Abstract
BACKGROUND:
We previously reported decreased transfusions and donor exposures in preterm infants randomized to Darbepoetin (Darbe) or erythropoietin (Epo) compared with placebo. As these erythropoiesis-stimulating agents (ESAs) have shown promise as neuroprotective agents, we hypothesized improved neurodevelopmental outcomes at 18 to 22 months among infants randomized to receive ESAs.
METHODS:
We performed a randomized, masked, multicenter study comparing Darbe (10 μg/kg, 1×/week subcutaneously), Epo (400 U/kg, 3×/week subcutaneously), and placebo (sham dosing 3×/week) given through 35 weeks’ postconceptual age, with transfusions administered according to a standardized protocol. Surviving infants were evaluated at 18 to 22 months’ corrected age using the Bayley Scales of Infant Development III. The primary outcome was composite cognitive score. Assessments of object permanence, anthropometrics, cerebral palsy, vision, and hearing were performed.
RESULTS:
Of the original 102 infants (946 ± 196 g, 27.7 ± 1.8 weeks’ gestation), 80 (29 Epo, 27 Darbe, 24 placebo) returned for follow-up. The 3 groups were comparable for age at testing, birth weight, and gestational age. After adjustment for gender, analysis of covariance revealed significantly higher cognitive scores among Darbe (96.2 ± 7.3; mean ± SD) and Epo recipients (97.9 ± 14.3) compared with placebo recipients (88.7 ± 13.5; P = .01 vs ESA recipients) as was object permanence (P = .05). No ESA recipients had cerebral palsy, compared with 5 in the placebo group (P < .001). No differences among groups were found in visual or hearing impairment.
CONCLUSIONS:
Infants randomized to receive ESAs had better cognitive outcomes, compared with placebo recipients, at 18 to 22 months. Darbe and Epo may prove beneficial in improving long-term cognitive outcomes of preterm infants.
Keywords: prematurity, erythropoietin, darbepoetin, cognitive outcome
What’s Known on This Subject:
Although a number of randomized controlled trials of erythropoietin administration to preterm infants have been performed, few studies have reported 2-year or longer neurodevelopmental outcomes, and no studies have evaluated neurodevelopmental outcomes of infants randomized to receive Darbepoetin.
What This Study Adds:
This is the first prospectively designed study to evaluate the neurocognitive outcomes of preterm infants randomized to receive Darbepoetin or erythropoietin compared with placebo. Infants in the ESA groups had significantly higher cognitive scores compared with the placebo group.
Erythropoiesis stimulating agents (ESAs) have been used clinically for over 20 years to stimulate red cell production. Erythropoietin (Epo) stimulates erythropoiesis and decreases transfusion requirements in adults and children who have anemia attributable to end-stage renal disease or cancer. In preterm infants, ESAs decrease the total number and volume of transfusions.1 Darbepoetin alfa (Darbe) is a biologically modified long-acting ESA.2 The increased half-life of Darbe allows for dosing every 1 to 4 weeks in adults who have anemia attributable to end-stage renal disease or cancer. Evaluation of single-dose pharmacokinetics in preterm infants revealed a similar prolonged half-life.3,4
Recent studies in animals and humans evaluating the non-hematopoietic effects of ESAs suggest a strong potential for neuroprotection via a variety of mechanisms, including oligodendrogenesis, decreased inflammation, decreased oxidative injury, and decreased apoptosis.5–9 Our group previously reported an increased mental developmental index in former extremely low birth weight (ELBW) infants who had serum Epo concentrations >500 mU/mL,10 and Neubauer et al reported improved developmental outcomes at 8 to 12 years in former Epo-treated preterm infants.11 Although major neurodevelopmental disabilities such as cerebral palsy (CP), mental disabilities, and learning and attention deficits during school age figure prominently in the outcomes of ELBW infants, successful neuroprotective interventions have yet to be developed.
We designed a prospective, randomized, masked study to evaluate ESAs during initial hospitalization and follow-up, and hypothesized that ESA recipients would receive fewer transfusions during initial hospitalization. We previously reported that preterm infants randomized to Epo and Darbe received half the number of transfusions and were exposed to half the donors compared with the placebo group.12 Based on its potential for neuroprotection, our study was designed to determine whether Darbe was also effective in improving developmental outcomes. We hypothesized that, compared with preterm infants administered placebo, preterm infants administered Darbe would have higher composite cognitive scores of the Bayley Scales of Infant Development III (BSID III)13 at 18 to 22 months’ adjusted age. Secondary analyses were performed to determine if there were group differences on the other BSID III scales, which included language and social-emotional scaled scores, as well as evaluation of object permanence (OP) and early working memory. The study was performed at 4 centers located above 4800 feet elevation (the University of New Mexico in Albuquerque, NM, McKay Dee Hospital in Ogden, UT, LDS Hospital/Intermountain Medical Center in Murray, UT, and the University of Colorado in Denver, CO).
