Abstract
Plants usually experience dynamic fluctuations of light intensities under natural conditions. However, the responses of mesophyll conductance, CO2 assimilation, and photorespiration to light fluctuation are not well understood. To address this question, we measured photosynthetic parameters of gas exchange and chlorophyll fluorescence in tobacco leaves at 2-min intervals while irradiance levels alternated between 100 and 1200 μmol photons m−2 s−1. Compared with leaves exposed to a constant light of 1200 μmol photons m−2 s−1, both stomatal and mesophyll conductances were significantly restricted in leaves treated with fluctuating light condition. Meanwhile, CO2 assimilation rate and electron flow devoted to RuBP carboxylation at 1200 μmol photons m−2 s−1 under fluctuating light were limited by the low chloroplast CO2 concentration. Analysis based on the C3 photosynthesis model indicated that, at 1200 μmol photons m−2 s−1 under fluctuating light, the CO2 assimilation rate was limited by RuBP carboxylation. Electron flow devoted to RuBP oxygenation at 1200 μmol photons m−2 s−1 under fluctuating light remained at nearly the maximum level throughout the experimental period. We conclude that fluctuating light restricts CO2 assimilation by decreasing both stomatal and mesophyll conductances. Under such conditions, photorespiration plays an important role in the regulation of photosynthetic electron flow.
Keywords: CO2 assimilation, fluctuating light, photorespiration, photosynthetic electron flow, mesophyll conductance
Introduction
In nature, plants grown in open habitats usually experience changes in light intensities because of clouds. Even on clear days, the leaves of understory plants are frequently exposed to short-term fluctuating light levels due to movements by the leaves and stems of other plants above them. To cope with fluctuating light conditions, plants must regulate their photosynthetic processes. Under constant low light, most of the absorbed light energy can be used to drive photosynthesis, even when stomatal conductance (gs) is reduced. Under constant high light, the rate of CO2 assimilation (An) is maintained at an elevated level due to high gs and mesophyll conductance (gm) (Yamori et al., 2010, 2011). Fluctuating light restricts both gs and CO2 assimilation rate (Fay and Knapp, 1993; Kirschbaum et al., 1998). However, it is unclear how gm and photorespiration respond to those changes in irradiance.
Under natural conditions, gm is an important determinant of the CO2 assimilation rate, especially at high light levels (Carriquí et al., 2015). Several environmental factors, such as water status and temperature, can affect gm (Flexas et al., 2002; Scafaro et al., 2011; Walker et al., 2013). For tobacco (Nicotiana tabacum) plants grown with adequate water and optimum temperature, gm is mainly dependent upon the growth light intensity (Yamori et al., 2010). Plants exposed to strong light have higher values of gm when compared with those grown under low light. When light levels are constant, gm does not appear to be dependent upon light intensity (Yamori et al., 2010). However, the effect of fluctuating light condition on gm is unclear. According to the model of Farquhar et al. (1980), CO2 assimilation in C3 plants is limited by either the carboxylation or the regeneration of ribulose-1,5-bisphosphate (RuBP). In tobacco, a model C3 plant, the rate of CO2 assimilation under high light is influenced by leaf nitrogen (N) content. CO2 assimilation rate under high light tends to be limited by RuBP regeneration for plants grown at high N concentration (Yamori et al., 2010, 2011). However, that presumption is based on high values of gs and gm. Once gs and gm decrease because of environmental stresses such as drought, CO2 assimilation rate is then partially constrained by RuBP carboxylation (Flexas et al., 2002; Flexas and Medrano, 2002). Therefore, if fluctuating light levels restrict gm, then An likely tends to be limited by RuBP carboxylation.
Photorespiration, an inevitable process in photosynthesis, plays a supporting role in photosynthetic CO2 assimilation (Timm et al., 2012; Busch et al., 2013; Weber and Bauwe, 2013). This process is initiated by the oxygenation of RuBP, in which one molecule of glycolate-2-phosphate and one molecule of glycerate-3-phosphateare are produced (Ogren, 1984). Although glycolate-2-phosphate cannot be used by plants for biosynthetic reactions, and is also a potential inhibitor of chloroplast functioning (Anderson, 1971), it can be converted into glycerate-3-phosphate through the photorespiratory pathway (Leegood et al., 1995). When gs and gm are diminished, the decreased chloroplast CO2 concentration increases the specificity of Rubisco to O2 and then induces a rise in the rate of RuBP oxygenation (Wingler et al., 1999, 2000).
Plants avoid those detrimental effects of glycolate-2-phosphate and other photorespiratory intermediates by activating the photorespiratory pathway when chloroplast CO2 concentration is low. In Arabidopsis thaliana plants grown under low irradiance, photorespiration plays a minor role in regulating photosynthetic electron flow after exposure to short-term fluctuating light (Kono et al., 2014). The growth light intensity significantly affects the development of the photorespiratory pathway (Huang et al., 2014). For example, plants such as tobacco grown under bright light have a greater capacity than those under low light (Huang et al., 2014). However, little is known about how the photorespiratory pathway functions in the acclimation to fluctuating light by plants grown under high light. Because this pathway is critical to the control of An and photosynthetic electron flow (Takahashi et al., 2007; Timm et al., 2012; Huang et al., 2014), it is important that research focused on photosynthetic regulation under fluctuating light should include growth light intensity as an experimental variable.
In this study, we measured the photosynthetic parameters of gas exchange and chlorophyll fluorescence to investigate the responses of gm, An, and photosynthetic electron flow to fluctuations in light levels. We also examined the limiting step of CO2 assimilation and the role the photorespiratory pathway has in modulating photosynthetic electron flow under alternating light conditions. Our objective was to improve our understanding of how photosynthesis is regulated when sun-grown plants are exposed to changes in irradiance. The following questions were addressed: (1) Is gm restricted by fluctuating light? (2) What is the limiting step of An under fluctuating light? and (3) Does the photorespiratory pathway play an important role in regulating photosynthetic electron flow under fluctuating light?
Materials and methods
Plant materials and growing conditions
Following seed germination, seedlings of tobacco cv. y87 were cultivated in phytotron for 7 weeks. Afterwards, they were grown in plastic pots in an open field at Kunming Institute of Botany, Yunnan, China (elevation 1900 m, 102°41′E, 25°01′N). During our experiment period (10 May to 24 June 2013), none of the plants experienced any water or nutrient stresses. The average temperature at Kunming was 20.9°C in May and 20.6°C in June. Fully expanded mature leaves on 13-week-old plants were used for photosynthetic measurements.
