals (CI) were calculated. The Cox regressions were modified to allow for the presence of death as a competing risk using the method described by Fine and Gray.18 Variables associated with incident AF in univariate analyses with a p<0.10 were entered as covariates in multivariate models. Cumulative incidence plots were used to assess cumulative AF incidence in different groups with AF-free death as a competing event, along with a test to compare the curves. The incremental value of GLS in predicting incident AF was assessed by the likelihood ratio test, and the Harrell’s C statistic was reported. The net reclassification improvement index (NRI) was calculated to assess the ability of GLS to reclassify AF risk (<2%, 2-5%, 5-10% >10%) in addition to risk factors.19, 20 For all statistical analyses, a two-tailed p<0.05 was considered significant. Statistical analyses were performed using Stata software version 12.0 (StataCorp., College Station, TX).
RESULTS
Population characteristics and incident AF
The study sample included 680 participants in sinus rhythm and with no history of AF. During a mean follow-up of 63.6±18.7 months, 37 new cases of AF were recorded, 5 of whom could not be confirmed by ECG or medical records and were therefore excluded from the analysis. The group of participants with suboptimal speckle-tracking analysis was older (73.2±8.2 vs. 71.1±9.3 years, p=0.02) and more frequently obese (29% vs 53%, p<0.001) than the group in which speckle-tracking was feasible, but the two groups did not significantly differ in AF incidence (4.7% vs 6.0%, p=0.524). Demographics and clinical characteristics of the study participants are shown in Table 1. Development of AF was significantly associated with older age (p<0.001) and obesity (p=0.024), and showed borderline association with hypertension (p=0.063). Among echocardiographic variables (Table 2), greater LV wall thickness, LV mass index, relative wall thickness, and LAVi were significantly associated with incident AF (all p≤0.001). E/A and e’ were significantly lower and E/e’ was higher in incident AF (all p≤0.001). LV diastolic dysfunction was also more frequent in the incident AF group (p=0.007).
Table 1.
Demographics and clinical characteristics of the study sample.
| Variable | Incident AF | P value | |
|---|---|---|---|
| No (n=643) | Yes (n=32) | ||
| Age, years | 70.8±9.2 | 77.1±7.4 | <0.001 |
| Women, n (%) | 391 (60.8) | 17 (53.1) | 0.386 |
| Obesity, n (%) | 182 (28.3) | 15 (46.9) | 0.024 |
| Systolic blood pressure, mmHg | 135.3±16.8 | 138.6±20.2 | 0.282 |
| Diastolic blood pressure, mmHg | 78.3±9.4 | 77.4±9.8 | 0.598 |
| Hypertension, n (%) | 492 (76.5) | 29 (90.6) | 0.063 |
| Anti-hypertensive treatment, n (%) | 442 (69.0) | 27 (84.4) | 0.064 |
| Diabetes, n (%) | 178 (27.7) | 9 (28.1) | 0.956 |
| Hypercholesterolemia, n (%) | 419 (65.3) | 24 (75.0) | 0.257 |
| Coronary artery disease, n (%) | 36 (5.6) | 3 (9.4) | 0.372 |
| History of heart failure, n (%) | 19 (3.0) | 0 (0) | 1.000 |
Data are presented as mean values ± standard deviation for continuous variables, or as absolute number and percentage for categorical variables.
AF: Atrial fibrillation.
Table 2.
Echocardiographic data.