Methods
Subjects
Infants were eligible for study if they were 500 to 1250 g birth weight, ≤48 hours of age, and were expected to survive the first days of life (as determined by the attending neonatologist). Infants who had trisomies, significant congenital anomalies (including known neurologic anomalies), hypertension, seizures, thromboses, hemolytic disease, or who were already receiving Epo were ineligible for study. The study was approved by the Institutional Review Boards at the Universities of New Mexico, Utah, and Colorado, and Intermountain Healthcare. Parental consent was obtained. An investigational new drug application was approved by the Federal Drug Administration (IND #100138).
Randomization was stratified by center using a computer-generated permuted block method. Multiples were randomized to the same treatment group. All caregivers and investigators (except the research pharmacists and coordinators administering the study medicine) were masked to the treatment assignment.
Dosing of Study Drug and Supplements
Methods for study drug and supplement dosing were previously published.12 Briefly, infants were randomized in masked fashion to 1 of 3 groups: Epo, 400 U/kg, given subcutaneously 3 times a week; Darbe, 10 μg/kg, given subcutaneously once a week, with sham dosing 2 other times per week; or placebo, consisting of 3 sham doses per week. Dosing continued until 35 completed weeks’ gestation, discharge, transfer to another hospital, or death. All infants received supplemental iron, folate, and vitamin E.
Follow-Up Examination
Infants were evaluated at 18 to 22 months’ adjusted age by examiners blinded to the treatment group. Demographic information and medical history were obtained from the family. Anthropometric measurements, a detailed neurologic evaluation, and the modified gross motor function classification system (GMFCS)14 (used to classify gross motor performance, with scores ranging from 0 [normal] to 5 [most impaired]) was administered by a neonatologist certified in the examination. Assessments of hearing impairment (characterized functionally as requirement of a hearing aid, unilateral or bilateral deafness) and visual impairment (defined as vision requiring correction) were based on examination and parental report. The BSID III was divided into 4 sections that included cognition, language, motor, and social-emotional skills. The BSID III was administered by certified examiners at each of the 4 sites who were blinded to the treatment group. The cognitive, social-emotional, and language scales (which included receptive and expressive subscales) of the BSID III were administered. In addition, 3 test items from the cognitive scale (items 40, 45, and 50) were used to calculate an OP score to provide a measure of early working memory.15 Neurodevelopmental impairment (NDI) was defined as 1 or more of the following: visual impairment requiring correction, hearing impairment requiring hearing aids, any CP (GMFCS 1–5), or BSID III cognitive score <80. Moderate NDI was defined as 1 or more of the following: visual impairment requiring correction, hearing impairment requiring hearing aids, any CP (GMFCS 1–5), or BSID III cognitive score <70.
Sample Size Estimate
The primary outcome was composite cognitive score. A total of 22 infants in each group were required to identify a difference of 15 ± 15 in BSID III composite cognitive score10 between the Darbe and placebo group, using an α of 0.05 and 80% power (the study was not powered to determine differences between Darbe and Epo). To account for deaths (15%) and loss to follow-up (10%), 34 infants per group were enrolled. Secondary outcomes included composite language, social-emotional and OP scores, neurodevelopmental impairment, and cognitive outcomes <85, <80, and <70 in each group.
Statistical Analysis
Baseline characteristics, growth, and medical outcomes were compared among treatment arms by using ANCOVA or Kruskal-Wallis tests (depending on distributional characteristics) for quantitative dependent variables, and 2-tailed Fisher’s exact tests for nominal and categorical dependent variables. For cognitive outcomes, comparisons were performed between the Darbe and Epo groups, and between ESA (Darbe and Epo groups combined) and placebo groups. Neurodevelopmental outcomes were analyzed by using a logistic regression model that included terms for treatment arm, gender, site, and maternal education. Mean BSID III scores reported for each treatment arm were adjusted for gender. All P values were 2-sided.
Results
The number of infants screened for the study, randomized to 1 of the 3 treatment arms, and evaluated at a corrected age of 18 to 22 months is shown in Fig 1. Reasons for non-randomization included parent refusal (39%), consent not requested (5%), or enrollment in other studies (37%). A total of 102 infants were enrolled in the original study. Three subjects were excluded from initial analysis (1 died before any study drug administration, 1 infant was determined after birth to have a structural brain defect and was not administered study drug, and 1 never received study drug). Of the 99 infants analyzed, 5 infants died during the hospital phase of the study. A total of 14 infants did not return for follow-up (2 moved outside of the country; 12 could not be contacted). Follow-up assessments began February 10, 2008 and ended March 17, 2012. All randomized subjects receiving at least 1 dose of study drug who were available for follow-up were included in the analysis. Outcomes for 86% of the infants were determined.
FIGURE 1.
