Abstract
Background
This study prospectively examines weight gain in breast cancer (BC) survivors compared to cancer-free women from a familial risk cohort.
Methods
Absolute and percent weight change over 4 years was compared among 303 BC survivors and 307 cancer-free women matched on age and menopausal status, from the same familial risk cohort. Linear and logistic regression was used to estimate the association between survivor status and weight gain.
Results
Overall, BC survivors gained significantly more weight (β = 3.06 pounds, 95% confidence interval (CI) 0.94, 5.17) than cancer-free women. Significant weight gain was observed in survivors diagnosed less than 5 years prior to baseline (β = 3.81 pounds, 95% CI 1.22, 6.29) and women with estrogen receptor (ER) negative tumors (β = 7.26 pounds, 95% CI 2.23, 12.30). Further, survivors treated with chemotherapy were 2.1 times more likely to gain at least 11 pounds during follow-up compared to cancer-free women (OR = 2.10, 95% CI 1.21, 3.63). Weight gain was even greater among survivors who took statins while undergoing chemotherapy treatment (p for interaction = 0.01).
Conclusion
This is the first study to demonstrate that weight gain is an important issue in BC survivors with a familial risk; further, that in the first five years post-treatment, BC survivors gain weight at a faster rate than cancer-free women, particularly after chemotherapy and statin use but not after hormone therapy alone.
Impact
Our findings provide support for the development of weight gain interventions for young BC survivors with a familial risk.
Keywords: breast cancer, survivors, family history, weight gain, breast cancer treatment
Introduction
Weight gain after breast cancer (BC) diagnosis has been commonly reported in studies of BC survivors,(1) although comparisons to cancer-free women are limited. It is still unclear whether BC survivors gain more weight than their cancer-free peers over the same period of time and to what degree factors such as early menopause or age confound this association. In cancer-free women, adult weight gain is an established risk factor for postmenopausal BC (2-4) and has been associated with increased risk for cardiovascular disease and diabetes(5, 6). In studies among BC survivors alone, the frequency of weight gain ranges from 27-100%, and is on average between 1 and 6 kg, over a follow-up time of a few months to seven years (1). Younger age at diagnosis and/or earlier age at menopause, and receipt of adjuvant chemotherapy, appear to increase risk for weight gain (7, 8).
Among survivors, weight gain and higher body mass index (BMI) have been associated with an increased risk for a second primary cancer(9) and weight gain has been shown to increase risk for BC recurrence (10-12) . Notably, chemotherapy is the treatment most consistently associated with weight gain in survivors, and may be due to a reduction in physical activity (13, 14) or metabolic disturbances such as insulin resistance (15-17) and increased inflammation(18, 19) . Only two studies, with mixed results, have compared weight gain in survivors to cancer-free women (20, 21).
Survivors with a family history of BC are a distinct subset of the BC survivor population that has been less studied. Comprising about 20% of survivors, they are often diagnosed at a young age or with hormone negative tumors and may undergo early menopause due to chemotherapy or ovarian suppression/removal (22, 23). Compared to older survivors, they are also more likely to experience depression(24), a risk factor for weight gain. On the other hand, these women are highly motivated when it comes to cancer screening, as they are at a higher risk of a second breast cancer(25).
In this study we examine whether BC survivors with a familial risk experience a greater weight gain trajectory post diagnosis compared to women without cancer.
Materials and Methods
Study Participants
The Breast and Ovarian Surveillance Service (BOSS) Cohort Study is an ongoing prospective study consisting of women and men with a familial risk for breast and/or ovarian cancer, recruited in 2005-2013 from the cancer genetics clinic at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. At enrollment, after informed consent was obtained, participants were asked to complete an extensive baseline questionnaire on demographic characteristics, weight, height, lifestyle factors including physical activity, and BC risk factors, cancer history and treatments, and medication use. A detailed family history and plasma, serum and DNA samples were obtained at this time. Follow-up questionnaires are administered every 3 to 4 years and have been completed for > 90% of participants enrolled in 2005 through 2010. All cancer diagnoses have been pathologically confirmed and all treatment information was confirmed by medical record.
Study Eligibility
Women who had enrolled in the cohort and completed a baseline questionnaire and at least 1 follow up questionnaire through December 31, 2013 were included in the study (n=938). In addition women had to have either: 1) a family history of breast or ovarian cancer, 2) a documented deleterious BRCA1/2 mutation, or 3) a diagnosis of breast cancer at age ≤ 40 years, and 4) provided height and weight on baseline questionnaire (n=911). Survivors were eligible if they had a personal history of BC (ductal carcinoma in situ [DCIS] or stage I-III BC) treated with surgery at any time prior to baseline (n=303). Eligible cancer-free women based on the study inclusion criteria were frequency matched to survivors on age and menopausal status (n=307), to ensure a similar distribution of these strong confounding factors between the two groups.