| Variable | Incident AF | P value | |
|---|---|---|---|
| No | Yes | ||
| LV end-diastolic volume, ml/m2 | 53.4±14.9 | 57.9±21.2 | 0.104 |
| LV end-systolic volume, ml/m2 | 19.9±9.5 | 22.8±18.9 | 0.115 |
| LV mass index, g/m2 | 101.1±23.9 | 116.5±22.5 | <0.001 |
| Relative wall thickness | 0.49±0.08 | 0.54±0.12 | 0.001 |
| LVEF, % | 63.5±6.7 | 63.6±10.8 | 0.933 |
| LVEF < 50%, n (%) | 27 (4.2) | 2 (6.3) | 0.577 |
| GLS, % | -17.2±3.0 | -15.2±4.1 | <0.001 |
| GLS >-14.7%, n (%) | 108 (16.8) | 15 (46.9) | <0.001 |
| LAVi, ml/m2 | 24.0±6.4 | 30.3±9.0 | <0.001 |
| LAVi >31.0 ml/m2, n (%) | 74 (12.2) | 12 (40.0) | <0.001 |
| E/A | 0.83±0.23 | 1.01±0.75 | <0.001 |
| E deceleration time, msec | 215.9±50.4 | 228.3±67.3 | 0.187 |
| e’, cm/s | 7.4±1.7 | 6.4±1.1 | 0.001 |
| E/e’ | 10.0±3.1 | 13.2±4.3 | <0.001 |
| LV diastolic dysfunction, n (%) | 346 (54.0) | 25 (78.1) | 0.007 |
Data are presented as mean values ± standard deviation for continuous variables, or as absolute number and percentage for categorical variables.
AF: Atrial fibrillation. LV: Left ventricular. LVEF: LV ejection fraction. GLS: global longitudinal strain. LAVi: Left atrial volume index.
LV function, LAVi, and incident AF
Among variables of LV systolic function, GLS was significantly lower in participants who developed AF compared to those who did not (-15.2±4.1% vs. -17.2±3.0%, p<0.001) (Table 2), whereas no difference in LVEF was seen between the two groups. In participants with normal GLS incidence of AF was 3.1%, whereas it was 12.2% in those with abnormal GLS (p<0.001). During follow-up, 63 participants died without developing AF and therefore death was used as a competing risk in all subsequent survival analyses. In univariate analysis (Table 3), GLS (HR/unit decrease=1.20, 95% CI=1.08-1.34, p=0.001), LAVi (HR/unit increase=1.11, 95% CI=1.07-1.16, p<0.001), and LV diastolic dysfunction (HR=2.93, 95% CI=1.27-6.74, p=0.012) were significantly associated with incident AF. In multivariable analysis (Table 3), lower GLS (p=0.015) and greater LAVi (p<0.001) remained significantly associated with incident AF, whereas LV diastolic dysfunction did not. As shown in Figure 1, cumulative AF incidence was significantly worse in participants with abnormal GLS than in those with normal GLS (p<0.001). Abnormal GLS was associated with incident AF with an HR=3.8 (95% CI=1.9-7.6, p<0.001) (Table 4). In a multivariable model also including LAVi, abnormal GLS remained strongly associated with incident AF (adjusted HR=3.2, 95% CI 1.4-7.5, p=0.007). Increased LAVi was also independently associated with incident AF with an adjusted HR=3.8 (95% CI=1.8-8.2, p<0.001) (Table 4).
Table 3.
Cox proportional hazards regression with death as a competing risk showing the association of LV systolic function, diastolic function and LA volume with incident AF.
| Univariate* | Multivariate† | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| GLS, per 1% decrease | 1.20 (1.08-1.34) | 0.001 | 1.22 (1.04-1.43) | 0.015 |
| LVEF, per 1% decrease | 1.00 (0.92-1.08) | 0.923 | 0.96 (0.90-1.02) | 0.176 |
| LAVi, per ml/m2 increase | 1.11 (1.07-1.16) | <0.001 | 1.12 (1.07-1.17) | <0.001 |
| LV diastolic dysfunction | 2.93 (1.27-6.74) | 0.012 | 1.19 (0.46-3.07) | 0.715 |
Each variable is entered in a separate model.
All variables are included in the same model. Covariates: age, obesity, hypertension, anti-hypertensive treatment, coronary artery disease, LV mass index, relative wall thickness.
HR: Hazard ratio. CI: Confidence interval. LV: Left ventricular. GLS: Global longitudinal strain. LVEF: LV ejection fraction. LAVi: Left atrial volume index.
Figure 1.