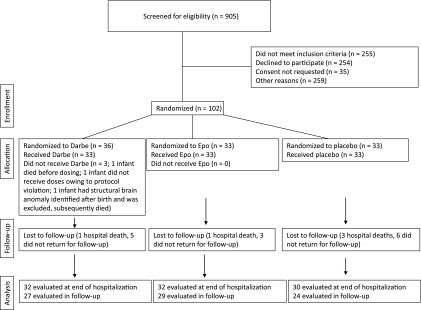
Numbers of infants who were screened for eligibility, were eligible for the study, were randomly assigned to receive Epo, Darbe, or placebo, and were followed to a corrected age of 18 to 22 months.
Characteristics of the 80 infants composing the present neurodevelopmental follow-up study are shown in Table 1. There were no significant differences among the 3 groups in maternal or neonatal characteristics aside from a greater number of females enrolled in the Darbe group. Subjects seen at follow-up were similar to the original population in terms of birth weight, gestation, and gender,12 and were not different for those same variables than the 14 subjects not seen at follow-up. All analyses during the follow-up phase were adjusted for gender and maternal education.
TABLE 1.
Characteristics of the Children and Their Families
| Characteristic | Darbe (N = 27) | Epo (N = 29) | Placebo (N = 24) |
|---|---|---|---|
| Birth wt (g) | 938.3 ± 176.5 | 947.2 ± 212.7 | 953.0 ± 210.0 |
| Median | 980.0 | 930.0 | 1005.0 |
| Interquartile range | 801.2–1090.0 | 778.2–1157.5 | 809.5–1133.0 |
| Gestation (wk) | 28.1 ± 1.8 | 27.8 ± 1.9 | 27.8 ± 1.6 |
| Median | 28.3 | 27.7 | 28.1 |
| Interquartile range | 26.5–29.5 | 26.2–29.1 | 27.1–28.8 |
| Corrected age at follow-up (mo) | 20.5 ± 1.1 | 21.2 ± 2.0 | 20.6 ± 1.9 |
| Median | 20.0 | 21.0 | 20.0 |
| Interquartile range | 20.0–21.0 | 20.0–22.0 | 19.5–21.0 |
| Female, N (%)* | 17 (63) | 10 (34) | 9 (38) |
| Mother’s race, N (%) | |||
| White | 20 (74) | 23 (79) | 18 (75) |
| Hispanic | 10 (37) | 9 (31) | 10 (42) |
| Native | 1 (4) | 3 (10) | 2 (8) |
| Pacific Islander | 2 (7) | 0 (0) | 0 (0) |
| Maternal education, N (%) | |||
| <7th grade | 0 (0) | 0 (0) | 1 (4) |
| 7th to 9th grade | 2 (8) | 1 (3) | 1 (4) |
| 10th to 12th grade | 5 (19) | 3 (10) | 5 (21) |
| High school diploma | 8 (31) | 10 (34) | 8 (33) |
| Some college or university | 6 (23) | 7 (24) | 4 (17) |
| University/college degree | 4 (15) | 6 (21) | 5 (21) |
| Graduate degree | 1 (4) | 2 (7) | 0 (0) |
| Income, N (%) | |||
| <$5000 | 1 (4) | 2 (7) | 1 (4) |
| $5000 to $9999 | 6 (23) | 2 (7) | 6 (25) |
| $10 000 to $19 999 | 5 (19) | 4 (14) | 4 (17) |
| $20 000 to $29 999 | 2 (8) | 7 (24) | 2 (8) |
| $30 000 to $39 999 | 1 (4) | 3 (10) | 5 (21) |
| $40 000 to $49 999 | 2 (8) | 4 (14) | 2 (8) |
| ≥$50 000 | 8 (31) | 5 (17) | 3 (12) |
| Not stated | 1 (4) | 2 (7) | 1 (4) |
| Children age <18 y at home, N | 2.2 ± 0.8 | 2.2 ± 1.3 | 2.6 ± 1.5 |
| Median | 2.0 | 2.0 | 2.0 |
| Interquartile range | 2.0–3.0 | 1.0–3.0 | 1.0–4.0 |
| Children age <6 y at home, N | 1.6 ± 0.7 | 1.6 ± 0.9 | 1.8 ± 1.0 |
| Median | 1.0 | 1.0 | 2.0 |
| Interquartile range | 1.0–2.0 | 1.0–2.0 | 1.0–2.0 |
| Number of household moves, N | 1.0 ± 1.0 | 0.7 ± 0.8 | 1.0 ± 1.4 |
| Median | 1.0 | 1.0 | 0.5 |
| Interquartile range | 0.0–2.0 | 0.0–1.0 | 0.0–2.0 |
| Visiting/home nurse, N (%) | 21 (78) | 21 (72) | 15 (62) |
| Occupational therapy, N (%) | 16 (59) | 21 (72) | 15 (62) |
| Physical therapy, N (%) | 16 (59) | 21 (72) | 15 (62) |
| Speech therapy, N (%) | 20 (74) | 16 (55) | 16 (67) |
| Early intervention, N (%) | 14 (52) | 14 (48) | 9 (38) |
| Social worker, N (%) | 22 (81) | 25 (86) | 22 (92) |
| Specialty medical clinic, N (%) | 16 (59) | 23 (79) | 19 (79) |
| Specialty babysitter, N (%) | 27 (100) | 29 (100) | 22 (92) |
| Family counseling, N (%) | 27 (100) | 27 (93) | 23 (96) |
P < .05
There were no differences among groups in family income, maternal education, or number of household members (Table 2). There were no deaths after discharge from the hospital, and no transfusions administered. The number of infants re-hospitalized was similar among groups (Table 2). There were no differences in intervention services (such as occupational therapy or home nursing) provided to subjects. Infants evaluated at follow-up were similar in corrected age (Table 2). Weight, length, head circumference, and the percent of infants <10th percentile in either weight or head circumference were similar among groups at 18 to 22 months (Table 2).