Analyses of gas exchange, chlorophyll fluorescence, and mesophyll conductance
Photosynthetic parameters for gas exchange and chlorophyll fluorescence were monitored with an open gas exchange system that incorporated infrared CO2 and water vapor analyzers (Li-6400XT; Li-Cor Biosciences, Lincoln, NE, USA) and a 2-cm2 measuring head (6400-40 Leaf Chamber Fluorometer; Li-Cor Biosciences). Measurements were made in a phytotron where relative air humidity (60%) and air temperature (25°C) were controlled. The atmospheric CO2 concentration was maintained at 400 μmol mol−1 by the Li-6400XT. To generate a light response curve, we initially exposed the mature leaves to strong irradiance (2000 μmol photons m−2 s−1) for 20 min to obtain steady, high levels of gs and CO2 assimilation. Afterward, photosynthetic parameters were evaluated at 2-min intervals at photosynthetic photon flux densities (PPFDs) of 2000, 1600, 1200, 800, 500, 300, 200, 100, 50, 20, or 0 μmol photons m−2 s−1. To investigate the responses of gs, gm, CO2 assimilation rate, and photosynthetic electron flow to fluctuating light, we also evaluated those photosynthetic parameters under light levels that alternated every 2 min between 100 and 1200 μmol photons m−2 s−1 after dark-adaptation for 30 min. Photosynthetic induction curves were also developed at 1200 μmol photons m−2 s−1 after 30 min of darkness. Values for those parameters were recorded automatically by the Li-6400XT at 2-min intervals.
The CO2 assimilation rate vs. chloroplast CO2 concentration (Cc) was examined at 1200 μmol photons m−2 s−1 (von Caemmerer and Farquhar, 1981). For each An/Cc curve, the photosynthetic rate reached a steady state at 400 μmol mol−1 CO2, then decreased to a lower limit of 50 μmol mol−1 before increasing stepwise to an upper limit of 1600 μmol mol−1. Each stepwise measurement was completed within 2–3 min. Using those An/Cc curves, we calculated the maximum rates of RuBP regeneration (Jmax) and RuBP carboxylation (Vcmax) according to the method of Long and Bernacchi (2003).
The fluorescence parameters Fo′, Fm′, and Fs were evaluated as previously described in Baker and Rosenqvist (2004). Here, Fo′ and Fm′ represented the minimum and maximum fluorescence after light-adaption, respectively. Fs indicated the light-adapted steady-state fluorescence. The maximum quantum yield of PSII after light adaptation (Fv′/Fm′) was calculated as (Fm′–Fo′)/Fm′. Coefficient of PSII photochemical quenching (qP) was calculated as (Fm′–Fs)/(Fm′–Fo′). Effective quantum yield of PSII (ΦPSII) was calculated as (Fm′–Fs)/Fm′ (Genty et al., 1989).
Total photosynthetic electron flow through PSII was calculated as JT = ΦPSII × PPFD × Labs × 0.5 (Krall and Edwards, 1992), where Labs represented leaf absorbance and was assumed to be 0.85 for sun-grown tobacco leaves that receive high-nitrogen nutrition (Miyake et al., 2005). The constant of 0.5 was applied based on the assumption that photons were equally distributed between photosystem I (PSI) and PSII (Miyake et al., 2005). Following the assumption that the water–water cycle is not a major alternative electron sink when CO2 assimilation is limited (Driever and Baker, 2011), we allocated the electron flow through PSII to RuBP carboxylation (JC) and oxygenation (JO). Values for JC and JO were estimated according to the method of Valentini et al. (1995):
where An was the net rate of CO2 assimilation and Rd represented the rate of mitochondrial respiration as measured after 30 min of dark-adaptation.
We recorded values for mesophyll conductance (gm) at 1200 μmol photons m−2 s−1 after plants were exposed to either fluctuating or constant light for 60 min. For our comparisons, gm was also estimated at 1200 μmol photons m−2 s−1 in light response curves. Values for gm were estimated through a combination analysis of gas exchange and chlorophyll fluorescence, and according to the following equation (Harley et al., 1992; Loreto et al., 1992; Warren and Dreyer, 2006; Yamori et al., 2010, 2011):
where An was the net rate of CO2 assimilation, Ci was the intercellular CO2 concentration, JT was total photosynthetic electron flow through PSII, Rd was the rate of mitochondrial respiration, and Γ* was the CO2 compensation point in the absence of daytime respiration (Farquhar et al., 1980; Brooks and Farquhar, 1985), with the latter assumed to be 32.2 at 25°C (Long and Bernacchi, 2003). Using the estimated gm, we calculated the chloroplast CO2 concentration with the following equation (Long and Bernacchi, 2003; Warren and Dreyer, 2006; Yamori et al., 2010, 2011):
where Ci was the intercellular CO2 concentration, An was the net rate of CO2 assimilation, and gm was mesophyll conductance. To identify the limiting step of CO2 assimilation under fluctuating light, we applied the method of Yamori et al. (2010, 2011) to determine Ctrans, the chloroplast CO2 concentration at which the transition from RuBP carboxylation to RuBP regeneration occurred:
where Kc (μmol mol−1) and Ko (mmol mol−1) were the Michaelis constants for CO2 and O2, respectively (Farquhar et al., 1980), and were assumed to be 406.7 μmol mol−1 and 277 mmol mol−1 at 25°C, respectively (Long and Bernacchi, 2003); Jmax was the maximum rate of RuBP regeneration; Vcmax was the maximum rate of RuBP carboxylation; and Γ* was the CO2 compensation point in the absence of daytime respiration. The limiting step of CO2 assimilation was then determined by comparing the values of Cc and Ctrans.
Statistical analysis
The results were displayed as mean values of four independent measurements. We used One-Way ANOVA and SPSS 16.0 software (SPSS Inc., Chicago, IL, USA) to examine differences among treatments involving fluctuating vs. constant light. Those differences were considered significant at P < 0.05.