Statistical Analysis
Baseline characteristics of survivors and cancer-free women were compared using t-tests for normally distributed continuous variables and Wilcoxon rank-sum test for continuous variables without a normal distribution. Categorical variables were compared using the Chi-squared or Fisher's exact tests.
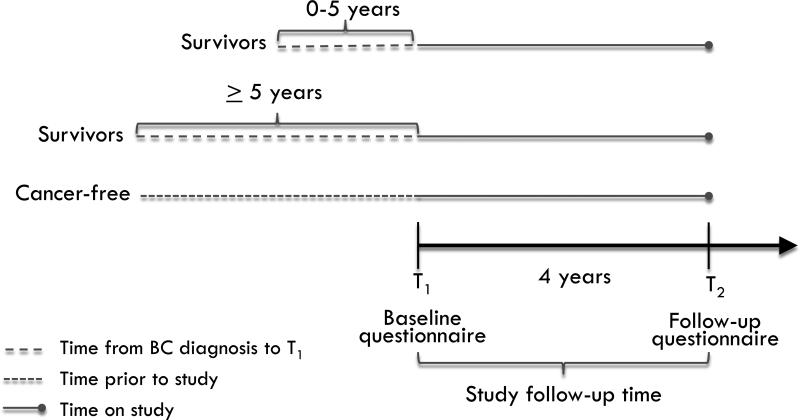
Figure 1 outlines the study design. Survivor status was stratified by the time (in years) that had elapsed between BC diagnosis and baseline questionnaire completion; two categories were defined by clinically relevant milestones in relation to recurrence risk: ≤ 5 years or > 5 years(26). Absolute weight change was calculated by subtracting baseline weight (T1) from follow-up weight (T2) for both survivors and cancer-free women. In addition, weight change was defined as percent weight change (follow-up weight – baseline weight/baseline weight) × 100, and as a binary outcome: weight gain of less than/greater than 5 kg. In a subset of participants, Pearson correlation coefficient was used to calculate correlation between baseline weight and clinic-measured weight (n=81).
Figure 1.
Study design schematic for timing of weight gain assessment among BC survivors.
Multivariable linear regression models were used to estimate the association of survivor status and change in weight. Logistic regression was used to estimate the association of survivor status with weight gain of ≥ 5 kg or < 5 kg, and ≥ 5% or < 5% weight gain. Potential confounders such as age, baseline body mass index (BMI), enrollment year, menopausal status, statin use, and baseline physical activity (measured by metabolic equivalence tasks [METs])(27) were included in adjusted models. Women who had a hysterectomy alone (i.e. without oophorectomy) before menopause (n=53) were assigned a menopausal age of 50 years, based on the mean age of natural menopause among cancer-free women. Stratified models were used to examine if the association between survivor status and change in weight differed by estrogen receptor (ER) tumor status, menopausal status at diagnosis versus baseline (premenopausal at both diagnosis and baseline; premenopausal at diagnosis and postmenopausal at baseline, or postmenopausal at diagnosis), and according to BC treatment category. An interaction term between family history and survivor status was included in the main models to account for modest differences in the family history of women with and without BC that are seen in high-risk clinics. Interaction terms were added to multivariable models to test for potential interactions between baseline BMI category (18.5-25 kg/m2 and ≥ 25 kg/m2) and survivor status on weight gain, as well as between statin use and survivor status on weight gain. The Wald test was used to evaluate statistical significance of all interaction terms. All analyses were performed using Stata (version 13; StataCorp LP, College Station, TX).
Results
The baseline characteristics and matching factors are described in Table 1. The average age of survivors and menopausal status was similar between the two groups. Twenty five percent of both groups were premenopausal. Age at menopause was younger than in the general population (28), but did not differ significantly between groups, with a mean of 48.0 years in survivors and 48.9 years in cancer-free women. BRCA1/2 mutation carrier status among those tested (n=357) was similar between groups. Sixty eight percent of survivors and 72% of cancer-free women reported baseline physical activity levels that met American College of Sports Medicine and the American Heart Association recommendations at baseline (at least 500 MET-minutes, or 8.3 MET-hours per week)(29), BMI category at T1 did not differ between groups; nor did the proportion of women who were overweight or obese at T1 or age 16. Statin use was reported by 19.8% of survivors and 22.8% of cancer-free women; duration of statin use did not differ significantly between groups.
Table 1.