TABLE 2.
Growth and Medical Outcomes
| Darbe | Epo | Placebo | |
|---|---|---|---|
| Corrected age at follow-up (mo) | 20.5 ± 1.1 | 21.2 ± 2.0 | 20.6 ± 1.9 |
| Median | 20.0 | 21.0 | 20.0 |
| Interquartile range | 20.0–21.0 | 20.0–22.0 | 19.5–21.0 |
| Corrected wt at follow-up (kg) | 11.2 ± 3.3 | 11.1 ± 2.1 | 11.0 ± 1.4 |
| Median | 10.9 | 10.8 | 11.1 |
| Interquartile range | 9.4–11.3 | 9.6–12.0 | 10.0–12.0 |
| Less than 10th percentile for wt, N (%) | 7 (25.9) | 6 (20.7) | 5 (20.8) |
| Recumbent length at follow-up (cm) | 79.9 ± 5.6 | 83.3 ± 4.0 | 83.4 ± 4.1 |
| Median | 80.5 | 84.0 | 82.4 |
| Interquartile range | 77.5–84.4 | 81.2–86.5 | 80.5–85.7 |
| Head circumference at follow-up (cm) | 46.7 ± 2.2 | 47.8 ± 2.2 | 47.1 ± 1.5 |
| Median | 46.6 | 47.5 | 47.0 |
| Interquartile range | 45.8–48.0 | 46.5–49.3 | 46.0–48.4 |
| Less than 10th percentile for head circumference, N (%) | 3 (11.1) | 3 (10.3) | 5 (20.8) |
| ROP | |||
| All stages | 10 (37.0) | 8 (27.6) | 8 (33.3) |
| Stage ≥3, regressed, N (%) | 0 (0) | 1 (3.5) | 2 (8.3) |
| Stage ≥3, requiring intervention, N (%) | 2 (7.4) | 0 (0) | 1 (4.2) |
| Intraventricular hemorrhage grade ≥3, N (%) | 2 (7.4) | 2 (6.9) | 5 (20.8) |
| Necrotizing enterocolitis, N (%) | 0 (0) | 1 (3.5) | 1 (4.2) |
| Bronchopulmonary dysplasia, N (%) | 17 (63.0) | 20 (69.0) | 14 (58.3) |
| Rehospitalized, N (%) | 13 (48.2) | 11 (37.9) | 9 (37.5) |
| Average readmissions, N (%) | 2.0 ± 1.5 | 1.4 ± 0.7 | 1.8 ± 1.2 |
| Median | 1.0 | 1.0 | 1.0 |
| Interquartile range | 1.0–2.2 | 1.0–2.0 | 1.0–3.0 |
| Surgically treated after initial discharge, N (%) | 6 (22.2) | 8 (27.6) | 6 (25) |
| ROP surgery, N (%) | 0 (0) | 1 (3.5) | 0 (0) |
| Shunt for hydrocephalus, N (%) | 0 (0) | 1 (3.5) | 0 (0) |
| Patent ductus arteriosus ligation, N (%) | 0 (0) | 1 (3.5) | 0 (0) |
| Other, N (%) | 1 (3.7) | 3 (10.3) | 2 (8.3) |
All differences are statistically insignificant.
Infants randomized to ESAs had significantly higher composite cognitive scores, and scored higher on the test for OP (Table 3), with a trend towards higher language scores (P = .06). Scores were similar between Epo and Darbe groups for all tests, with the exception of OP, for which scores in the Darbe group were higher than those in the Epo group (P = .01). None of the infants in the Darbe group had a cognitive score <85. The number of infants in the placebo group who had BSID III composite cognitive score <85 was greater than the number of infants in the ESA groups (P < .05; Table 4). There were no differences among groups in the number of infants who had BSID III composite cognitive score <70. The incidence of visual or hearing impairment was not different between groups (Table 4). CP (GMFCS = 2 in all cases) was seen in 5 of the 24 infants (20.8%) in the placebo group, compared with 0 of the 56 infants in the ESA groups (P < .001).