Results
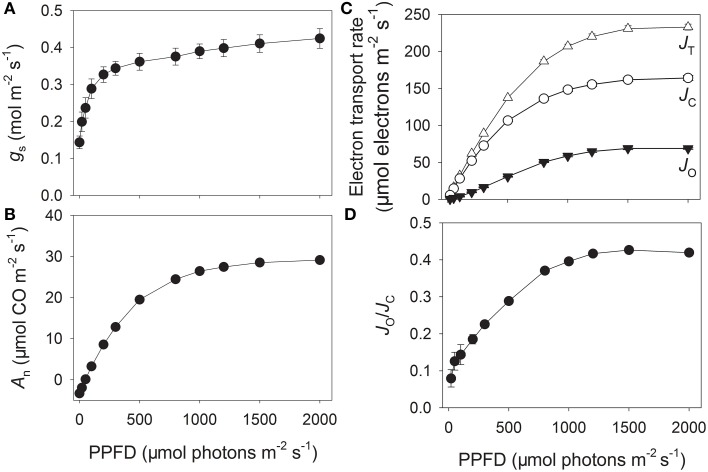
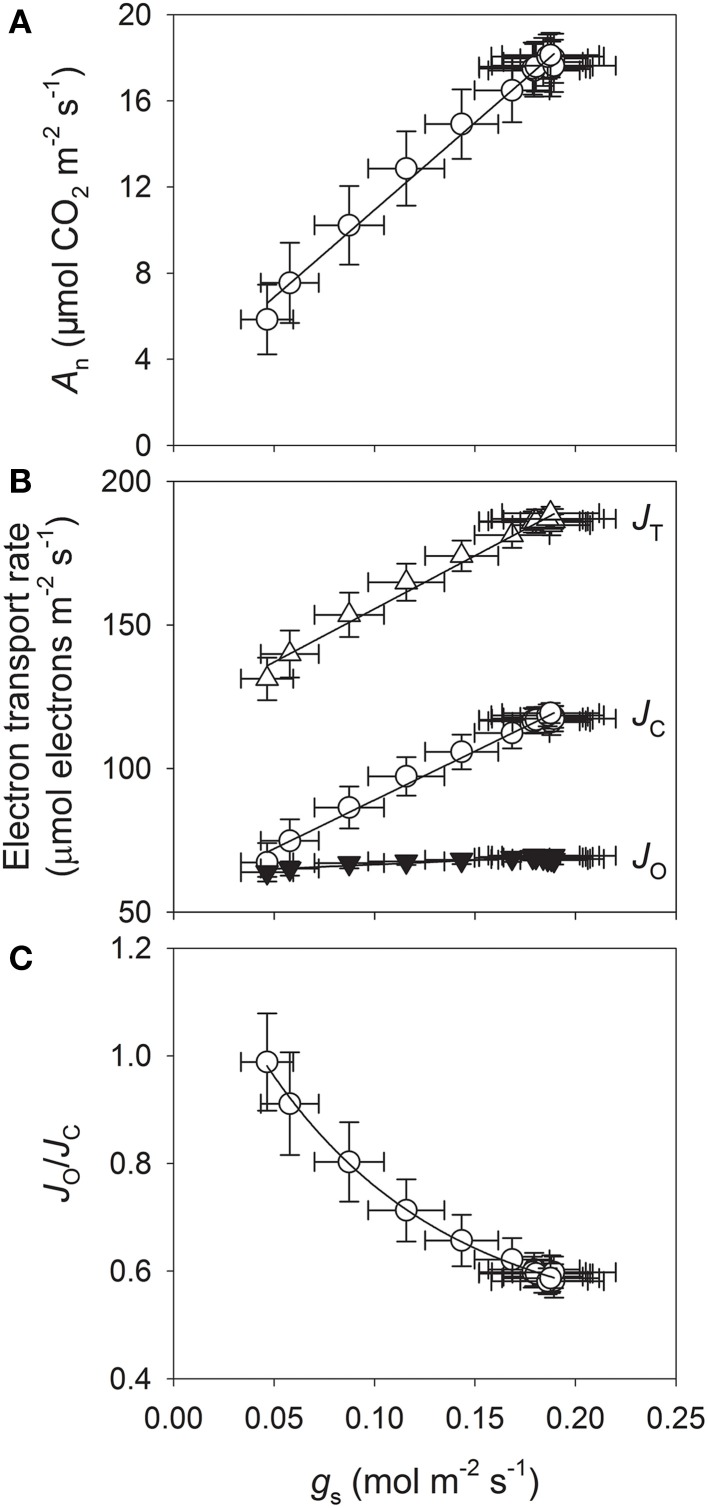
Light response curves indicated that gs was maintained at high levels (>0.3 mol m−2 s−1) when plants were exposed to light intensities above 100 μmol photons m−2 s−1 (Figure 1A). When light levels were reduced from 100 to 0 μmol photons m−2 s−1, values for gs decreased sharply from 0.29 to 0.14 mol m−2 s−1 within 6 min (Figure 1A). This indicated that stomatal conductance in sun-grown tobacco leaves is very sensitive to light intensity in sun-grown tobacco leaves. Under strong irradiance, i.e., 1500 μmol photons m−2 s−1, An was 28.5 μmol CO2 m−2 s−1 (Figure 1B). At levels below 1500 μmol photons m−2 s−1, values for JT, JC, JO, and JO/JC gradually rose with increasing PPFD, peaking at 233 μmol electrons m−2 s−1, 164 μmol electrons m−2 s−1, 69 μmol electrons m−2 s−1, and 0.43, respectively (Figures 1C,D).
Figure 1.
Light response changes in stomatal conductance (gs) (A), CO2 assimilation (An) (B), total electron flow through PSII (JT) (C), electron flow devoted to RuBP carboxylation (JC) (C), electron flow devoted to RuBP oxygenation (JO) (C), and JO/JC ratio for leaves of tobacco (D). Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
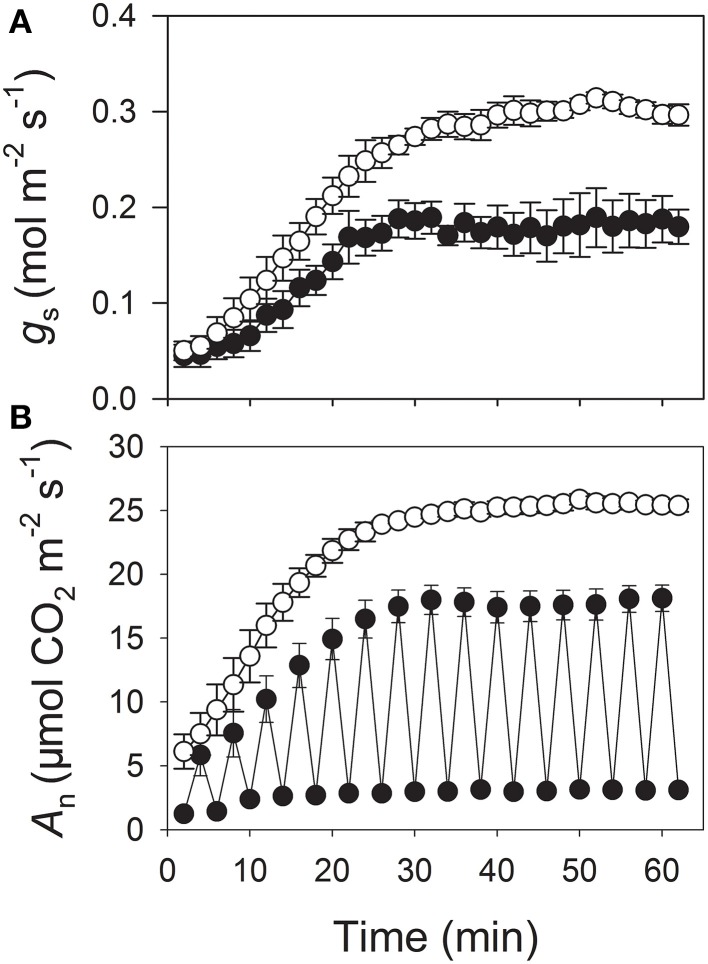
Fluctuating light conditions significantly restricted the opening of stomata. After plants were alternately exposed to 100 and 1200 μmol photons m−2 s−1 every 2 min for 60 min, gs was 0.18 mol m−2 s−1 (Figure 2A). However, when plants were illuminated at a constant 1200 μmol photons m−2 s−1 for 60 min, gs was 0.30 mol m−2 s−1. After 60 min of fluctuating light, the CO2 assimilation rate at 1200 μmol photons m−2 s−1 was 18.1 μmol CO2 m−2 s−1 vs. 25.4 μmol CO2 m−2 s−1 after exposure to 1200 μmol photons m−2 s−1 for 60 min (Figure 2B). Those values for gs and An differed significantly between constant and fluctuating-light treatments, demonstrating that the latter condition inhibited gs as well as CO2 assimilation. This finding was consistent with those reported previously (Fay and Knapp, 1993; Kirschbaum et al., 1998).