Baseline and follow-up characteristics of cancer-free women and breast cancer survivors from the BOSS Cohort Study.
| Cancer-free (n=307) | Survivors (n=303) | p-value | |
|---|---|---|---|
| Age, mean y (SD) | 53.8 (11.2) | 54.0 (9.9) | 0.8 |
| Menopausal status, % | 0.69 | ||
| Premenopausal | 26.7 | 25.1 | |
| Postmenopausal, non surgical | 55.7 | 59.1 | |
| Postmenopausal, surgical | 17.6 | 15.8 | |
| Age at menopause, mean y (SD) | 48.9 (6.6) | 48.0 (5.3) | 0.11 |
| Race, % | 0.39 | ||
| Black | 5.2 | 4.6 | |
| White | 93.2 | 92.1 | |
| Other | 1.6 | 3.3 | |
| Education, 4-year college or greater, % | 73.6 | 75.8 | 0.55 |
| BRCA status1, % | 0.24 | ||
| Negative | 64.7 | 71.3 | |
| Positive | 34.5 | 26.7 | |
| Variant of uncertain significant | 0.9 | 2.1 | |
| BMI category (kg/m2): | 0.22 | ||
| Normal (18.5-24.9) | 43.3 | 51.5 | |
| Overweight (25-30) | 34.2 | 30.4 | |
| Obese (≥ 30) | 20.9 | 16.5 | |
| BMI category at age 16 y (kg/m2): | 0.09 | ||
| Normal (18.5-24.9) | 73.4 | 71.4 | |
| Overweight (25-30) | 5.4 | 2.1 | |
| Obese (≥ 30) | 0.3 | 1.1 | |
| Missing | 3.3 | 6.6 | |
| Physical activity at baseline, mean MET-hrs/week (SD) | 27.3 (30.1) | 25.5 (31.8) | 0.47 |
| Physical activity at baseline: ≥ 8.3 MET-hrs/week2, % | 71.7 | 68.3 | 0.37 |
| Hypothyroid disease, % | 15 | 11 | 0.28 |
| Diabetes, % | 6.5 | 5 | 0.41 |
| High cholesterol, % | 35.8 | 33.3 | 0.81 |
| Statins3 ever use, % | 22.8 | 19.8 | 0.37 |
| Duration of statin use, mean y (SD) | 6.6 (5.9) | 5.1 (4.7) | 0.13 |
| Age at BC diagnosis, mean y (SD) | - | 48.2 (9.9) | - |
| Time from diagnosis to baseline, mean y (SD) | - | 5.6 (6.5) | - |
| Invasive cancer (stage I-III), % | - | 81.9 | - |
| Estrogen receptor status4, %: | |||
| Positive | - | 70.6 | - |
| Negative | - | 23.4 | - |
| Missing/Untested | - | 6.3 | - |
| Triple negative BC5, % | - | 15.9 | |
| BC treatment, %: | |||
| Surgery | - | 100 | - |
| Hormonal therapy | - | 68 | - |
| Radiation | - | 53.8 | - |
| Chemotherapy, any | - | 51.5 | - |
| Chemotherapy, by regimen, %: | |||
| AC | 27.1 | ||
| AC-T, TAC, AC-TH | 44.3 | ||
| Other6 | 28.6 | ||
Abbreviations: BC, breast cancer; BMI, body mass index; MET, metabolic equivalence task; AC, doxorubicin plus cyclophosphamide; AC-T, doxorubicin plus cyclophosphamide followed by paclitaxel; TAC, docetaxel, doxorubicin, and cyclophosphamide; AC-TH, doxorubicin plus cyclophosphamide followed by paclitaxel plus trastuzumab
among women who were tested, n=356
meeting or exceeding physical activity recommendations of American Heart Association(29)
statins includes: atorvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin, and ezetimibe/simvastatin
among invasive cases only (n=248)
among invasive cases diagnosed in 2003 or later
other regimens includes: 5-fluorouracil, epirubicin, plus cyclophosphamide; docetaxel plus cyclophosphamide; docetaxel, carboplatin, and trastuzumab; cyclophosphamide, methotrexate, and 5-fluourouracil; and 5-fluourouracil, doxorubicin and cyclophosphamide.
Half of all survivors reported receiving chemotherapy, and approximately two-thirds received hormonal therapy (Table 1). In addition to self-reported weight, we also had information on measured weight obtained from clinic visits within 3 months of T1 for 81 survivors. There was a high correlation between the self-reported and measured weight, irrespective of time since diagnosis (r = 0.98).