TABLE 3.
Bayley Scales of Infant Development Scores
| Darbe, n = 27 | Epo, n = 29 | P | ESAa, n = 56 | Placebo, n = 24 | P | |
|---|---|---|---|---|---|---|
| Composite cognitive score | 96.2 ± 7.3 | 97.9 ± 14.3 | .61 | 96.5 ± 11.2 | 88.7 ± 13.5 | .01 |
| Median (interquartile range) | 95.0 (90.0–100.0) | 95.0 (90.0–106.2) | 95.0 (90.0–105.0) | 90.0 (82.5–100.0) | ||
| Composite language score | 92.4 ± 13.2 | 89.9 ± 18.4 | .56 | 90.7 ± 15.4 | 83.6 ± 13.1 | .06 |
| Median (interquartile range) | 91.0 (83.0–102.2) | 89.0 (78.0–100.0) | 91.0 (79.0–100.0) | 86.0 (71.8–94.0) | ||
| Composite social/emotional | 103.5 ± 19.2 | 99.6 ± 20.4 | .47 | 100.9 ± 19.3 | 96.7 ± 19.4 | .38 |
| Median (interquartile range) | 100.0 (95.0–115.0) | 97.5 (82.5–107.5) | 100.0 (85.0–110.0) | 95.0 (85.0–103.8) | ||
| OP score | 2.8 ± 0.4 | 2.4 ± 0.9 | .01 | 2.6 ± 0.7 | 2.2 ± 1.0 | .05 |
| Median (interquartile range) | 3.0 (3.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–3.0) | 3.0 (1.5–3.0) |
All means and standard deviations (denoted by ±) have been adjusted for gender. Medians and interquartile ranges are unadjusted. All P values have been adjusted for gender.
Treated groups are combined.
TABLE 4.
Neurodevelopmental Outcomes
| Darbe | Epo | ESAa | Placebo | Unadjusted | Adjusted for Gender, Maternal Education | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 27 | n = 29 | P* | n = 56 | n = 24 | Odds Ratio (95% CI) | P* | Odds Ratio (95% CI) | P* | |
| Cognitive score <85 | 0 (0) | 3 (10.3) | 0.12 | 3 (5.4) | 6 (25.0) | 0.17 (0.04–0.75) | 0.02 | 0.18 (0.04–0.82) | 0.03 |
| Cognitive score <80 | 0 (0) | 3 (10.3) | 0.12 | 3 (5.4) | 5 (20.8) | 0.22 (0.05–0.99) | 0.05 | 0.24 (0.05–1.13) | 0.07 |
| Cognitive score <70 | 0 (0) | 1 (3.5) | 0.50 | 1 (1.8) | 2 (8.3) | 0.20 (0.02–2.32) | 0.20 | 0.24 (0.02–2.93) | 0.26 |
| NDI,b N (%) | 3 (11.1) | 4 (13.8) | 0.88 | 7 (12.5) | 10 (41.7) | 0.20 (0.06–0.62) | 0.005 | 0.21 (0.07–0.68) | 0.009 |
| CP,c | 0 (0) | 0 (0) | 1.00 | 0 (0) | 5 (20.8) | N/A | 0.002 | N/A | <0.001 |
| Visual deficit | 2 (7.4) | 0 (0) | 0.27 | 2 (3.6) | 1 (4.2) | 0.85 (0.07–9.87) | 0.91 | 0.72 (0.06–8.74) | 0.80 |
| Hearing deficit | 0 (0) | 1 (3.5) | 0.50 | 1 (1.8) | 1 (4.2) | 0.42 (0.03–6.98) | 0.54 | 0.48 (0.03–8.59) | 0.62 |
| NDI or death, N (%) | 4/28 (14.3) | 5/30 (16.7) | 0.92 | 9/58 (15.5) | 13/27 (48.2) | 0.20 (0.07–0.56) | 0.002 | 0.22 (0.07–0.70) | 0.01 |
| Moderate NDI,d N (%) | 3 (11.1) | 2 (6.9) | 0.45 | 5 (8.9) | 9 (37.5) | 0.16 (0.05–0.56) | 0.004 | 0.18 (0.05–0.63) | 0.008 |
| Moderate NDI or death, N (%) | 4/28 (14.3) | 3/30 (10.0) | 0.48 | 7/58 (12.1) | 12/27 (44.4) | 0.17 (0.06–0.51) | 0.002 | 0.20 (0.06–0.67) | 0.009 |
P values for comparisons between Darbe and Epo were computed using 2 methods depending on characteristics of the data. For the NDI measures, P values were computed using logistic regression with gender and maternal education as covariates. For the remaining measures, P values were computed using ANCOVA with only gender as a covariate. Percentages for NDI or death include deaths during initial hospitalization. CI, confidence interval. N/A, not applicable.