Figure 2.
Responses of stomatal conductance (gs) (A) and CO2 assimilation (An) (B) to either light fluctuations between 100 and 1200 μmol photons m−2 s−1 at 2-min intervals (closed symbols) or constant light of 1200 μmol photons m−2 s−1 (open symbols) in tobacco leaves after 30 min of dark-adaptation. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
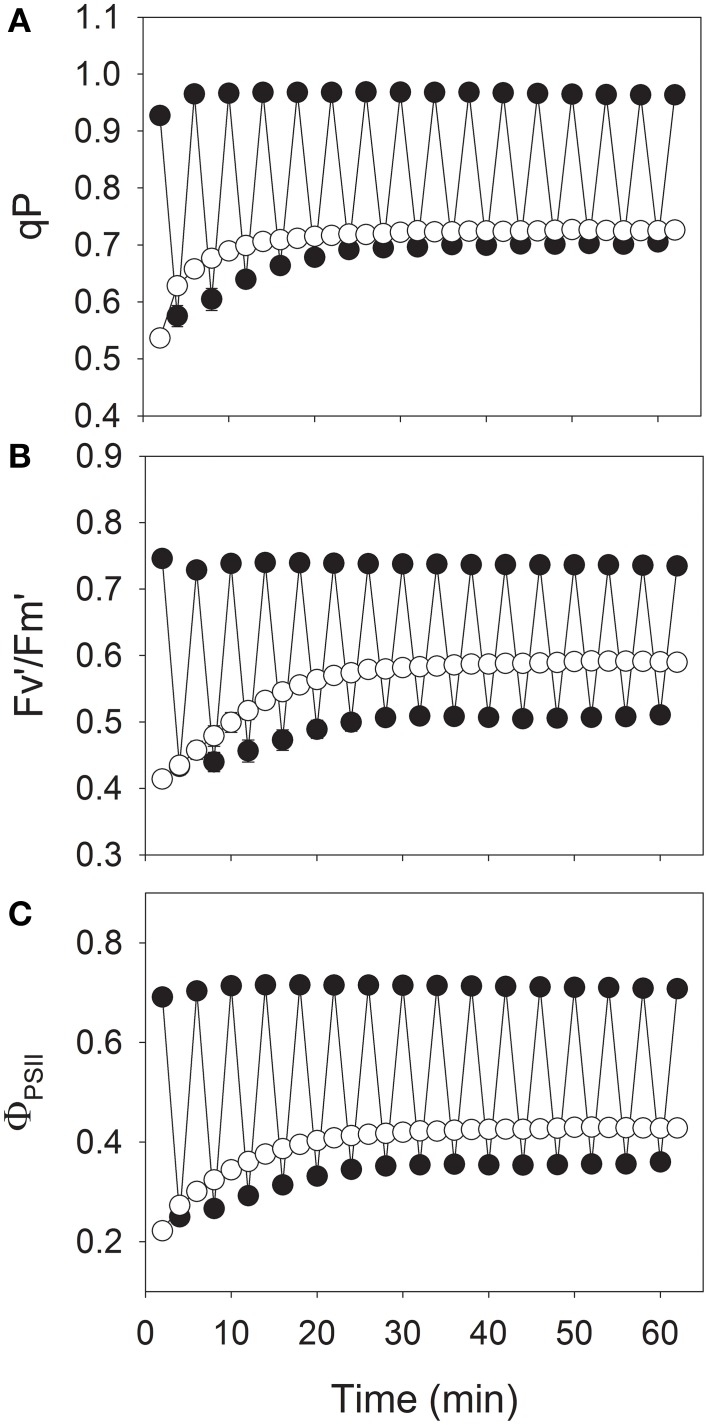
By contrast, values for qP at 1200 μmol photons m−2 s−1 differed only slightly between the constant and fluctuating light treatments (Figure 3A), while Fv′/Fm′ and ΦPSII at 1200 μmol photons m−2 s−1 were significantly lower under fluctuating light (P < 0.001; Figures 3B,C). The parameter Fv′/Fm′ represents the maximum efficiency of PSII when all reaction centers are “open,” and qP is the factor that relates maximum PSII efficiency to the operating PSII efficiency (Farage et al., 2006). Because ΦPSII is the product of qP and Fv′/Fm′, the difference in ΦPSII that we found between fluctuating light and constant light resulted from the change in Fv′/Fm′. These results suggested that although fluctuating light had little effect on the coefficient of PSII photochemical quenching, it induced a significant decline in the maximum efficiency of PSII.
Figure 3.
Responses of coefficient of PSII photochemical quenching (qP) (A), maximum quantum yield of PSII after light-adaptation (Fv′/Fm′) (B), and effective quantum yield of PSII (ΦPSII) (C) to fluctuating light levels (closed symbols) or constant bright light (open symbols). Treatment protocol followed that described for Figure 2. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
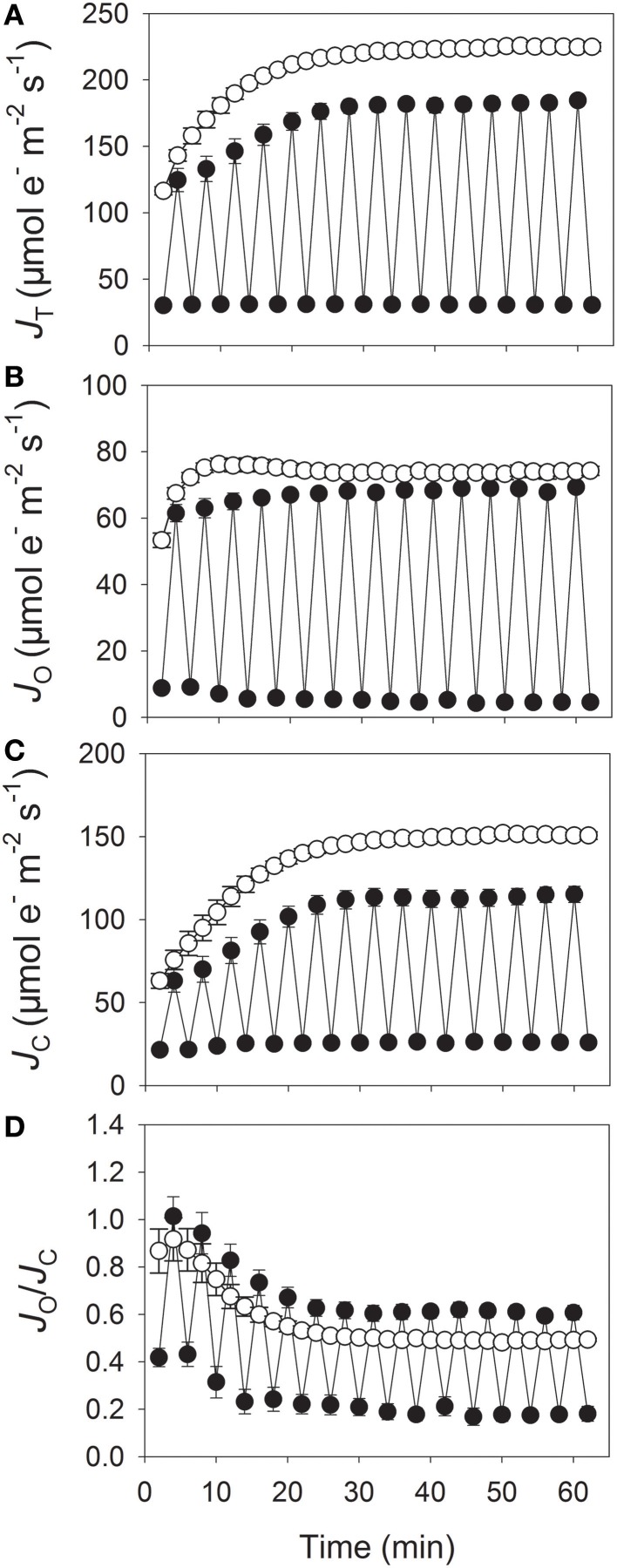
After 60 min of treatment, total electron flow through PSII (JT) at 1200 μmol photons m−2 s−1 was significantly higher under constant illumination than under fluctuating light, i.e., 225 vs. 185 μmol electrons m−2 s−1, respectively (Figure 4A). During that time period, the value for electron flow devoted to RuBP oxygenation (JO) at 1200 μmol photons m−2 s−1 changed only slightly between constant- and fluctuating-light treatments (Figure 4B). By contrast, electron flow devoted to RuBP carboxylation (JC) at 1200 μmol photons m−2 s−1 was higher under constant light (151 μmol electrons m−2 s−1) than under fluctuating light (115 μmol electrons m−2 s−1) (Figure 4C). Consequently, the ratio JO/JC at 1200 μmol photons m−2 s−1 was higher for plants treated with fluctuating light because of the lower value for JC (Figure 4D). These results indicated that fluctuations in irradiance levels suppressed photosynthetic electron flow, primarily by restricting electron flow devoted to RuBP carboxylation. By comparison, electron flow devoted to RuBP oxygenation was hardly affected by fluctuating light conditions.