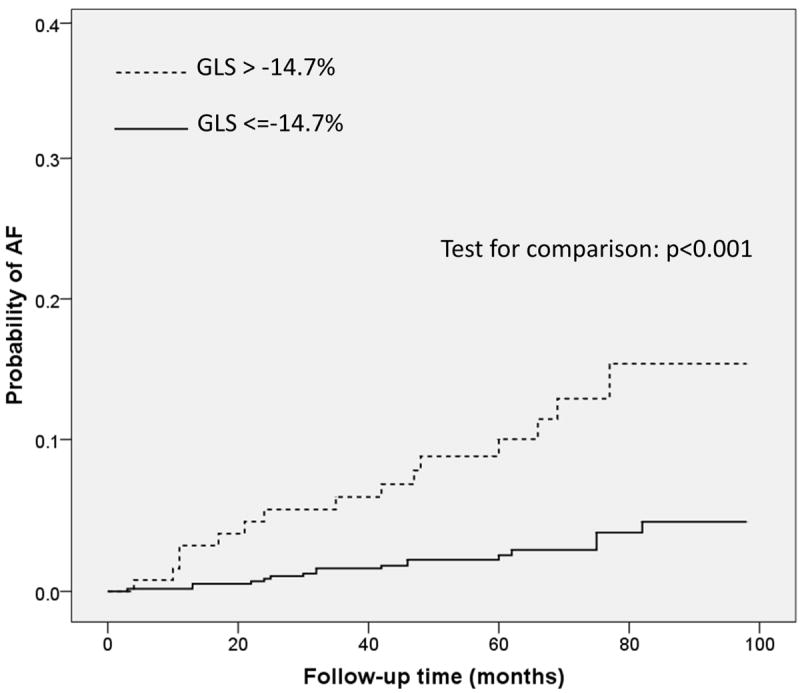
Cumulative AF incidence in participants with normal and reduced GLS. AF cumulative incidence was significantly worse in participants with GLS>-14.7% compared to those with GLS≤-14.7% (p<0.001).
Table 4.
Cox proportional hazards regression with death as a competing risk showing the association of abnormal GLS and LAVi with incident AF.
| Univariate* | Multivariate† | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| GLS > -14.7% | 3.8 (1.9-7.6) | <0.001 | 3.2 (1.4-7.5) | 0.007 |
| LAVi > 31 ml/m2 | 5.2 (2.5-10.9) | <0.001 | 3.8 (1.8-8.2) | <0.001 |
Each variable is entered in a separate model.
Both variables are included in the same model. Covariates: LVEF, diastolic dysfunction, age, obesity, hypertension, anti-hypertensive treatment, coronary artery disease, LV mass index, relative wall thickness.
HR: Hazard ratio. CI: Confidence interval. GLS: Global longitudinal strain. LAVi: Left atrial volume index.
Figure 2A shows cumulative AF incidence in participants stratified by GLS and LAVi categories. Cumulative AF incidence was intermediate in the groups with either abnormal GLS or LAVi, and was significantly worse in the abnormal GLS/abnormal LAVi group (p<0.001). Participants with normal GLS and normal LAVi experienced the lowest rate of AF during follow-up (2.0%), those with either impaired GLS or increased LAVi showed intermediate rates of AF development (9.3% and 11.1% respectively), whereas subjects with both impaired GLS and increased LAVi had the highest AF rate (28.6%, p<0.001 for the overall comparison) (Figure 2B). Compared to subjects with both normal GLS/normal LAVi, those with abnormal GLS/normal LAVi had an adjusted HR for incident AF=3.3 (95% CI=1.2-9.1, p=0.020), those with normal GLS/abnormal LAVi had an adjusted HR=4.0 (95% CI=1.5-10.5, p=0.005), and those with abnormal GLS/abnormal LAVi showed the highest risk of AF (adjusted HR=12.1, 95% CI=3.3-44.8, p<0.001) (Table 5).
Figure 2.