Treated groups are combined.
NDI is defined as having either CP, visual deficit, hearing deficit, or a cognitive score <85.
Given the absence of CP cases in the ESA treatment group, odds ratios cannot be accurately estimated. P values given for CP were computed using Fisher’s exact tests for the unadjusted P value and using ANCOVA with gender as the only covariate for the remaining P values.
Moderate NDI is defined as having either CP, visual deficit, hearing deficit, or a cognitive score <70.
The incidence of NDI was significantly greater in the placebo group compared with the ESA groups (P < .01). The adjusted odds ratio for NDI (visual impairment, hearing impairment, CP, or composite cognitive score <85) was 0.21 (95% confidence interval 0.07–0.68) for infants in the ESA groups, and the adjusted odds ratio for moderate NDI was 0.18 (95% confidence interval 0.05–0.63).
Discussion
This is the first prospective, randomized, masked, multicenter study evaluating the effect of Darbe and Epo on neurodevelopmental outcomes of preterm infants. For the hospital phase of the study we hypothesized that Darbe recipients would receive fewer transfusions than placebo recipients,12 and in the follow-up phase we hypothesized that Darbe recipients would have higher cognitive scores than placebo recipients. We found that ESA-treated infants had significantly higher composite cognitive scores and higher on tests of OP, and language scores that neared statistical significance. Our previous follow-up of ELBW infants randomized to Epo administration16 reported fewer infants who had a head circumference <10th percentile, but no differences, compared with placebo recipients, in mean mental developmental index or psychomotor index as measured by the BSID II. The current study evaluated Darbe, but also the same dose of Epo used in the previous National Institute of Child Health and Human Development (NICHD) network study.17 The improvement in cognitive outcome of Epo recipients in this study compared with previous studies may be attributable in part to differences in study design. In the current study we administered the study drugs earlier, before 60 hours of life (12–24 hours earlier than previous studies). Also, ESA recipients in the current study were exposed to fewer transfusions, which possibly impacted their neurodevelopmental outcomes.18,19
In animal models, ESAs are protective in the developing brain, suggesting the possibility that they might be of benefit to very premature infants who are at risk for intraventricular hemorrhage, hypoxic-ischemic injury, and developmental delay. The neuroprotective mechanisms of ESAs include decreased neuronal apoptosis, decreased inflammation, promotion of oligodendrocyte differentiation and maturation, and improved white matter survival.5–9,20 In addition, ESAs are angiogenic and neurogenic. Recent clinical studies also suggest a potential for neonatal neuroprotection.7,8,21,22 European investigators reported improved developmental outcomes at age 8 to 12 years in former Epo-treated preterm infants, especially those who had significant brain injury during initial hospitalization.11 Brown and colleagues reported improved cognitive outcomes in preterm infants exposed to higher cumulative doses of Epo,21 and our group reported an improved cognitive outcome in former ELBW infants who had serum Epo concentrations greater than 500 mU/mL10 McAdams et al randomized ELBW infants to 3 doses of 500 to 2500 U/kg Epo and found improved cognitive and motor outcomes compared with concurrent controls.22 Our present study is the first to report the effects of repeated doses of Darbe on cognitive outcome.
We found that OP was greater in ESA recipients, and was the only test in which a significant difference between Epo and Darbe groups was observed. OP appears to be a more sensitive measure of early working memory. Woodward and colleagues found that a measure of early working memory was associated with cerebral white matter abnormalities on term MRI.23 These same children at age 4 and 6 years who had moderate to severe white matter abnormalities had impairment in measures of intelligence, language, and executive function.24 Impairment in executive function (including measures of impulsivity, attention, planning, and organization) is highly associated with later difficulties in academics and behavioral problems.25 Executive function deficiencies, attention deficits, and academic difficulties are all problems in children born very low birth weight that can continue into young adulthood.26
The incidence of CP in the placebo group in this study (5/24) was similar to the placebo group in the previous NICHD Epo study (9/51). All of the infants in the current study who had CP had a gross motor function classification scale score of 2. This study was not powered to determine this specific neurodevelopmental impairment, and further studies will be required to confirm the effects of early ESAs on CP. Although not the primary outcome of this study, NDI was significantly different between ESA and placebo groups, mainly owing to differences in cognitive scores and the incidence of CP. Our data indicate that 4 infants would need to be treated with ESAs to prevent 1 adverse outcome at 18 to 22 months.