Figure 4.
Responses of total electron flow (JT) (A), electron flow devoted to RuBP carboxylation (JC) (B), electron flow devoted to RuBP oxygenation (JO) (C), and JO/JC (D) to fluctuating light levels (closed symbols) or constant bright light (open symbols). Treatment protocol followed that described for Figure 2. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4).
After pooling the photosynthesis data collected at 1200 μmol photons m−2 s−1 under fluctuating light, we determined that gs was linearly and positively correlated with An, JT, and JC (Figures 5A,B). We found it interesting that JO was independent of gs (Figure 5B), which implied that RuBP carboxylation and RuBP oxygenation responded differently to gs. Under fluctuating light, JO remained at nearly the maximum level throughout the experimental period. In the initial stage of fluctuating light treatment, electron flow attributed to RuBP oxygenation contributed largely to the total electron transport through PSII (Figure 5C).
Figure 5.
Relationships between parameters derived from simultaneous measurements of gas exchange and chlorophyll fluorescence at 1200 μmol photons m−2 s−1 under fluctuating-light conditions. Analyzed data are those depicted in Figures 2, 4. Comparisons were made between (A) gs and An; (B) gs and JT, JC, or JO; and (C) gs and JO/JC.
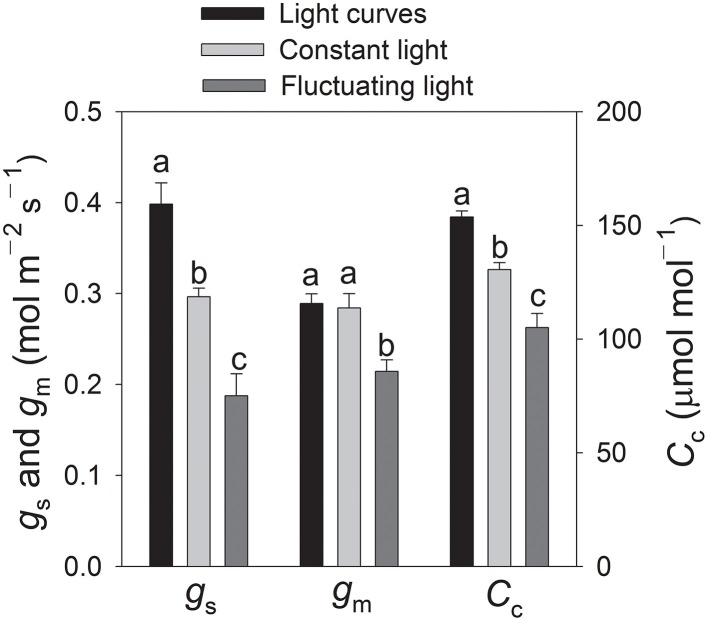
To analyze the limiting step of CO2 assimilation under fluctuating light, we examined the relationship between photosynthesis and chloroplast CO2 concentration. Here, the ratio of the maximum rate of RuBP regeneration (Jmax) to that of RuBP carboxylation (Vcmax) was 0.92, and the chloroplast CO2 concentration at which the transition from RuBP carboxylation to RuBP regeneration occurred (Ctrans) was 135 μmol mol−1 (Figure 6). After exposure to fluctuating light conditions for 60 min, gm at 1200 μmol photons m−2 s−1 was 0.21 mol m−2 s−1, which was significantly lower than that found with light curves (0.29 mol m−2 s−1) or under constant light (0.28 mol m−2 s−1) (Figure 7). For the light curves, Cc at 1200 μmol photons m−2 s−1 was 154 μmol mol−1. After exposure to fluctuating light or constant light for 60 min, the value for Cc was 105 or 131 μmol mol−1, respectively. This indicated that fluctuating light not only decreased gs but also restricted gm, leading to a decline in Cc. Because Cc was significantly lower than Ctrans (P < 0.0001), the rate of CO2 assimilation at 1200 μmol photons m−2 s−1 under fluctuating light was limited by RuBP carboxylation.
Figure 6.