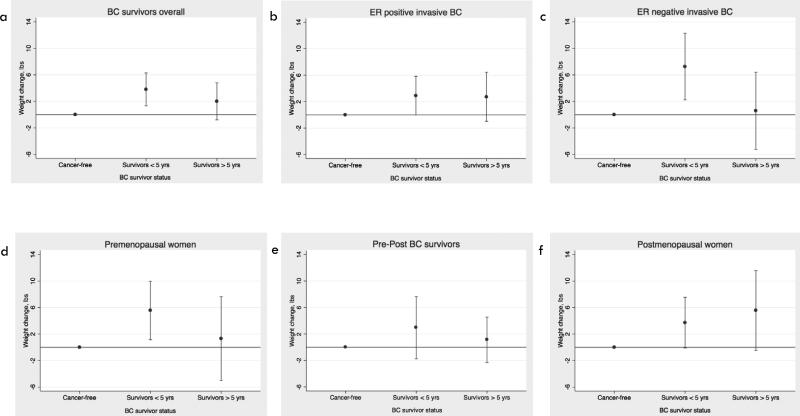
In age-adjusted linear regression models, survivors had a higher mean weight gain than cancer-free women (β = 2.84 pounds, 95% CI 0.75, 4.93). This difference persisted in multivariable models (β = 3.24 pounds, 95% CI 0.63, 5.85) after adjusting for age, menopausal status, BMI at T1, enrollment year, statin use, physical activity, and family history (Figure 2a). In analyses stratified by time since diagnosis, a significant weight gain of 3.81 more pounds (95% 1.33, 6.29) was observed in women diagnosed with BC in the last 5 years only. In survivors diagnosed with ER- invasive disease, weight gain was also significantly greater than in cancer-free women (β = 4.45 pounds, 95% CI 0.45, 8.45). The greatest weight gain was seen in survivors diagnosed with ER− invasive BC in the past 5 years compared to cancer-free women (β = 7.26 pounds, 95% CI 2.23, 12.30) (Fig. 2c). This same pattern was not observed in ER+ invasive BC survivors, for whom modest weight gain persisted over time (Figure 2b).
Figure 2.
Adjusted 4-year weight gain in survivors, stratified by time since diagnosis, compared to cancer-free women. Estimates for weight gain in (a) all survivors, (b) survivors diagnosed with ER positive invasive BC, (c) survivors diagnosed with ER negative invasive BC, (d) premenopausal survivors and cancer-free women, (e) survivors who became postmenopausal between diagnosis and baseline and postmenopausal cancer-free women, and (f) postmenopausal survivors and cancer-free women.
In logistic regression models, survivors diagnosed less than 5 years prior to T1 were twice as likely as cancer-free women to have gained at least 11 pounds over follow-up, (OR = 2.07, 95% CI 1.22, 3.50), whereas survivors diagnosed > 5 years prior to T1 had no significantly increased risk (Table 2). To compare our findings with other studies we also evaluated association based on a 5% weight gain. Survivors diagnosed with BC within 5 years prior to T1 had a 66% increased risk for gaining at least 5% of their baseline weight. Of note, sensitivity analyses, excluding survivors who had been diagnosed with a new primary cancer (n=77), yielded similar results to the main analyses.
Table 2.
Adjusted OR and 95% CI for weight gain of ≥ 11 lbs (5 kg), and ≥ 5% weight gain, in survivors and cancer-free women.
| Absolute weight gain | Percent weight gain | |||||
|---|---|---|---|---|---|---|
| < 11 lbs | ≥ 11 lbs | OR (95% CI) | < 5% | ≥ 5% | OR (95% CI) | |
| Cancer-free | 272 | 35 | referent | 251 | 56 | referent |
| Survivors < 5 yrs | 143 | 37 | 2.07 (1.22-3.50) | 129 | 21 | 1.66 (1.05-2.62) |
| Survivors ≥ 5 yrs | 111 | 12 | 0.96 (0.47-1.96) | 95 | 28 | 1.51 (0.89-2.56) |
Abbreviations: OR, odds ratio; CI, confidence interval; lbs, pounds adjusted for age, baseline BMI, menopausal status, enrollment year, physical activity, and statin use
Next we examined weight gain in survivors versus cancer-free women, stratified by menopausal status at diagnosis. Premenopausal survivors gained significantly more weight than premenopausal cancer-free women (p = 0.03); particularly those women diagnosed in the 5 years prior to T1 (β = 5.55 pounds, 95% CI 1.13, 9.98) (Figure 2d). Survivors who were postmenopausal at time of diagnosis gained 4.17 more pounds (0.71, 7.63) than postmenopausal cancer-free women over follow-up (Figure 2f). There was no difference in weight gain in survivors who became postmenopausal after diagnosis compared to postmenopausal cancer-free women (Figure 2e).