Another factor that might have impacted developmental outcomes was iron supplementation during the hospital phase of the study. Adequate iron is vital to brain development, and previous investigators have postulated that infants receiving Epo would be at risk for neurodevelopmental impairment owing to available iron being shunted to red cells at the expense of the brain.27 Despite monitoring ferritin during the study and adjusting dosing accordingly, infants in the ESA groups had lower ferritin at 14 and 42 days.12 The improved outcomes in ESA-treated infants, despite reduced ferritin values, suggest that ESA treatment may be beneficial even in the setting of reduced iron availability, and may be even more beneficial when adequate iron stores are achieved.
All infants in the study were transfused under a very restrictive protocol.12 Guidelines were well tolerated at high altitude, and might be even better tolerated at sea level. Conflicting results have been reported in the developmental outcomes of infants randomized to specific transfusion strategies. Kirpalani and colleagues28 randomized 451 infants to a low or high threshold hemoglobin strategy and reported no difference in neurologic morbidities between groups; however, analyses at 2 years of age revealed an increased number of infants who had BSID II mental developmental index <80 in the low hemoglobin threshold group.29 Conversely, improved cognitive and MRI outcomes were reported in the restrictively transfused group of the Iowa study by Bell et al,18,19,30 underscoring the need for long-term evaluation and more comprehensive study. Of note, none of the infants in either the Iowa or Preterm Infants in Need of Transfusion (PINT) studies received ESAs.
Similar to our previous randomized trials of Epo administration to preterm infants, we saw no difference in the incidence of any stage of retinopathy of prematurity (ROP) during the hospital phase of the study, and no differences in visual impairment at 2 years.12,17,31,32 Recently revised meta-analyses of “early” Epo (administered in the first week of life) compared with “late” Epo now show no differences in Stage 3 or greater ROP on Epo-treated infants.33,34 Close ophthalmologic evaluation of all ESA-treated infants continues as a priority in current and proposed studies.
Conclusions
Darbe and Epo administration to preterm infants resulted in higher cognitive, and OP scores at 18 to 22 months’ corrected age. Importantly, Darbe provided this effect with weekly dosing. Given its significantly longer half-life,3,4 Darbe may be the more attractive and practical choice of ESA for preterm infants. There were no differences in hearing or visual impairment, but significant differences in the incidence of CP were noted, in that only infants in the placebo group had CP identified by examiners masked to the treatment group. As such, Epo and Darbe may serve as a beneficial therapy for preterm infants not only in the acute hospitalization in which the risk for anemia exists, but also as possible neuroprotective agents to improve neurodevelopmental outcomes.
Acknowledgments
The authors are indebted to the parents for their willingness to allow their infants to participate in this study. We wish to acknowledge the faculty, staff, and nurses in the Newborn Intensive Care Units at the University of New Mexico, Presbyterian Hospital in Albuquerque, the University of Colorado, the LDS Hospital and Intermountain Medical Center in Salt Lake City, and McKay Dee hospital in Ogden, Utah for their support and contributions to this study. We also wish to thank Dr Ronald Schrader, Dr Subramani Mani, Sarah Peceny, and the G-CRC/CTSC Research Coordinators (Carol Hartenberger, Sandra Brown, Christine Reed, Donna Rodden, Kathy Hale, and Lucy Fashaw) for their support and contributions to this study, and we thank members of the Data Safety Monitoring Board for their time and effort during the study. Finally, the authors thank the Thrasher Research Fund for their grant support.
Glossary
- BSID III
Bayley Scales of Infant Development, Third Edition
- CI
confidence interval
- CP
cerebral palsy
- Darbe
darbepoetin alfa
- ELBW
extremely low birth weight
- Epo
erythropoietin
- ESA
erythropoiesis stimulating agents
- GMFCS
gross motor function classification system
- ICH
intracranial hemorrhage
- NDI
neurodevelopmental impairment
- OP
object permanence
- ROP
retinopathy of prematurity
Footnotes
Dr Ohls conceptualized and designed the study, performed analyses, and drafted the initial manuscript; Dr Kamath-Rayne served as co-Principal Investigator and supervised data collection at the Colorado site, and critically reviewed and revised the manuscript; Dr Christensen conceptualized and designed the study, was the Principal Investigator at the Ogden site, and reviewed and revised the manuscript; Dr Wiedmeier was the Principal Investigator and supervised data collection at the Salt Lake City site and reviewed the final manuscript as submitted; Dr Rosenberg was the Principal Investigator and supervised data collection at the Colorado site and critically reviewed the manuscript; Dr Fuller performed follow-up analyses and data collection at the New Mexico site and critically reviewed and revised the manuscript; Ms Lacy and Ms Roohi coordinated the study and performed data collection at the New Mexico site and critically reviewed and revised the manuscript; Ms Lambert coordinated the study and performed data collection at the Ogden site and critically reviewed the final manuscript as submitted; Ms Burnett coordinated the study and performed data collection at the Salt Lake City site and critically reviewed the final manuscript as submitted; Ms Pruckler coordinated the study and performed data collection at the Colorado site and critically reviewed the final manuscript as submitted; Ms Peceny performed data collection at the New Mexico site, managed data collection for the entire study, and critically reviewed the final manuscript as submitted; Mr Cannon performed the statistical analyses and critically reviewed the final manuscript as submitted; Dr Lowe performed statistical analyses and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (NCT 00334737; IND 100138).