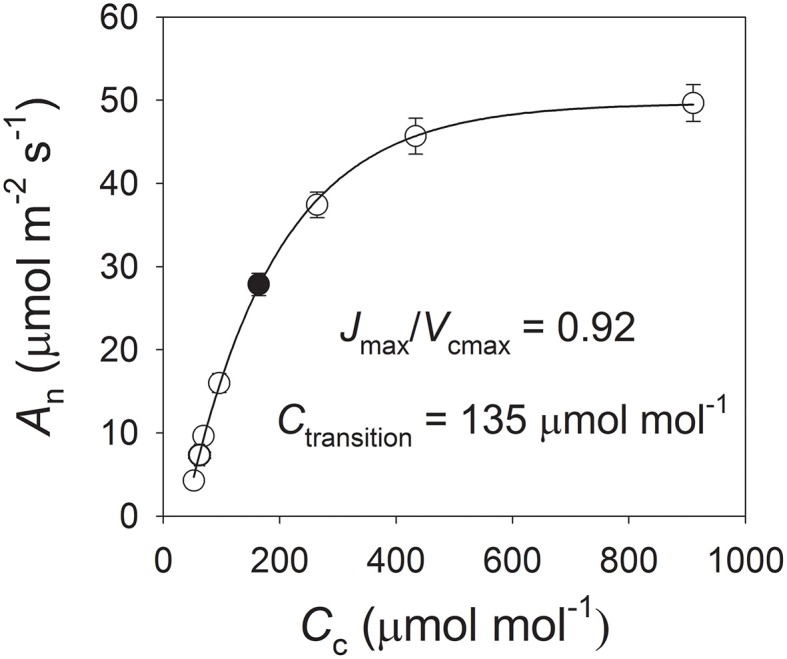
Response of CO2 assimilation rate (An) to incident chloroplast CO2 concentration (Cc) at 25°C and 1200 μmol photons m−2 s−1. Maximum rates of RuBP regeneration (Jmax) and RuBP carboxylation (Vcmax) were calculated according to method of Long and Bernacchi (2003). Chloroplast CO2 concentration at which RuBP carboxylation transitions to RuBP regeneration (Ctrans), as determined by method of Yamori et al. (2010); Yamori et al. (2011). Solid symbol, An at atmospheric CO2 concentration of 400 μmol mol−1.
Figure 7.
Values for stomatal conductance (gs), mesophyll conductance (gm), and chloroplast CO2 concentration (Cc) at 1200 μmol photons m−2 s−1 analyzed in light response curves, constant vs. fluctuating light levels after 60 min of treatment. Measurements were conducted at 25°C and 400 μmol mol−1 CO2. Values are means ± SE (n = 4). For each treatment type, different letters indicate significant differences among light treatments (P < 0.05), based on Tukey's multiple comparison tests.
Discussion
The limiting step of An is mainly determined by the relative values of Ctrans and Cc. For tobacco plants supplied with high concentrations of nitrogen, photosynthesis tends to be limited by RuBP regeneration because Cc is higher than Ctrans (Yamori et al., 2010, 2011). Such previous conclusions have been based on experiments that involved high levels of gs and gm under constant strong light. Although gs and An can be significantly inhibited under fluctuating light, the limiting step of An under such conditions has been unknown. Here, our results indicate that both gs and gm are significantly restricted under fluctuating light, leading to the decrease in Cc. After exposure to fluctuating light for 60 min, Cc was 105 vs. 135 μmol mol−1 for Ctrans. Those data provided evidence that, at 1200 μmol photons m−2 s−1, the photosynthetic process is limited by RuBP carboxylation under fluctuating light. Meanwhile, the high activation of photorespiration contributed largely to the regulation of photosynthetic electron flow.
Carriquí et al. (2015) have demonstrated that gm plays an important role in determining the CO2 assimilation rate, especially at high light intensities. Nevertheless, the response of gm to light intensity remains controversial. For example, in sclerophylls such as Banksia integrifolia, B. serrata, and B. paludosa, the average gm under ambient CO2 concentration is 22% lower at 500 than at 1500 μmol photons m−2 s−1 (Hassiotou et al., 2009). However, the average gm calculated for wheat leaves is not affected by light intensity (Tazoe et al., 2009). In tobacco leaves, gm is significantly lower at 250 than at 1000 μmol photons m−2 s−1 (Flexas et al., 2007). By contrast, Yamori et al. (2010) have shown that gm differs little between constant high light and constant low light in tobacco leaves. Our results indicated that gm at 1200 μmol photons m−2 s−1 under the fluctuating light was 25% lower than the level calculated at constant light of 1200 μmol photons m−2 s−1 (Figure 7). We believe that this difference was caused by the use of a low light regime (100 μmol photons m−2 s−1) in fluctuating light. We found that, under fluctuating light, gm was regulated by both high and low light levels; i.e., although the former induced an increase in gm, this effect could be partially reversed when plants were then exposed to reduced irradiance.
According to the photosynthesis model of Farquhar et al. (1980), CO2 assimilation in C3 plants is constrained by RuBP carboxylation and/or RuBP regeneration. Therefore, based on that model, the limiting step can be altered in two ways: (1) adjustments in the balance between the maximum rates of RuBP regeneration and RuBP carboxylation, or (2) changes in the chloroplast CO2 concentration (Hikosaka et al., 2006; Yamori et al., 2011). For example, in research with tobacco plants, Yamori et al. (2011) have reported that the CO2 assimilation rate at 380 μmol mol−1 CO2 and 1500 μmol photons m−2 s−1 (A380) depends upon the leaf-N content and is mainly determined by Jmax/Vcmax. Furthermore, at high leaf-N content, A380 is limited by RuBP regeneration due to the low ratio of Jmax/Vcmax (Yamori et al., 2010, 2011). However, those conclusions have been drawn from experiments with plants that had high values for both gs and gm, and which did not consider the effects of fluctuating light levels. By comparison, our photosynthetic data for gs, An, JT, and Jmax/Vcmax ratio are very similar to those that describe the performance of plants grown with a high nitrogen supply (Yamori et al., 2011), indicating that plants grown with high N concentration were used in the present study. Furthermore, CO2 assimilation rate at 1200 μmol photons m−2 s−1 under constant light was limited by RuBP regeneration. When plants were exposed to fluctuating light, the declines in gs and gm resulted in a decrease in Cc. The low light regimes under fluctuating light decreased the Rubisco activation state (Yamori et al., 2012), which further restricted the Calvin cycle. The rate of CO2 assimilation under high light during the fluctuating-light treatment tended to be limited by RuBP carboxylation. Fluctuating light has altered the limiting step of CO2 assimilation in tobacco plants with high leaf-N content.
Previous studies with A. thaliana have investigated the roles of cyclic electron flow (CEF) and O2-dependent alternative electron sinks in regulating photosynthetic electron flow under fluctuating light (Suorsa et al., 2012; Kono et al., 2014). It is believed that CEF is essential for proper acclimation of PSI to such light condition (Suorsa et al., 2012). However, the contribution of photorespiration to photodamage under fluctuating light is small in Arabidopsis leaves sampled from plants exposed to low light (Kono et al., 2014). In tobacco, the capacity of the photorespiratory pathway is strongly influenced by the growth light intensity, with sun leaves up-regulating this pathway to control CO2 assimilation and photosynthetic electron flow (Huang et al., 2014). However, it is unknown what role the photorespiratory pathway has in enabling plants normally grown under high light to adapt to fluctuating light conditions. Our data demonstrated that, when plants were exposed to fluctuating light, the reduction in Cc meant that less electron flow could be devoted to RuBP carboxylation. However, we found that the flow devoted to RuBP oxygenation was completely and highly activated under such conditions.
