Abstract
Auxin regulates numerous aspects of plant growth and development. For many years, investigating roles for AUXIN BINDING PROTEIN1 (ABP1) in auxin response was impeded by the reported embryo lethality of mutants defective in ABP1. However, identification of a viable Arabidopsis thaliana TILLING mutant defective in the ABP1 auxin binding pocket (abp1-5) allowed inroads into understanding ABP1 function. During our own studies with abp1-5, we observed growth phenotypes segregating independently of the ABP1 lesion, leading us to sequence the genome of the abp1-5 line described previously. We found that the abp1-5 line we sequenced contains over 8000 single nucleotide polymorphisms in addition to the ABP1 mutation and that at least some of these mutations may originate from the Arabidopsis Wassilewskija accession. Furthermore, a phyB null allele in the abp1-5 background is likely causative for the long hypocotyl phenotype previously attributed to disrupted ABP1 function. Our findings complicate the interpretation of abp1-5 phenotypes for which no complementation test was conducted. Our findings on abp1-5 also provide a cautionary tale illustrating the need to use multiple alleles or complementation lines when attributing roles to a gene product.
INTRODUCTION
The plant hormone auxin regulates cell division and cell expansion to affect all aspects of plant growth (reviewed in Perrot-Rechenmann, 2010). Auxin regulates a wide range of developmental processes, and tight control of auxin response is maintained by multiple modes of regulation. These include regulating auxin biosynthesis and metabolism, transport, and signaling (reviewed in Enders and Strader, 2015). To date, nuclear auxin signaling components have been well characterized (reviewed in Chapman and Estelle, 2009; Salehin et al., 2015). In addition, a nontranscriptional auxin response pathway has been proposed, with AUXIN BINDING PROTEIN1 (ABP1) acting in the apoplast as an auxin receptor and transmitting a cytoplasmic signal to regulate auxin responses such as auxin transport and cytoskeletal rearrangements (reviewed in Shi and Yang, 2011; Sauer et al., 2013).
Study of ABP1 has a long, complicated history. Experiments demonstrating auxin binding activity for ABP1 were published as early as the 1980s (reviewed in Jones, 1994). Reverse genetics proved to be a complicated approach to study ABP1 function in Arabidopsis thaliana, as two independently generated abp1 mutants, presumed to be null alleles, appeared to be embryo lethal (Chen et al., 2001; Tzafrir et al., 2004; Meinke et al., 2008; Sassi et al., 2014). Knockdown lines of ABP1 provided some ability to study ABP1 function by reverse genetics. Arabidopsis lines expressing an inducible ABP1 antisense transcript or an inducible single-chain fragment variable scFv12 (David et al., 2007) from a monoclonal antibody raised to ABP1 (Leblanc et al., 1999), targeted to either the apoplast (SS12S) or the endoplasmic reticulum (SS12K), led to phenotypes consistent with decreased auxin activity (Braun et al., 2008; Tromas et al., 2009). Furthermore, the abp1-5 mutation, first described 5 years ago, provided a much needed tool to study ABP1 function. This ethyl methanesulfonate (EMS)-generated TILLING (Henikoff et al., 2004) allele carries a histidine-to-tyrosine point mutation at position 94 in the auxin binding pocket of ABP1, which presumably alters auxin binding affinity (Robert et al., 2010; Xu et al., 2010). Studies using the ABP1 antisense lines, the inducible monoclonal antibody lines, and abp1-5 have uncovered many auxin-related roles for ABP1.
Decreased ABP1 activity in the antisense lines, antibody-expressing lines, and abp1-5 has been reported to result in both morphological and molecular phenotypes. Reported morphological changes in these lines include small epinastic cotyledons and leaves caused by decreased cell size and infrequent cell divisions (Braun et al., 2008), reduced root growth (Tromas et al., 2009), decreased epidermal pavement cell lobing (Xu et al., 2010), reduced hypocotyl elongation in dark-grown seedlings (Paque et al., 2014), and long hypocotyls when grown under low red:far-red light conditions (Effendi et al., 2013). On a molecular level, affecting ABP1 function using antisense, antibodies, or the abp1-5 allele has been reported to result in decreased auxin transcriptional responses (Tromas et al., 2009), reduced activation of ROP small GTPases (Xu et al., 2010), enhanced auxin efflux protein internalization in epidermal pavement cells (Xu et al., 2010), reduced Brefeldin A-induced internalization of auxin efflux proteins in root cells (Robert et al., 2010; Chen et al., 2012), altered auxin-responsive rearrangement of microtubules (Chen et al., 2014), and altered xyloglucan structure (Paque et al., 2014). These phenotypes suggest roles for ABP1 in many processes.