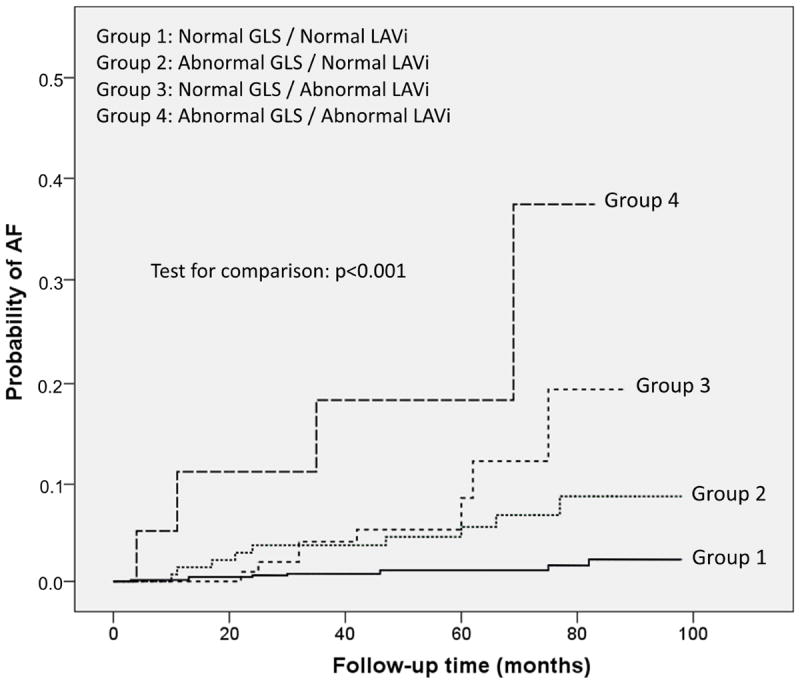
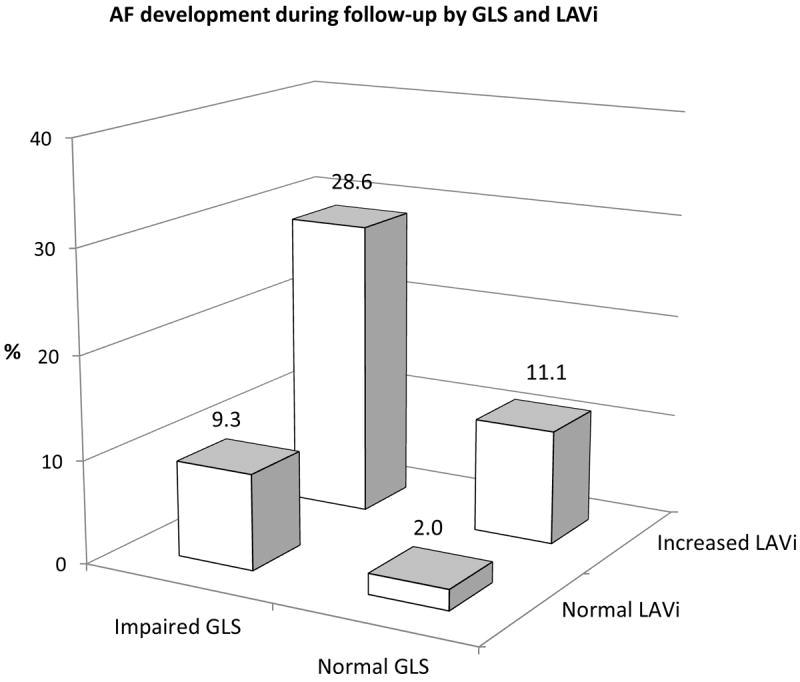
AF development during follow-up according to the combination of GLS and LAVi categories. A: Cumulative AF incidence in the four groups (p<0.001). B: Subjects with abnormal GLS and abnormal LAVi had an incidence of new-onset AF over 10-fold greater than the group with both normal parameters. Subjects with only one abnormality had an intermediate incidence of AF.
Table 5.
Cox proportional hazards regression with death as a competing risk showing the risk of incident AF in different GLS and LAVi categories.
| Univariate | Multivariate† | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Normal GLS / Normal LAVi | Reference | - | Reference | - |
| Abnormal GLS / Normal LAVi | 4.4 (1.7-11.0) | 0.002 | 3.3 (1.2-9.1) | 0.020 |
| Normal GLS / Abnormal LAVi | 6.6 (2.6-17.3) | <0.001 | 4.0 (1.5-10.5) | 0.005 |
| Abnormal GLS / Abnormal LAVi | 17.6 (5.4-57.5) | <0.001 | 12.1 (3.3-44.8) | <0.001 |
Covariates: LVEF, diastolic dysfunction, age, obesity, hypertension, anti-hypertensive treatment, coronary artery disease, LV mass index, relative wall thickness.