Suppression of CO2 fixation can cause over-acidification of lumen in the thylakoid membrane, which then activates non-photochemical quenching (NPQ) to dissipate excess light energy harmlessly as heat (Flexas and Medrano, 2002; Takahashi et al., 2007; Huang et al., 2012). At 1200 μmol photons m−2 s−1, Fv′/Fm′ were lower under fluctuating light than under constant light. Because Fv′/Fm′ is inversely related to NPQ, this result was evidence of the higher activation of NPQ under fluctuating light. Furthermore, an increase in the proton gradient across the thylakoid membrane can limit linear electron flow (LEF) via cytochrome b6/f (Tikkanen and Aro, 2014). Consumption of photochemical energy, such as ATP and NADPH, through the photorespiratory pathway is thought to alleviate such over-acidification. Especially in the initial stage of our fluctuating-light period, electron flow that was consumed by the photorespiratory pathway largely contributed to the operation of LEF. Therefore, for tobacco plants grown under full sunlight, photorespiratory pathway would be essential for regulating photosynthetic electron flow under fluctuating light, even though our findings contradict a previous report concerning low-light-grown A. thaliana (Kono et al., 2014).
Although photorespiratory intermediates such as glycine and glycerate inhibit the Calvin cycle (Chastain and Ogren, 1989; Eisenhut et al., 2007; Timm et al., 2012), they can be converted to glycerate-3-phosphate through the photorespiratory pathway (Peterhansel and Maurino, 2011). This process is critical for photosynthesis and photoprotection (Takahashi et al., 2007). Under fluctuating light, a reduction in Cc will accelerate RuBP oxygenation and, ultimately, the production of those intermediates. If the photorespiratory pathway is maintained at a low level under such conditions, the accumulation of those intermediates inhibits CO2 assimilation as well as photosynthetic electron flow, causing acceleration of photodamage (Chastain and Ogren, 1989; Eisenhut et al., 2007; Takahashi et al., 2007). In plants with a high rate of CO2 assimilation, rapid acceleration of photorespiratory pathway results in low glycine and glycerate contents (Timm et al., 2012). Therefore, to overcome those detrimental effects of photorespiratory intermediates, this pathway is highly activated under fluctuating light, which then benefits photosynthetic CO2 assimilation and photosynthetic electron flow. In addition, the operation of this pathway is necessary for the regeneration of RuBP (Takahashi et al., 2007). To optimize photosynthetic CO2 fixation, the rates of RuBP oxygenation and RuBP regeneration through photorespiratory pathway must be balanced. Therefore, under fluctuating light conditions, strong activation of the photorespiratory pathway accelerates RuBP regeneration, preventing a decrease in the RuBP pool and favoring the Calvin cycle.
In summary, our results provide evidence that, for sun-grown tobacco leaves, fluctuating light conditions significantly decrease both stomatal and mesophyll conductances, as well as chloroplast CO2 concentration. Consequently, the rate of CO2 assimilation is limited by RuBP carboxylation under such conditions. Meanwhile, the photorespiratory pathway is highly activated to regulate photosynthetic electron flow and benefit photosynthetic CO2 fixation. Thus, strong activation of this pathway is an important strategy by which sun-grown plants adapt to fluctuating light.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant 31300332), funding from the China Postdoctoral Science Foundation to WH (2014T70892), and the following scientific foundations from the Yunnan Tobacco Academy of Agriculture: 110201101003 (TS-03), 2011YN02, 2011YN03.
Glossary
Abbreviations
- An
CO2 assimilation rate
- Cc
chloroplast CO2 concentration
- Fv′/Fm′
the maximum quantum yield of PSII after light adaptation
- Ctrans
the chloroplast CO2 concentration at which the transition from RuBP carboxylation to RuBP regeneration occurred
- ΦPSII
effective quantum yield of PSII
- gm
mesophyll conductance
- gs
stomatal conductance
- JC
electron flow devoted to RuBP carboxylation
- JO
electron flow devoted to RuBP oxygenation
- JT
total electron flow through PSII
- Jmax
the maximum rate of RuBP regeneration
- NPQ
non-photochemical quenching
- PSI
photosystem I
- PSII
photosystem II
- qP
coefficient of PSII photochemical quenching
- Vcmax
the maximum rate of RuBP carboxylation.
References
- Anderson L. E. (1971). Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta 235, 237–244. 10.1016/0005-2744(71)90051-9 [DOI] [PubMed] [Google Scholar]
- Baker N. R., Rosenqvist E. (2004). Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J. Exp. Bot. 55, 1607–1621. 10.1093/jxb/erh196 [DOI] [PubMed] [Google Scholar]
- Brooks A., Farquhar G. D. (1985). Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165, 397–406. 10.1007/BF00392238 [DOI] [PubMed] [Google Scholar]
- Busch F. A., Sage T. L., Cousins A. B., Sage R. F. (2013). C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 36, 200–212. 10.1111/j.1365-3040.2012.02567.x [DOI] [PubMed] [Google Scholar]
- Carriquí M., Cabrera M., Conesa M. À., Coopman R. E., Douthe C., Gago J., et al. (2015). Diffusional limitations explain the lower photosynthetic capacity of ferns as compared with angiosperms in a common garden study. Plant Cell Environ. 38, 448–460. 10.1111/pce.12402 [DOI] [PubMed] [Google Scholar]
- Chastain C. J., Ogren W. L. (1989). Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation state in vivo. Plant Cell Physiol. 30, 937–944. [Google Scholar]
- Driever S. M., Baker N. R. (2011). The water-water cycle in leaves is not a major alternative electron sink for dissipation of excess excitation energy when CO2 assimilation is restricted. Plant Cell Environ. 34, 837–846. 10.1111/j.1365-3040.2011.02288.x [DOI] [PubMed] [Google Scholar]
- Eisenhut M., Bauwe H., Hagemann M. (2007). Glycine accumulation is toxic for the cyanobacterium Synechocystis sp. strain PCC 6803, but can be compensated by supplementation with magnesium ions. FEMS Microbiol. Lett. 277, 232–237. 10.1111/j.1574-6968.2007.00960.x [DOI] [PubMed] [Google Scholar]
- Farage P. K., Blowers D., Long S. P., Baker N. R. (2006). Low growth temperatures modify the efficiency of light use by photosystem II for CO2 assimilation in leaves of two chilling-tolerant C4 species, Cyperus longus L. and Miscanthus × giganteus. Plant Cell Environ. 29, 720–728. 10.1111/j.1365-3040.2005.01460.x [DOI] [PubMed] [Google Scholar]
- Farquhar G. D., von Caemmerer S., Berry J. A. (1980). A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90. 10.1007/BF00386231 [DOI] [PubMed] [Google Scholar]
- Fay P. A., Knapp A. K. (1993). Photosynthetic and stomatal responses of Avena sativa (Poaceae) to a variable light environment. Am. J. Bot. 80, 1369–1373. 10.2307/2445664 [DOI] [Google Scholar]
- Flexas J., Bota J., Escalona J. M., Sampol B., Medrano H. (2002). Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct. Plant Biol. 29, 461–471. 10.1071/PP01119 [DOI] [PubMed] [Google Scholar]