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants from the Thrasher Research Fund, the University of New Mexico CLINICAL Translational Science Center, and the University of Colorado Clinical and Translational Sciences Institute (UL1 TR000154; UL1 TR000041).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33(1):29–34 [DOI] [PubMed] [Google Scholar]
- 2.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer. 2001;84(Suppl 1):3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warwood TL, Ohls RK, Wiedmeier SE, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25(11):725–730 [DOI] [PubMed] [Google Scholar]
- 4.Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300 [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Chopp M, Zhang RL, et al. Erythropoietin amplifies stroke-induced oligodendrogenesis in the rat. PLoS One. 2010;5:e11016 [DOI] [PMC free article] [PubMed]
- 6.Iwai M, Stetler RA, Xing J, et al. Enhanced oligodendrogenesis and recovery of neurological function by erythropoietin after neonatal hypoxic/ischemic brain injury. Stroke. 2010;41(5):1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul SE, Beyer RP, Bammler TK, McPherson RJ, Wilkerson J, Farin FM. Microarray analysis of high-dose recombinant erythropoietin treatment of unilateral brain injury in neonatal mouse hippocampus. Pediatr Res. 2009;65(5):485–492 [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59(4):419–425 [DOI] [PubMed] [Google Scholar]
- 9.Vairano M, Dello Russo C, Pozzoli G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16(4):584–592 [DOI] [PubMed] [Google Scholar]
- 10.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e635 [DOI] [PubMed] [Google Scholar]
- 11.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–666 [DOI] [PubMed] [Google Scholar]
- 12.Ohls RK, Christensen RD, Kamath-Rayne BD, et al. A randomized, masked, placebo controlled study of darbepoetin administered to preterm infants. Pediatrics. 2013;132(1):119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayley N. Bayley Scales of Infant and Toddler Development. Third Edition. San Antonio, TX: Harcourt Assessment; 2006 [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and validation of a gross motor function classification system for children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223 [DOI] [PubMed] [Google Scholar]
- 15.Lowe J, MacLean PC, Shaffer ML, Watterberg K. Early working memory in children born with extremely low birth weight: assessed by object permanence. J Child Neurol. 2009;24(4):410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohls RK, Ehrenkranz RA, Das A, et al. National Institute of Child Health and Human Development Neonatal Research Network . Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114(5):1287–1291 [DOI] [PubMed] [Google Scholar]
- 17.Ohls RK, Ehrenkranz RA, Wright LL, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics. 2001;108(4):934–942 [DOI] [PubMed] [Google Scholar]
- 18.McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17(4):347–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nopoulos PC, Conrad AL, Bell EF, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165(5):443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391–403 [DOI] [PubMed] [Google Scholar]
- 21.Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/4/e681 [DOI] [PubMed] [Google Scholar]
- 22.McAdams RM, McPherson RJ, Mayock DE, Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33(3):226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128(Pt 11):2578–2587 [DOI] [PubMed] [Google Scholar]
- 24.Woodward LJ, Clark CA, Pritchard VE, Anderson PJ, Inder TE. Neonatal white matter abnormalities predict global executive function impairment in children born very preterm. Dev Neuropsychol. 2011;36(1):22–41 [DOI] [PubMed] [Google Scholar]
- 25.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51–58 [DOI] [PubMed] [Google Scholar]
- 26.Aarnoudse-Moens CS, Oosterlaan J, Duivenvoorden HJ, van Goudoever JB, Weisglas-Kuperus N. Development of preschool and academic skills in children born very preterm. J Pediatr. 2011;158(1):51–56 [DOI] [PubMed] [Google Scholar]
- 27.Fretham SJ, Carlson ES, Georgieff MK. The role of iron in learning and memory. Adv Nutr. 2011;2(2):112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307 [DOI] [PubMed] [Google Scholar]
- 29.Whyte RK, Kirpalani H, Asztalos EV, et al. PINTOS Study Group . Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123(1):207–213 [DOI] [PubMed] [Google Scholar]
- 30.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohls RK, Roohi M, Peceny HM, Schrader R, Bierer R. A randomized, masked study of weekly erythropoietin dosing in preterm infants. J Pediatr. 2012;160(5):790–795, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double-blind, placebo-controlled study. J Pediatr. 1997;131(5):661–665 [DOI] [PubMed] [Google Scholar]
- 33.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014, In press [DOI] [PubMed] [Google Scholar]
- 34.Aher S, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2014, In press [DOI] [PubMed] [Google Scholar]


