Abstract
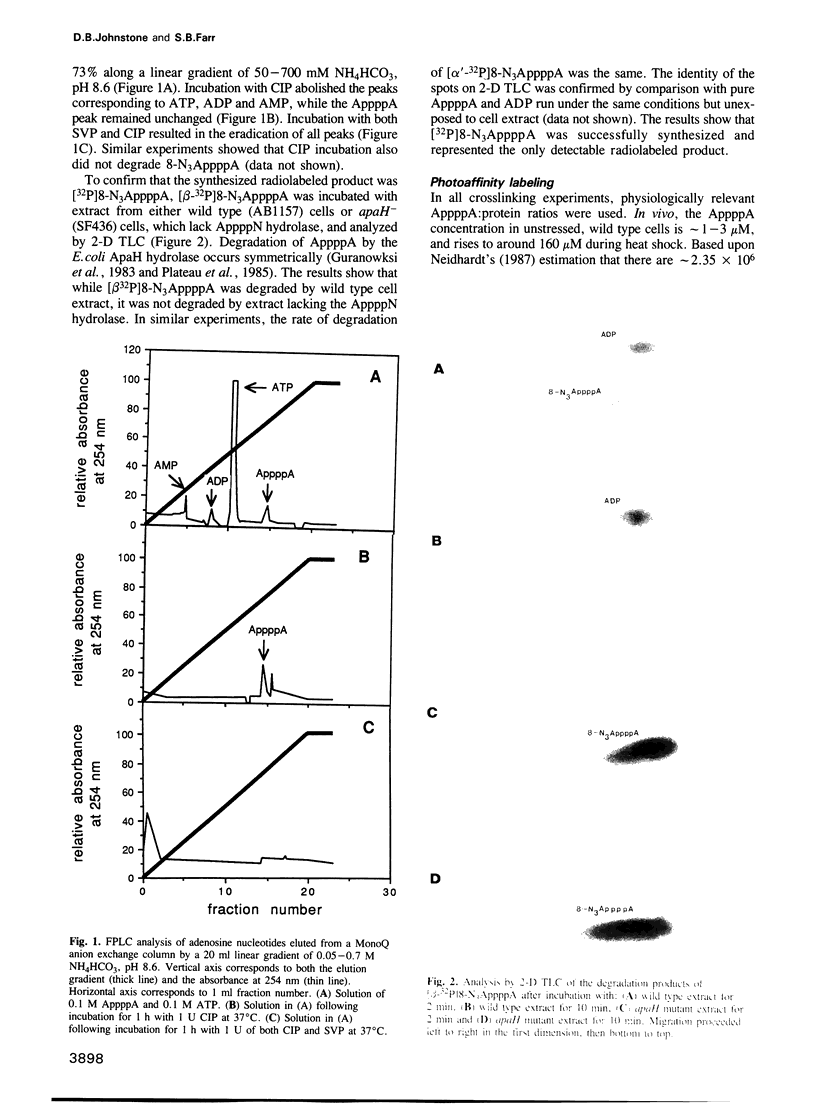
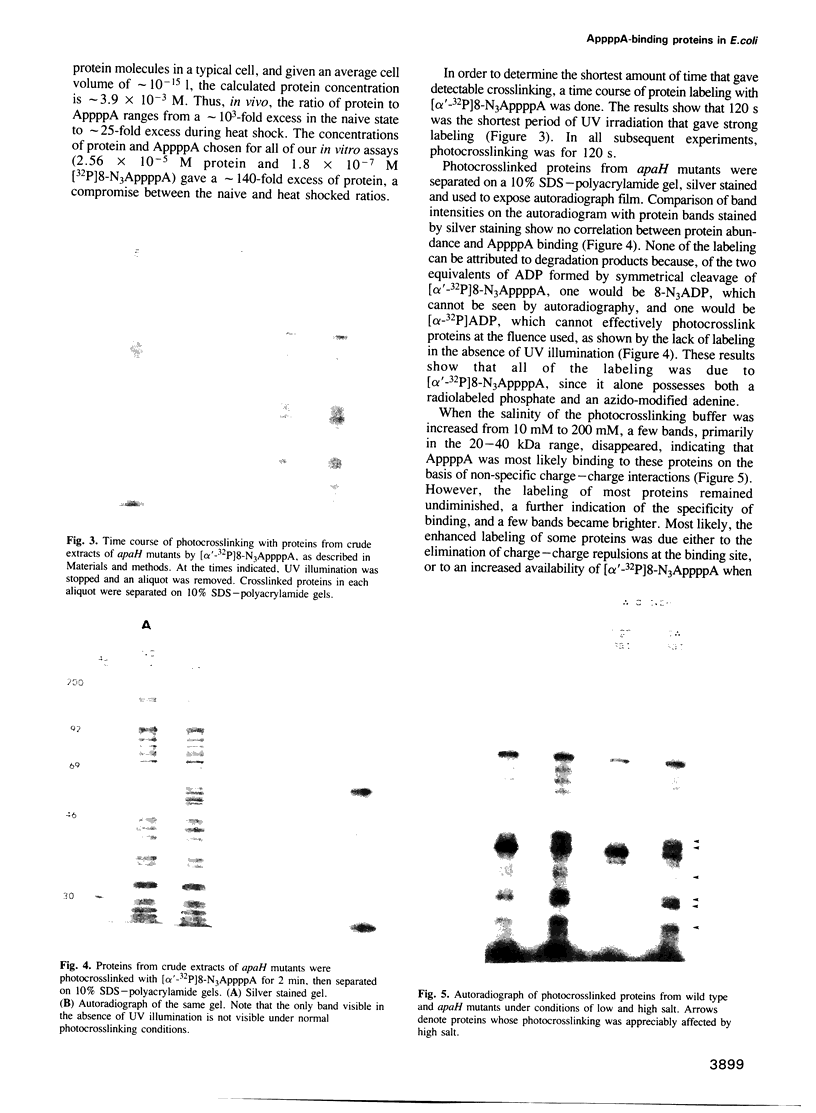
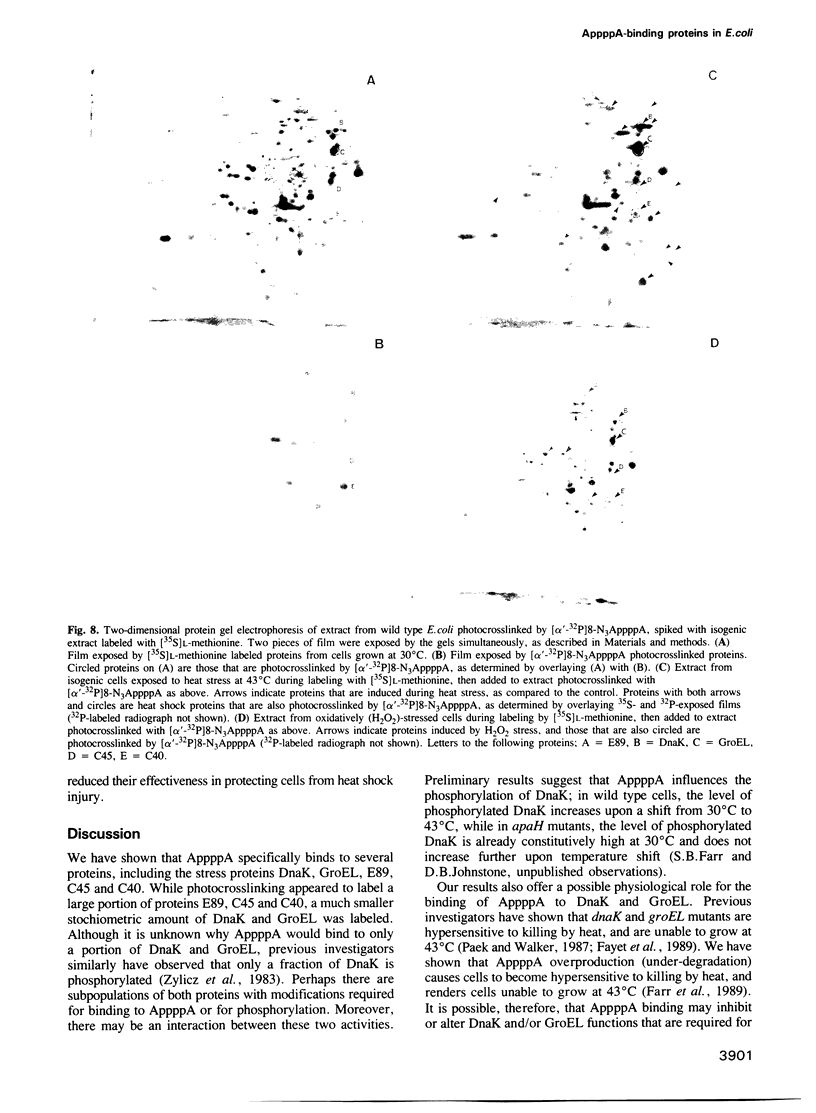
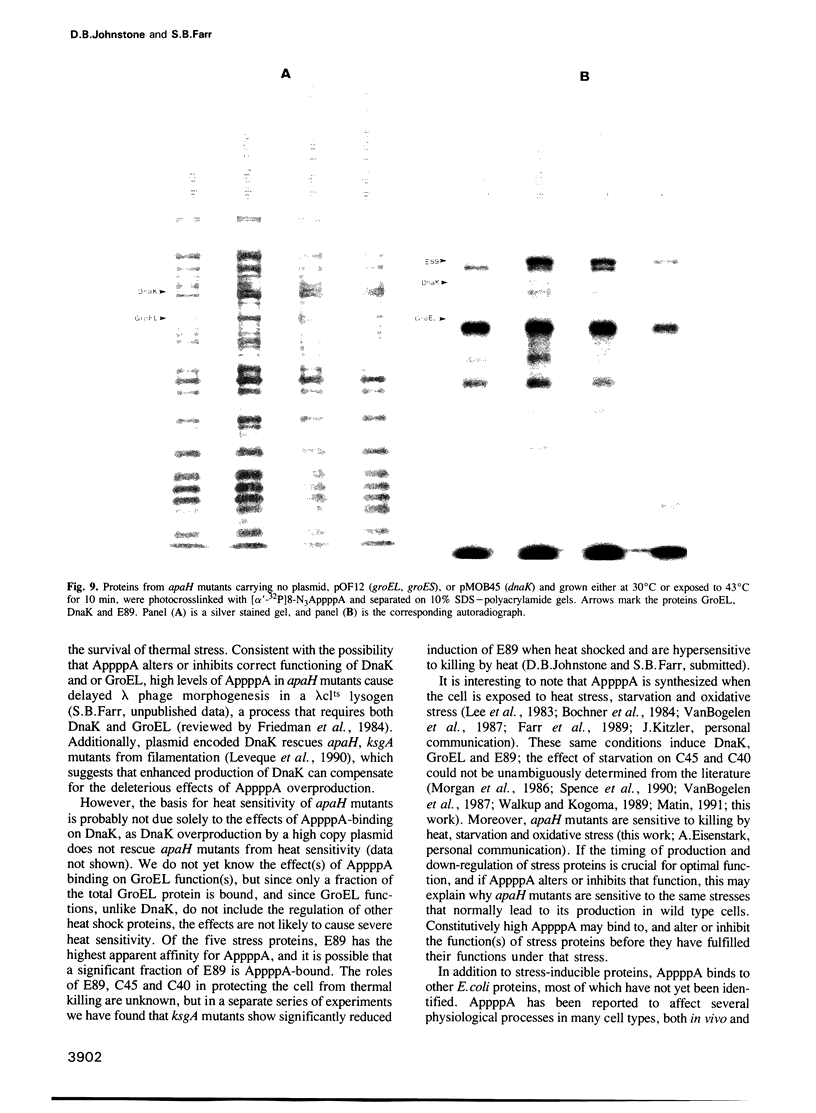
The dinucleotide AppppA (5',5'''-P1, P4-diadenosine tetraphosphate) is rapidly synthesized in cells exposed to heat stress or oxidative stress. Stress-induced AppppA accumulation has been observed in all cell types studied to date. In order to study the function(s) of AppppA, we created a mutation in the Escherichia coli gene that encodes the sole AppppN hydrolase (apaH). High levels of AppppA have subsequently been shown to affect many cellular processes, including expression of catabolite repressible genes and the ability to survive starvation, oxidative stress and near-UV irradiation. Nevertheless, the precise role of AppppA remains undefined. In order to better understand the mechanism by which AppppA exerts its effects, we attempted to determine which proteins bind to AppppA by synthesizing (alpha'-32P) 8-N3AppppA for use in photocrosslinking experiments with extract derived from cells with different genetic backgrounds and exposed to various stress conditions. We report here that several E. coli proteins bind AppppA, including the heat shock and oxidative stress proteins DnaK, GroEL, E89, C45 and C40. In addition, we show that apaH mutants, which have high basal levels of AppppA, are hypersensitive to killing by heat.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Ames B. N. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J Biol Chem. 1982 Aug 25;257(16):9759–9769. [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Zylicz M., Georgopoulos C. Escherichia coli DnaK protein possesses a 5'-nucleotidase activity that is inhibited by AppppA. J Bacteriol. 