Inconsistent with research suggesting multiple roles for ABP1 throughout plant development, Gao et al. (2015) recently reported that two independent CRISPR and T-DNA insertion alleles of abp1, which fail to accumulate ABP1 protein, display no obvious phenotypes. These findings cannot be reconciled easily with the embryo lethal phenotypes of the two original lethal T-DNA insertion lines (Chen et al., 2001; Tzafrir et al., 2004; Meinke et al., 2008; Sassi et al., 2014), the phenotypes of the ABP1 antisense line (Braun et al., 2008; Chen et al., 2014), the phenotypes of the lines expressing anti-ABP1 monoclonal antibody fragments (Braun et al., 2008; Tromas et al., 2009; Paque et al., 2014), or the phenotypes of the abp1-5 TILLING allele (Robert et al., 2010; Xu et al., 2010; Effendi et al., 2013; Chen et al., 2014) and thus require explanation. The situation is far from clear at present and might be explained, for example, by off-target effects in the antibody and antisense expression lines in conjunction with background mutations in the knockout lines as well as in the abp1-5 allele. Or, for the opposite interpretation, these differences may be explained by background suppressor mutations or compensatory systems in the CRISPR knockout line. The differences observed between the various T-DNA lines remain confusing (Chen et al., 2001; Tzafrir et al., 2004; Meinke et al., 2008; Sassi et al., 2014; Gao et al., 2015). Resolving these differences in reported abp1 phenotypes will be an important task for the community in the coming years.
In our studies using abp1-5, we found that the long hypocotyl phenotypes ascribed to abp1-5 segregated independently of the abp1-5 lesion. We therefore performed whole-genome sequencing of the abp1-5 line (originally described in Xu et al., 2010) and found numerous additional mutations, some of which may account for the phenotype differences between the two recently reported abp1 null alleles (Gao et al., 2015) and abp1-5.
abp1-5 LIGHT SIGNALING DEFECTS ARE LIKELY CAUSED BY A SECOND-SITE MUTATION IN PHYB
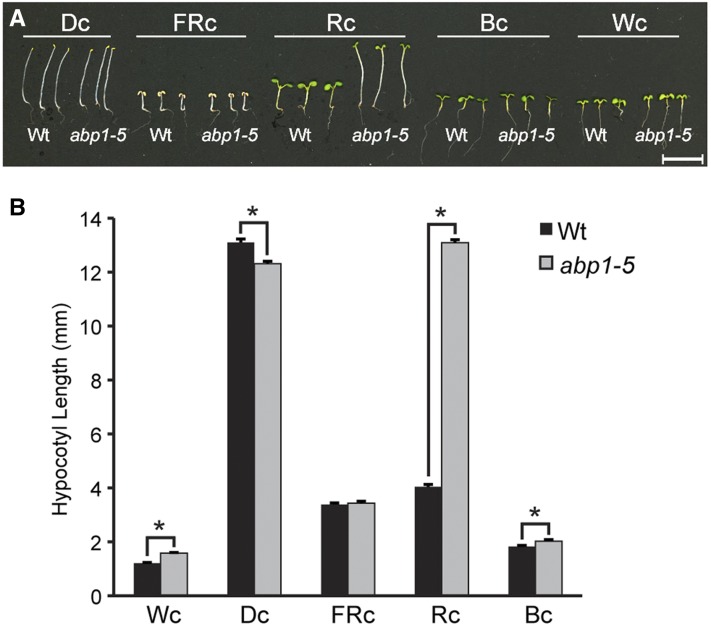
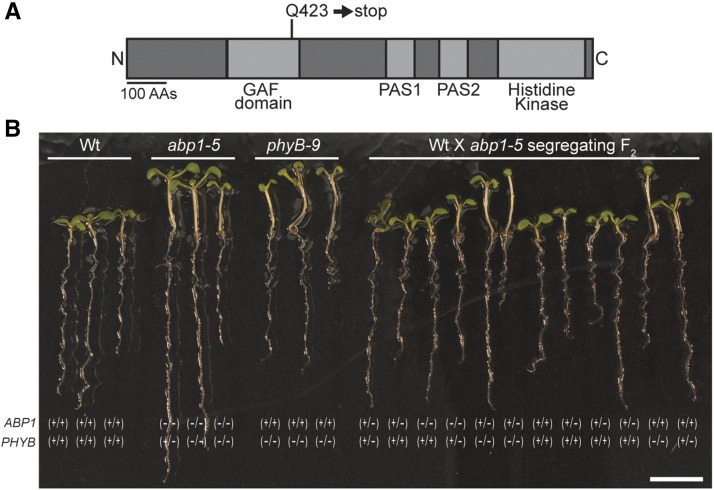