HR: Hazard ratio. CI: Confidence interval. GLS: Global longitudinal strain. LAVi: Left atrial volume index.
Incremental prognostic value of GLS for AF prediction
The incremental prognostic value of GLS for incident AF is shown in Figure 3. When added to a model including clinical risk factors (-2 LOG likelihood=211.289), LAVi significantly increased the model predictive value [-2 LOG likelihood=199.374, chi-square change=11.914 vs. previous step with 1 degree of freedom (df), p=0.001], whereas the addition of LVEF did not (-2 LOG likelihood=199.333, chi-square change from previous step=0.041 with 1 df, p=0.802). The further addition of GLS resulted in a significant incremental improvement in the predictive value of the model (-2 LOG likelihood=190.025, chi-square change from previous step=9.308 with 1 df, p=0.004). C-statistic was 0.75 in the model with risk factors, 0.78 after adding LAVi, and 0.81 after adding GLS. The NRI for GLS in addition to the model including LA volume and risk factors was 0.29 (SE 0.13, p=0.028).
Figure 3.
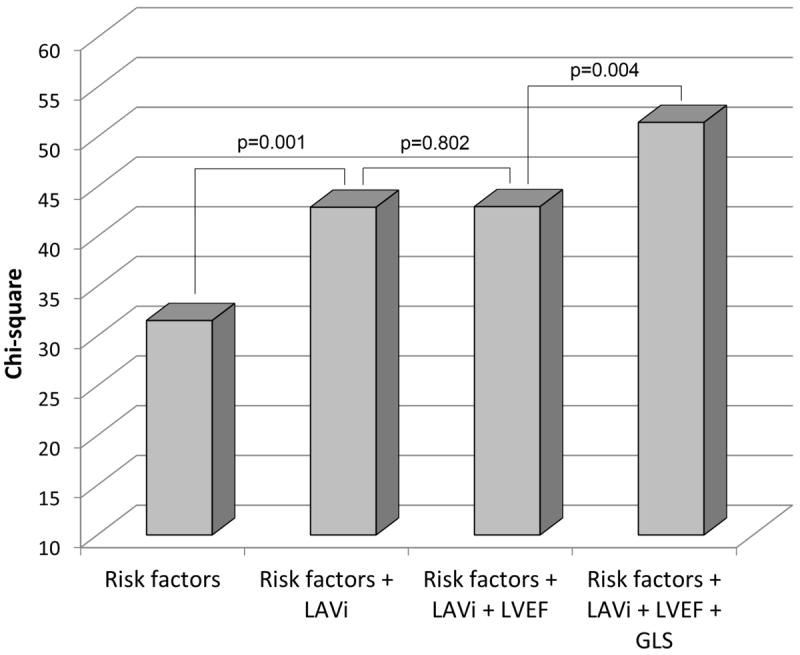
Incremental value of GLS over risk factors, LAVi, and LVEF in predicting AF development (likelihood ratio test). Risk factors include age, obesity, hypertension, anti-hypertensive treatment, coronary artery disease, LV mass index, relative wall thickness, and LV diastolic dysfunction.
DISCUSSION
In this study, we assessed the value of GLS in predicting future development of AF in predominantly elderly individuals from the community. We found that impaired GLS was a strong independent predictor of AF development. Over a mean follow-up period of 5.3 years, AF incidence in participants with an abnormal GLS was 4 times higher than in those with normal GLS (12.2% vs. 3.1%). Traditional assessment of LV systolic function by LVEF did not show any association with incident AF. We also confirmed the independent association of increased LA volume with the development of AF. Furthermore, our study showed that GLS and LAVi predicted incident AF independently of each other, and of other risk factors. Most importantly, we demonstrated that the coexistence of both abnormal GLS and abnormal LAVi was associated with significantly higher incidence of AF (28.6%), whereas the presence of either impaired GLS or dilated LAVi was associated with an intermediate risk of AF during follow-up.