1986 Nov;168(2):931–935. doi: 10.1128/jb.168.2.931-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr S. B., Arnosti D. N., Chamberlin M. J., Ames B. N. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5010–5014. doi: 10.1073/pnas.86.13.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayet O., Ziegelhoffer T., Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989 Mar;171(3):1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R., Georgopoulos C., Tilly K., Herskowitz I., Banuett F. Interactions of bacteriophage and host macromolecules in the growth of bacteriophage lambda. Microbiol Rev. 1984 Dec;48(4):299–325. doi: 10.1128/mr.48.4.299-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Waltl G., Jantzen H. M., Hamprecht K., Huebscher U., Kuenzle C. C. Diadenosine 5',5'''-P1,P4-tetraphosphate, a ligand of the 57-kilodalton subunit of DNA polymerase alpha. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6081–6085. doi: 10.1073/pnas.76.12.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A., Jakubowski H., Holler E. Catabolism of diadenosine 5',5"'-P1,P4-tetraphosphate in procaryotes. Purification and properties of diadenosine 5',5"'-P1,P4-tetraphosphate (symmetrical) pyrophosphohydrolase from Escherichia coli K12. J Biol Chem. 1983 Dec 25;258(24):14784–14789. [PubMed] [Google Scholar]
- Léveque F., Blanchin-Roland S., Fayat G., Plateau P., Blanquet S. Design and characterization of Escherichia coli mutants devoid of Ap4N-hydrolase activity. J Mol Biol. 1990 Mar 20;212(2):319–329. doi: 10.1016/0022-2836(90)90127-8. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Perry M. E., Levy B. T. P1,P4-Di(adenosine-5')tetraphosphate inhibits phosphorylation of immunoglobulin G by Rous sarcoma virus pp60src. J Biol Chem. 1983 Apr 10;258(7):4055–4058. [PubMed] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Mechulam Y., Fromant M., Mellot P., Plateau P., Blanchin-Roland S., Fayat G., Blanquet S. Molecular cloning of the Escherichia coli gene for diadenosine 5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. J Bacteriol. 1985 Oct;164(1):63–69. doi: 10.1128/jb.164.1.63-69.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R. W., Christman M. F., Jacobson F. S., Storz G., Ames B. N. Hydrogen peroxide-inducible proteins in Salmonella typhimurium overlap with heat shock and other stress proteins. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8059–8063. doi: 10.1073/pnas.83.21.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Phillips T. A., VanBogelen R. A., Smith M. W., Georgalis Y., Subramanian A. R. Identity of the B56.5 protein, the A-protein, and the groE gene product of Escherichia coli. J Bacteriol. 1981 Jan;145(1):513–520. doi: 10.1128/jb.145.1.513-520.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paek K. H., Walker G. C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987 Jan;169(1):283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R., Gallagher P. M., Boyko W. L., Ganschow R. E. Genetic control of levels of murine kidney glucuronidase mRNA in response to androgen. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7596–7600. doi: 10.1073/pnas.80.24.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Blanquet S. Heat shock and hydrogen peroxide responses of Escherichia coli are not changed by dinucleoside tetraphosphate hydrolase overproduction. J Bacteriol. 