- Flexas J., Diaz-Espejo A., Galmés J., Kaldenhoff R., Medrano H., Ribas-Carbo M. (2007). Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ. 30, 1284–1298. 10.1111/j.1365-3040.2007.01700.x [DOI] [PubMed] [Google Scholar]
- Flexas J., Medrano H. (2002). Energy dissipation in C3 plants under drought. Funct. Plant Biol. 29, 1209–1215. 10.1071/FP02015 [DOI] [PubMed] [Google Scholar]
- Genty B., Briantais J. M., Baker N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 99, 87–92. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Harley P. C., Loreto F., Marco G. D., Sharkey T. D. (1992). Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol. 98, 1429–1436. 10.1104/pp.98.4.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassiotou F., Ludwig M., Renton M., Veneklaas E. J., Evans J. R. (2009). Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J. Exp. Bot. 60, 2303–2314. 10.1093/jxb/erp021 [DOI] [PubMed] [Google Scholar]
- Hikosaka K., Ishikawa K., Borjigidai A., Muller O., Onoda Y. (2006). Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 57, 291–302. 10.1093/jxb/erj049 [DOI] [PubMed] [Google Scholar]
- Huang W., Yang S. J., Zhang S. B., Zhang J. L., Cao K. F. (2012). Cyclic electron flow plays an important role in photoprotection for the resurrection plant Paraboea rufescens under drought stress. Planta 235, 819–828. 10.1007/s00425-011-1544-3 [DOI] [PubMed] [Google Scholar]
- Huang W., Zhang S. B., Hu H. (2014). Sun leaves up-regulate the photorespiratory pathway to maintain a high rate of CO2 assimilation in tobacco. Front. Plant Sci. 5:688. 10.3389/fpls.2014.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum M. U. F., Küppers M., Schneider H., Giersch C., Noe S. (1998). Modelling photosynthesis in fluctuating light with inclusion of stomatal conductance, biochemical activation and pools of key photosynthetic intermediates. Planta 204, 16–26. 10.1007/s004250050225 [DOI] [Google Scholar]
- Kono M., Noguchi K., Terashima I. (2014). Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol. 55, 990–1004. 10.1093/pcp/pcu033 [DOI] [PubMed] [Google Scholar]
- Krall J. P., Edwards G. E. (1992). Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 86, 180–187. 10.1111/j.1399-3054.1992.tb01328.x24318756 [DOI] [Google Scholar]
- Leegood R. C., Lea P. J., Adcock M. D., Hausler R. E. (1995). The regulation and control of photorespiration. J. Exp. Bot. 46, 1397–1414. 10.1093/jxb/46.special_issue.1397 [DOI] [Google Scholar]
- Long S. P., Bernacchi C. J. (2003). Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 54, 2393–2401. 10.1093/jxb/erg262 [DOI] [PubMed] [Google Scholar]
- Loreto F., Harley P. C., Marco G. D., Sharkey T. D. (1992). Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol. 98, 1437–1443. 10.1104/pp.98.4.1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake C., Horiguchi S., Makino A., Shinzaki Y., Yamamoto H., Tomizawa K. (2005). Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of chl fluorescence in tobacco leaves. Plant Cell Physiol. 46, 1819–1830. 10.1093/pcp/pci197 [DOI] [PubMed] [Google Scholar]
- Ogren W. L. (1984). Photorespiration: pathways, regulation, and modification. Annu. Rev. Plant Physiol. 35, 415–442. 10.1146/annurev.pp.35.060184.00221526070643 [DOI] [Google Scholar]
- Peterhansel C., Maurino V. G. (2011). Photorespiration redesigned. Plant Physiol. 155, 49–55. 10.1104/pp.110.165019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scafaro A. P., Von Caemmerer S., Evans J. R., Atwell B. J. (2011). Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ. 34, 1999–2008. 10.1111/j.1365-3040.2011.02398.x [DOI] [PubMed] [Google Scholar]
- Suorsa M., Järvi S., Grieco M., Nurmi M., Pietrzykowska M., Rantala M., et al. (2012). PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948. 10.1105/tpc.112.097162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Bauwe H., Badger M. R. (2007). Impairment of the photorespiratory pathway accelerates photoinhibition of photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol. 144, 487–494. 10.1104/pp.107.097253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazoe Y., von Caemmerer S., Badger M. R., Evans J. R. (2009). Light and CO2 do not affect the mesophyll conductance to CO2 diffusion in wheat leaves. J. Exp. Bot. 60, 2291–2301. 10.1093/jxb/erp035 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Aro E. M. (2014). Integrative regulatory network of plant thylakoid energy transduction. Trends. Plant Sci. 19, 10–17. 10.1016/j.tplants.2013.09.003 [DOI] [PubMed] [Google Scholar]
- Timm S., Florian A., Arrivault S., Stitt M., Fernie A. R., Bauwe H. (2012). Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 586, 3692–3697. 10.1016/j.febslet.2012.08.027 [DOI] [PubMed] [Google Scholar]
- Valentini R., Epron D., De Angelis P., Matteucci G., Dreyer E. (1995). In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant Cell Environ. 18, 631–640. 10.1111/j.1365-3040.1995.tb00564.x [DOI] [Google Scholar]
- von Caemmerer S., Farquhar G. D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. 10.1007/BF00384257 [DOI] [PubMed] [Google Scholar]
- Walker B., Ariza L. S., Kaines S., Badger M. R., Cousins A. B. (2013). Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant Cell Environ. 36, 2108–2119. 10.1111/pce.12166 [DOI] [PubMed] [Google Scholar]
- Warren C. R., Dreyer E. (2006). Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. J. Exp. Bot. 57, 3057–3067. 10.1093/jxb/erl067 [DOI] [PubMed] [Google Scholar]
- Weber A. P., Bauwe H. (2013). Photorespiration – a driver for evolutionary innovations and key to better crops. Plant Biol. 15, 621–623. 10.1111/plb.12036 [DOI] [PubMed] [Google Scholar]
- Wingler A., Lea P. J., Quick W. P., Leegood R. C. (2000). Photorespiration: metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. B Biol. Sci. 355, 1517–1529. 10.1098/rstb.2000.0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A., Quick W. P., Bungard R. A., Bailey K. J., Lea P. J., Leegood R. C. (1999). The role of photorespiration during drought stress: an analysis utilising barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 22, 361–373. 10.1046/j.1365-3040.1999.00410.x [DOI] [Google Scholar]
- Yamori W., Evans J. R., von Caemmerer S. (2010). Effects of growth and measurement light intensities on temperature dependence of CO2 assimilation rate in tobacco leaves. Plant Cell Environ. 33, 332–343. 10.1111/j.1365-3040.2009.02067.x [DOI] [PubMed] [Google Scholar]
- Yamori W., Masumoto C., Fukayama H., Makino A. (2012). Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 71, 871–880. 10.1111/j.1365-313X.2012.05041.x [DOI] [PubMed] [Google Scholar]
- Yamori W., Nagai T., Makino A. (2011). The rate-limiting step for CO2 assimilation at different temperatures is influenced by the leaf nitrogen content in several C3 crop species. Plant Cell Environ. 34, 764–777. 10.1111/j.1365-3040.2011.02280.x [DOI] [PubMed] [Google Scholar]