Although it is known that the presence of overt congestive heart failure is associated with AF,21 and that impaired LVEF predicts AF occurrence in subjects with coronary artery disease,22 our study is the first to describe the association between a parameter of subclinical LV dysfunction and incident AF in a general elderly population. The mechanisms behind the association of GLS with incident AF, however, are not known. GLS is a measure of the contraction of the longitudinally oriented myocardial fibers, which are mostly located in the subendocardial region of the left ventricle. Since the LV subendocardium is especially vulnerable to ischemic injury and hemodynamic overload,23 GLS can document myocardial dysfunction at a stage when LVEF is still normal as the decrease in longitudinal strain can be compensated by either an increase in circumferential fibers contraction or by the development of myocardial hypertrophy.24-26 The advantage of using GLS over LVEF lies therefore in its ability to detect the early isolated systolic impairment of the longitudinal myocardial contraction component, often the first event in the natural history of LV dysfunction. At this stage LVEF is generally preserved, thus explaining its lack of prognostic value in population studies, in which LVEF is mostly in the normal range. In this context, longitudinal strain can be a useful tool to re-stratify the risk of subjects without overt LV dysfunction. The detection of subclinical LV dysfunction by GLS is a sign of a more advanced stage of LV disease, rather than just a risk factor, and therefore its strong association with AF, and with cardiovascular events in general, is likely related to the fact that it is the evidence of an already existing myocardial damage, and at least in part of a more advanced disease process. GLS has been shown to be associated with several cardiovascular risk factors also linked to AF such as hypertension, LV hypertrophy, and diabetes,27 and to predict cardiovascular events in addition to and independent of LVEF.9 In previous studies, we demonstrated that GLS, but not LVEF, was reduced in subjects with increased arterial stiffness,28 a marker of atherosclerosis.29 Consistently, in the Multi-Ethnic Study of Atherosclerosis (MESA) study, it was reported an association of myocardial strain with coronary artery calcium score.30 In a previous study, we demonstrated that a reduction in GLS was independently associated with silent brain infarctions and with white matter disease,17 further strengthening the hypothesis that a reduced LV longitudinal strain might be the expression of a generalized underlying macrovascular and microvascular involvement. In this light, the association of GLS with subclinical atherosclerosis and microvascular disease may be one hypothesis for the association with incident AF through chronic hypoperfusion and subsequent fibrosis of the LA tissue and sinus node, conditions both favoring reentry mechanisms and development of AF.31-33 Another possible explanation for the association between GLS and AF may be found in the relationship between GLS and LA function. It is known that the LV longitudinal systolic function is a major determinant of the LA reservoir function through its effect on the systolic descent of the mitral plane.34 We previously demonstrated that a reduction in GLS can be in fact associated with a lower LA reservoir function,35 which has in turn been demonstrated to be a strong predictor of development of first-onset AF.36 In line with our findings, a previous study reported lower GLS but similar LVEF in patients with paroxysmal AF compared to healthy controls;37 therefore it is possible that an impaired GLS may in some cases be associated with undiagnosed paroxysmal AF, progressing toward permanent AF over time, an hypothesis that deserves further investigation.
We performed an annual follow-up by actively contacting the study participants and using a standardized questionnaire to detect new-onset AF. This approach could lead to an underestimation of the AF detection rate, a problem common to other population studies using similar methodology, due to the impossibility of detecting asymptomatic AF or paroxysmal episodes. ECG monitoring devices would provide a better AF detection including paroxysmal and asymptomatic episodes, but this methodology was not feasible in our study. AF detection however, was very specific, as new-onset AF cases were confirmed by ECG or medical records, and unconfirmed cases were excluded. Further studies are needed to investigate the GLS ability to predict asymptomatic AF and undiagnosed paroxysmal AF.