We then evaluated the effect of treatment on weight gain. Significant weight gain was seen in survivors who had received adjuvant chemotherapy +/− hormone therapy (β = 4.26, 95% CI 1.49, 7.02) when compared to cancer-free women. Treatment with chemotherapy alone was associated with even greater weight gain (7.86 [95% CI 1.85, 13.87] pounds). Survivors treated with chemotherapy within 5 years prior to T1 gained 5.58 (95% CI 2.13, 9.03) more pounds than cancer-free women, whereas no significant weight gain was observed in survivors treated with chemotherapy more than 5 years prior to T1 (Table 3). Weight gain in survivors who had received hormone therapy alone, or surgery alone, did not differ significantly from that seen in cancer-free women.
Table 3.
Adjusted multiple linear regression coefficients for change in weight (lbs) during follow-up stratified by type of treatment.
| Surgery only | Chemotherapy, with or without hormonal therapy* | Hormonal therapy* Alone | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | β | 95% CI | n | β | 95% CI | n | β | 95% CI | |
| Cancer-free | 307 | 0 | referent | 307 | 0 | referent | 307 | 0 | referent |
| Survivors diagnosed ≤ 5 yrs | 26 | 1.8 | −3.63-7.24 | 86 | 5.58 | 2.13-9.03 | 67 | 1.62 | −1.99-5.24 |
| Survivors diagnosed > 5 yrs | 26 | −1.15 | −6.57-4.27 | 70 | 2.72 | −0.94-6.38 | 27 | 2.48 | −2.77-7.73 |
| All survivors | 52 | 0.32 | −3.67-4.32 | 156 | 4.26 | 1.49-7.02 | 94 | 1.88 | −1.26-5.01 |
Abbreviations: CI, confidence interval; lbs, pounds
adjusted for age, baseline BMI, menopausal status, enrollment year, physical activity, and statin use.
hormonal therapy includes selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs).
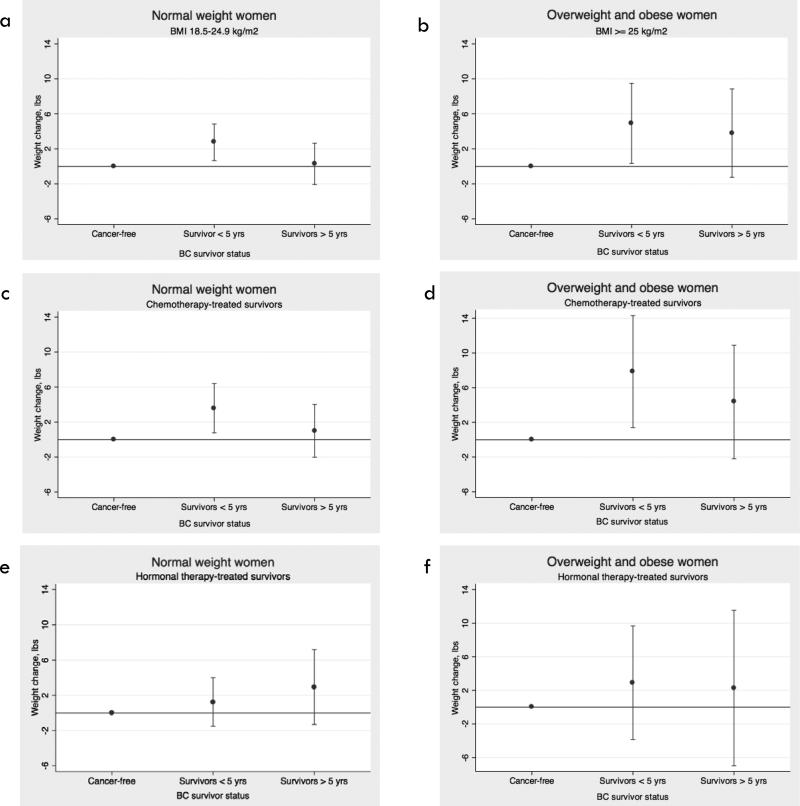
Among women who were overweight/obese at T1, survivors appeared to have a more sustained weight gain over time compared to cancer free women (Figures 3a and 3b). This was observed particularly among chemotherapy-treated survivors (Figures 3c and 3d), and not among survivors receiving hormonal therapy alone (Figures 3e and 3f). We were unable to evaluate the effect of chemotherapy versus hormone therapy alone on weight gain among survivors, as there was very little overlap in predictors of each treatment type (i.e. ER status, age at diagnosis, and stage). In additional analyses stratified by BRCA status, we found that in BRCA positive women, survivors gained significantly more weight than cancer-free women; the point estimate was higher than what we observed overall, but the confidence intervals for these estimates overlapped (Supplementary Table S1).