1987 Aug;169(8):3817–3820. doi: 10.1128/jb.169.8.3817-3820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Brevet A., Gesquière A., Blanquet S. Catabolism of bis(5'-nucleosidyl) oligophosphates in Escherichia coli: metal requirements and substrate specificity of homogeneous diadenosine-5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. Biochemistry. 1985 Feb 12;24(4):914–922. doi: 10.1021/bi00325a016. [DOI] [PubMed] [Google Scholar]
- Prescott M., McLennan A. G. Synthesis and applications of 8-azido photoaffinity analogs of P1,P3-bis(5'-adenosyl)triphosphate and P1,P4-bis(5'-adenosyl)tetraphosphate. Anal Biochem. 1990 Feb 1;184(2):330–337. doi: 10.1016/0003-2697(90)90690-b. [DOI] [PubMed] [Google Scholar]
- Spence J., Cegielska A., Georgopoulos C. Role of Escherichia coli heat shock proteins DnaK and HtpG (C62.5) in response to nutritional deprivation. J Bacteriol. 1990 Dec;172(12):7157–7166. doi: 10.1128/jb.172.12.7157-7166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Matsunami N., Yoshihara K. Inhibition of ADP-ribosylation of histone by diadenosine 5', 5"' -p (1), p(4)-tetraphosphate. Biochem Biophys Res Commun. 1981 Apr 15;99(3):837–843. doi: 10.1016/0006-291x(81)91240-7. [DOI] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tilly K., VanBogelen R. A., Georgopoulos C., Neidhardt F. C. Identification of the heat-inducible protein C15.4 as the groES gene product in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1505–1507. doi: 10.1128/jb.154.3.1505-1507.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshavsky A. Diadenosine 5', 5"'-P1, P4-tetraphosphate: a pleiotropically acting alarmone? Cell. 1983 Oct;34(3):711–712. doi: 10.1016/0092-8674(83)90526-3. [DOI] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]