Our study has potential clinical implications. The finding that impaired GLS was independently associated with a significant risk of incident AF, whereas neither LVEF nor LV diastolic dysfunction were, suggests that the use of speckle-tracking echocardiography to detect subclinical LV systolic dysfunction may help identify subjects at high risk of developing AF. Furthermore, we demonstrated that the combined use of GLS and LAVi was able to identify subjects at different risk of future AF, with the group having both abnormalities being at an extremely high risk. The abnormal LAVi cut-off identified in our reference group (31 ml/m2) was slightly smaller than the 34 ml/m2 recommended by recent guidelines. Our population is composed of subjects from three race-ethnic groups, mostly Hispanic, and with high mean body size, a factor that might have contributed to the slightly lower LA volume once indexed by body size. Additionally, it is not clear to what extent 3D echo software from different vendors is interchangeable, as there is a lack of data regarding inter-vendor differences in 3D LA volumes. Our approach, however, allowed the re-stratification of new-onset AF risk with significant net reclassification improvement. Whether the high-risk groups might benefit from preventive strategies to stop or delay the progression to AF and from closer follow-up for early detection of AF is a possibility that needs to be investigated.
Strengths and limitations
The main strengths of our study are the prospective design, the long follow-up duration, the confirmation of AF by ECG and medical records, the large number of subjects studied with modern imaging techniques, the wide range of cardiovascular risk profiles present in our study population, and the confirmation of our findings in multivariable models and in competing risks analyses. However, our study has also limitations. The study sample included subjects over age 50 with a large representation of Hispanic ethnicity, which might preclude the generalization of our findings to populations with different demographic composition. However, since the frequency of AF is extremely low below age 50, our study cohort was an optimal setting to explore this topic. Another limitation is that the AF detection during follow-up was based on participants’ self-report; therefore, although only ECG-confirmed AF episodes were included in the analysis, the possibility of AF under-detection cannot be excluded. Finally, analyses stratified by gender and race-ethnicity were not performed because the number of events limited the feasibility of subgroup analysis.
Conclusions
Subclinical LV systolic dysfunction by GLS was a powerful and independent predictor of future development of AF in this predominantly elderly community cohort. The prognostic value of GLS was independent of cardiovascular risk factors, and incremental to established markers of AF risk such as LAVi. The combined assessment of GLS and LAVi identified subjects at different risk for developing AF. The re-stratification of subjects at low, intermediate, and high risk of AF might be guidance for different treatment and monitoring strategies.
Supplementary Material
Acknowledgments
The authors wish to thank Janet De Rosa, MPH (project manager), Rafi Cabral, MD, Michele Alegre, RDCS, and Palma Gervasi-Franklin (collection and management of the data).
SOURCES OF FUNDING
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [grant numbers R01 NS36286 to M.D.T., and R37 NS29993 to R.L.S./M.S.V.E.].
Footnotes
DISCLOSURES
None.
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 3.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 5.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. doi: 10.4065/76.5.467. [DOI] [PubMed] [Google Scholar]
- 7.Ng AC, Delgado V, Bertini M, van der Meer RW, Rijzewijk LJ, Shanks M, Nucifora G, Smit JW, Diamant M, Romijn JA, de Roos A, Leung DY, Lamb HJ, Bax JJ. Findings from left ventricular strain and strain rate imaging in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2009;104:1398–1401. doi: 10.1016/j.amjcard.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 8.Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011;24:322–332. doi: 10.1016/j.echo.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Russo C, Jin Z, Elkind MS, Rundek T, Homma S, Sacco RL, Di Tullio MR. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Motoki H, Borowski AG, Shrestha K, Troughton RW, Tang WH, Thomas JD, Klein AL. Incremental prognostic value of assessing left ventricular myocardial mechanics in patients with chronic systolic heart failure. J Am Coll Cardiol. 2012;60:2074–2081. doi: 10.1016/j.jacc.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, Di Tullio MR, Homma S, Elkind MS, Paik MC. Improving Global Vascular Risk Prediction With Behavioral and Anthropometric Factors The Multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009;54:2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. doi: 10.1161/01.cir.55.4.613. [DOI] [PubMed] [Google Scholar]