Figure 3.
Adjusted 4-year weight gain in survivors, stratified by time since diagnosis, treatment category, and BMI category at T1, compared to cancer-free women. Estimates for weight gain among (a) women with BMI between 18.5 kg/m2 and 25 kg/m2, (b) women with BMI ≥ 25 kg/m2, (c) survivors treated with chemotherapy and cancer-free women, with BMI between 18.5 kg/m2 and 25 kg/m2, (d) survivors treated with chemotherapy and cancer-free women, with BMI ≥ 25 kg/m2, (e) survivors treated with hormone therapy alone and cancer-free women, with BMI between 18.5 kg/m2 and 25 kg/m2, (f) survivors treated with hormone therapy alone and cancer-free women, with BMI ≥ 25 kg/m2.
Finally, we examined the effect of statin use, a drug with anti-inflammatory properties, in survivors treated with chemotherapy, as a potential preventive agent (Supplementary Table S2). We observed that chemotherapy-treated survivors who used statins had the greatest weight gain when compared to cancer-free women who used statins, chemotherapy treated survivors who had never used statins and cancer free women who never used a statin (p for interaction = 0.01).
Discussion
The prevalence of overweight/obesity in women with a familial breast cancer risk, irrespective of whether they had BC, was high (55.1% of cancer-free women and 46.9% of survivors). In this prospective study we observed that BC survivors gained weight at a greater rate than their cancer-free peers, particularly if they received chemotherapy for an ER negative tumor or were within 5 years of their diagnosis. Women who completed chemotherapy within 5 years of enrollment were more than twice as likely as cancer-free women over the same period to have gained at least 11 pounds. This amount of weight gain has been associated with a significantly increased risk for coronary heart disease and diabetes in women. Post diagnosis weight change in survivors alone has been associated with prognosis (BC recurrence, BC specific- or overall mortality)(11, 12, 30-33).
This is one of only a few studies that have been able to compare weight change in BC survivors to cancer-free women within the same cohort of women, and the first study to evaluate weight change in women with a familial risk of BC. The design of this study enabled us to examine the association of prior BC treatment and its sequelae with weight gain, independent of the impact of advancing age, which is a strong confounder. Two prior studies conducted in the general population have examined weight gain in BC survivors compared to cancer-free women (20, 21). While both studies observed weight gain over time for the overall study population, neither study found significantly greater weight gain in the survivors compared to cancer-free women. The first of these studies compared weight change in 20 women diagnosed with BC and undergoing chemotherapy to 51 healthy controls during a 6-month follow-up period. A modest amount of weight gain was observed in the premenopausal survivors during the 6-month post-treatment follow-up, but this weight gain did not differ significantly from that in controls (20). While the authors did find significant changes in body composition among the BC patients, including increase in body fat percentage and decrease in fat-free body mass, the same measurements were not done on the controls. The second study compared weight change over a mean of 6 years of follow-up in Hispanic and non-Hispanic White BC survivors (n=305) and cancer-free women (n=345) (21). The authors analyzed weight gain as ≥ 5% increase from baseline body weight, and found no significant difference between women with and without cancer history in adjusted models irrespective of treatment. The women in this study were less likely to have received chemotherapy compared to our study population (43% versus 52% in our study), were older at baseline (mean age 57 years versus 53 years in our study) and were more likely to be overweight and obese at baseline. Previous studies of BC survivors alone have observed greater weight gain among women at a lower BMI at the time of diagnosis (8); however, in analyses stratified by BMI category, we did not observe a significant difference in estimates for weight gain between the groups.
The greatest weight gain in our study was seen among chemotherapy-treated survivors, and in particular those survivors treated within 5 years prior to T1, This finding corroborates some previous studies limited to survivors only, (reviewed in (1)), and confirms that the weight gain is not related to increasing age or change in menopausal status. We observed a greater than 11 pound weight gain in 21% of the women treated with chemotherapy. This amount of weight gain is shown to have serious implications for future risk of coronary heart disease. In a study of healthy women, adult weight gain of at least 5 kg from age 18 years was associated with a 25% or greater risk for coronary heart disease (5). In comparison with weight maintenance, weight gain of 5-10 kg during adulthood has also been associated with significantly increased risks for hypertension(34), and type 2 diabetes in women (6, 34). The mechanisms underlying chemotherapy-induced weight gain have not been fully elucidated. One line of evidence suggests that chemotherapy may result in unfavorable changes in body composition such as sarcopenic obesity, which is associated with unfavorable metabolic alterations(35) including decreased growth hormone production, increased insulin resistance and inflammation(36)(18) or direct damage to tissues.
Our finding that survivors taking statins actually gained more weight than both cancer-free women taking statins, as well as non-statin users, deserves further study. The differential weight change by survivor status for statin users (survivors gained weight while cancer-free women lost weight) appears to be predominantly in women who received chemotherapy. Although there is evidence that statins can suppress inflammation(37), they also appear to modestly increase risk of diabetes(38), potentially through decrease in insulin sensitivity. In a small, randomized trial of otherwise healthy women with polycystic ovarian disease, the group treated with atorvastatin was found to have a significant decrease in C reactive protein (CRP), but also a significant decrease in insulin sensitivity compared to women who received placebo(39). It is plausible that statins exert the same effect on BC survivors who are treated with chemotherapy and this counteracts the positive effect statins have on obesity-associated inflammation.
Limitations of our study include the fact that the vast majority of our cohort is white, preventing us from making inferences about change in weight in high-risk survivors of other racial or ethnic background. In addition we relied on self-reported weight, which may be subject to bias or measurement error. Previous large studies however have shown that while self-report of weight compared to measured weight may not be fully accurate, they are highly correlated, and most discrepancies are small, averaging 3 to 4 pounds among women (40, 41) and the majority of misclassification of self-report versus measured BMI falls within one BMI unit (42, 43). In the subset of our participants that we were able to compare self-report weight with measured weight the correlation was high. Importantly, in this high-risk cohort we believe that self-reported weight is unlikely to differ by exposure group given that the majority of women participate in regular annual and bi-annual screenings for cancer and other chronic diseases, regardless of cancer history. Our study has several strengths, including its prospective nature and the direct comparison to a cancer-free group recruited from the same cohort.
In summary, this is the first study to demonstrate that weight gain is also a concern among BC survivors with a familial risk, where the focus is often on prophylactic surgeries and/or intensive screening. Further, in this high-risk population, BC survivors gain weight at a faster rate than cancer-free women with the same familial risk. The finding that survivors treated with chemotherapy are at greatest risk of rapid weight gain compared to cancer-free women after controlling for age and menopausal status suggest that the underlying etiology is likely to be related to the treatment. Weight gain interventions should also be evaluated in this high-risk population. Further follow up of this high-risk population is ongoing.
Supplementary Material
Acknowledgement of Research Support
K.Visvanathan, Breast Cancer Research Foundation
A.L.Gross, NIH T32 CA009314, R.B.S.Roden. P50CA098252, K.Visvanathan, P30CA006973
We would like to thank all the participants in the ongoing BOSS cohort as well as staff over the years that have worked on this study.
Footnotes
No conflict of interest to disclose
References
- 1.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–94. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 2.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 3.Magnusson C, Baron J, Persson I, Wolk A, Bergstrom R, Trichopoulos D, et al. Body size in different periods of life and breast cancer risk in post-menopausal women. Int J Cancer. 1998;76:29–34. doi: 10.1002/(sici)1097-0215(19980330)76:1<29::aid-ijc6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 4.Lahmann PH, Schulz M, Hoffmann K, Boeing H, Tjonneland A, Olsen A, et al. Long-term weight change and breast cancer risk: the European prospective investigation into cancer and nutrition (EPIC). Br J Cancer. 2005;93:582–9. doi: 10.1038/sj.bjc.6602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willett WC, Manson JE, Stampfer MJ, Colditz GA, Rosner B, Speizer FE, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA. 1995;273:461–5. doi: 10.1001/jama.1995.03520300035033. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–22. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, et al. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–9. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 8.Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13:258–65. doi: 10.1111/j.1524-4741.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Trentham-Dietz A, Newcomb PA, Nichols HB, Hampton JM. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat. 2007;105:195–207. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- 10.Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8:1327–34. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 11.Thivat E, Therondel S, Lapirot O, Abrial C, Gimbergues P, Gadea E, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648. doi: 10.1186/1471-2407-10-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–8. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 13.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–9. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 14.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makari-Judson G, Braun B, Jerry DJ, Mertens WC. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J Clin Oncol. 2014;5:272–82. doi: 10.5306/wjco.v5.i3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell KE, Di Sebastiano KM, Vance V, Hanning R, Mitchell A, Quadrilatero J, et al. A comprehensive metabolic evaluation reveals impaired glucose metabolism and dyslipidemia in breast cancer patients early in the disease trajectory. Clin Nutr. 2014;33:550–7. doi: 10.1016/j.clnu.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Guinan EM, Connolly EM, Healy LA, Carroll PA, Kennedy MJ, Hussey J. The development of the metabolic syndrome and insulin resistance after adjuvant treatment for breast cancer. Cancer Nurs. 2014;37:355–62. doi: 10.1097/NCC.0b013e3182a40e6d. [DOI] [PubMed] [Google Scholar]