- 14.Russo C, Hahn RT, Jin Z, Homma S, Sacco RL, Di Tullio MR. Comparison of echocardiographic single-plane versus biplane method in the assessment of left atrial volume and validation by real time three-dimensional echocardiography. J Am Soc Echocardiogr. 2010;23:954–960. doi: 10.1016/j.echo.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR. Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation. 2013;128:1105–1111. doi: 10.1161/CIRCULATIONAHA.113.001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31:101–113. doi: 10.1002/sim.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundstrom J, Byberg L, Gedeborg R, Michaelsson K, Berglund L. Useful tests of usefulness of new risk factors: tools for assessing reclassification and discrimination. Scand J Public Health. 2011;39:439–441. doi: 10.1177/1403494810396556. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 22.Stamboul K, Zeller M, Fauchier L, Gudjoncik A, Buffet P, Garnier F, Guenancia C, Lorgis L, Beer JC, Touzery C, Cottin Y. Incidence and prognostic significance of silent atrial fibrillation in acute myocardial infarction. Int J Cardiol. 2014;174:611–617. doi: 10.1016/j.ijcard.2014.04.158. [DOI] [PubMed] [Google Scholar]
- 23.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 24.Palmon LC, Reichek N, Yeon SB, Clark NR, Brownson D, Hoffman E, Axel L. Intramural myocardial shortening in hypertensive left ventricular hypertrophy with normal pump function. Circulation. 1994;89:122–131. doi: 10.1161/01.cir.89.1.122. [DOI] [PubMed] [Google Scholar]
- 25.Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26:195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 26.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Relationship of multidirectional myocardial strain with radial thickening and ejection fraction and impact of left ventricular hypertrophy: a study in a community-based cohort. Echocardiography. 2013;30:794–802. doi: 10.1111/echo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 28.Russo C, Jin Z, Takei Y, Hasegawa T, Koshaka S, Palmieri V, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and subclinical left ventricular systolic dysfunction. J Hypertens. 2011;29:574–582. doi: 10.1097/HJH.0b013e328342ca56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Popele NM, Grobbee DE, Bots ML, Asmar R, Topouchian J, Reneman RS, Hoeks AP, van der Kuip DA, Hofman A, Witteman JC. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454–460. doi: 10.1161/01.str.32.2.454. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes VR, Polak JF, Edvardsen T, Carvalho B, Gomes A, Bluemke DA, Nasir K, O’Leary DH, Lima JA. Subclinical atherosclerosis and incipient regional myocardial dysfunction in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47:2420–2428. doi: 10.1016/j.jacc.2005.12.075. [DOI] [PubMed] [Google Scholar]
- 31.Davies MJ, Pomerance A. Pathology of atrial fibrillation in man. Br Heart J. 1972;34:520–525. doi: 10.1136/hrt.34.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willeit K, Pechlaner R, Egger G, Weger S, Oberhollenzer M, Willeit J, Kiechl S. Carotid atherosclerosis and incident atrial fibrillation. Arterioscler Thromb Vasc Biol. 2013;33:2660–2665. doi: 10.1161/ATVBAHA.113.302272. [DOI] [PubMed] [Google Scholar]
- 33.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Rooij FJ, Lip GY, Witteman JC. Subclinical atherosclerosis and risk of atrial fibrillation: the rotterdam study. Arch Intern Med. 2007;167:382–387. doi: 10.1001/archinte.167.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100:427–436. doi: 10.1161/01.cir.100.4.427. [DOI] [PubMed] [Google Scholar]
- 35.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart. 2012;98:813–820. doi: 10.1136/heartjnl-2011-301388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TS. Left atrial reservoir function as a potent marker for first atrial fibrillation or flutter in persons > or = 65 years of age. Am J Cardiol. 2008;101:1626–1629. doi: 10.1016/j.amjcard.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 37.Reant P, Lafitte S, Bougteb H, Sacher F, Mignot A, Douard H, Blanc P, Hocini M, Clementy J, Haissaguerre M, Roudaut R, Jais P. Effect of catheter ablation for isolated paroxysmal atrial fibrillation on longitudinal and circumferential left ventricular systolic function. Am J Cardiol. 2009;103:232–237. doi: 10.1016/j.amjcard.2008.08.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