- 18.Morris PG, Zhou XK, Milne GL, Goldstein D, Hawks LC, Dang CT, et al. Increased levels of urinary PGE-M, a biomarker of inflammation, occur in association with obesity, aging, and lung metastases in patients with breast cancer. Cancer Prev Res. 2013;6:428–36. doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross AL, Newschaffer CJ, Hoffman-Bolton J, Rifai N, Visvanathan K. Adipocytokines, inflammation, and breast cancer risk in postmenopausal women: a prospective study. Cancer Epidemiol Biomarkers Prev. 2013;22:1319–24. doi: 10.1158/1055-9965.EPI-12-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89:2248–53. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 21.Sedjo RL, Hines LM, Byers T, Giuliano AR, Marcus A, Vadaparampil S, et al. Long-term weight gain among Hispanic and non-Hispanic White women with and without breast cancer. Nutr Cancer. 2013;65:34–42. doi: 10.1080/01635581.2013.741750. [DOI] [PubMed] [Google Scholar]
- 22.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncologica. 2011;50:187–93. doi: 10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- 23.Gordon AM, Hurwitz S, Shapiro CL, LeBoff MS. Premature ovarian failure and body composition changes with adjuvant chemotherapy for breast cancer. Menopause. 2011;18:1244–8. doi: 10.1097/gme.0b013e31821b849b. [DOI] [PubMed] [Google Scholar]
- 24.Howard-Anderson J, Ganz PA, Bower JE, Stanton AL. Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:386–405. doi: 10.1093/jnci/djr541. [DOI] [PubMed] [Google Scholar]
- 25.Reiner AS, John EM, Brooks JD, Lynch CF, Bernstein L, Mellemkjaer L, et al. Risk of asynchronous contralateral breast cancer in noncarriers of BRCA1 and BRCA2 mutations with a family history of breast cancer: a report from the Women's Environmental Cancer and Radiation Epidemiology Study. J Clin Oncol. 2013;31:433–9. doi: 10.1200/JCO.2012.43.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson RW. Surveillance of patients following primary therapy. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast. 4th ed. Lippincott Williams and Wilkins; Philadelphia: 2010. [Google Scholar]
- 27.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr., Montoye HJ, Sallis JF, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol. 1998;51:1271–6. doi: 10.1016/s0895-4356(98)00119-x. [DOI] [PubMed] [Google Scholar]
- 29.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 30.Caan BJ, Kwan ML, Hartzell G, Castillo A, Slattery ML, Sternfeld B, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403–9. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bradshaw PT, Ibrahim JG, Stevens J, Cleveland R, Abrahamson PE, Satia JA, et al. Postdiagnosis change in bodyweight and survival after breast cancer diagnosis. Epidemiology. 2012;23:320–7. doi: 10.1097/EDE.0b013e31824596a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, et al. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105:1344–54. doi: 10.1093/jnci/djt223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett WC, Dietz WH, Colditz GA. Guidelines for healthy weight. N Engl J Med. 1999;341:427–34. doi: 10.1056/NEJM199908053410607. [DOI] [PubMed] [Google Scholar]
- 35.Gadea E, Thivat E, Planchat E, Morio B, Durando X. Importance of metabolic changes induced by chemotherapy on prognosis of early-stage breast cancer patients: a review of potential mechanisms. Obes Rev. 2012;13:368–80. doi: 10.1111/j.1467-789X.2011.00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 38.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 39.Puurunen J, Piltonen T, Puukka K, Ruokonen A, Savolainen MJ, Bloigu R, et al. Statin therapy worsens insulin sensitivity in women with polycystic ovary syndrome (PCOS): a prospective, randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2013;98:4798–807. doi: 10.1210/jc.2013-2674. [DOI] [PubMed] [Google Scholar]
- 40.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–8. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 41.Hattori A, Sturm R. The obesity epidemic and changes in self-report biases in BMI. Obesity. 2013;21:856–60. doi: 10.1002/oby.20313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stommel M, Schoenborn CA. Accuracy and usefulness of BMI measures based on self-reported weight and height: findings from the NHANES & NHIS 2001-2006. BMC Public Health. 2009;9:421. doi: 10.1186/1471-2458-9-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stommel M, Osier N. Temporal changes in bias of body mass index scores based on self-reported height and weight. Int J Obesity. 2013;37:461–7. doi: 10.1038/ijo.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.