abp1-5 displays red light phenotypes consistent with ABP1 roles in light signaling (Effendi et al., 2013, 2015). In particular, abp1-5 displays longer hypocotyls under red light (Effendi et al., 2013; Figure 1). Although less dramatic, abp1-5 also displays shorter hypocotyls than the wild type when grown in darkness and longer hypocotyls than the wild type under white light and blue light (Figure 1A). Because these abp1-5 phenotypes were strikingly similar to phenotypes seen in phytochrome B (phyB) mutants (Reed et al., 1993), we sequenced the PHYB gene in abp1-5 to rule out the possibility that these phenotypes were caused by a second-site mutation in PHYB. Rather than ruling out PHYB mutations as a contributor to the abp1-5 phenotypes, we found that the PHYB gene in abp1-5 contains a C-to-T base change at position 1267 (where the A of the ATG is at position 1) that causes a Glu-423-to-stop mutation (Figure 2A). Because this premature stop is in the GAF domain, the PHYB gene in abp1-5 is unlikely to encode a functional protein (Reed et al., 1993; Bradley et al., 1996; Chen et al., 2003).
Figure 1.
abp1-5 Displays Altered Light Responses.
(A) Photographs of 7-d-old wild-type (Col-0) and abp1-5 seedlings grown at 22°C on Phytoblend medium supplemented with 1% sucrose under darkness (Dc), FRc (5 µmol m−2 s−1), Rc (50 µmol m−2 s−1), Bc (25 µmol m−2 s−1), or Wc (100 µmol m−2 s−1) light conditions. Bar = 1 mm.
(B) Mean hypocotyl lengths (±se; n = 75) of 7-d-old wild-type (Col-0) and abp1-5 seedlings grown at 22°C on Phytoblend medium supplemented with 1% sucrose under Dc (darkness), FRc (5 µmol m−2 s−1), Rc (50 µmol m−2 s−1), Bc (25 µmol m−2 s−1), or Wc (100 µmol m−2 s−1) light conditions. abp1-5 hypocotyls were significantly longer than wild-type hypocotyls under Wc, Rc, and Bc conditions (P ≤ 0.005) in two-tailed t tests assuming unequal variance. abp1-5 hypocotyls were significantly shorter than wild-type hypocotyls under Dc conditions (P ≤ 1 × 10−6) in two-tailed t tests assuming unequal variance.
Figure 2.
The Long Hypocotyl Phenotype of abp1-5 Segregates Independently of the abp1-5 Mutation.
(A) Examination of the PHYB gene in abp1-5 revealed a C-to-T base change at position 1267 (where the A of the ATG is at position 1) that caused a Glu-423-to-stop mutation in the GAF domain.
(B) Images of 7-d-old wild-type (Col-0), abp1-5, phyB-9, and F2 progeny from the abp1-5 backcross to Col-0 seedlings grown under yellow light conditions. Genotyping results for ABP1 and PHYB mutations are indicated below each imaged seedling. Bar = 7 mm.
Because the ABP1 and PHYB genes are located on different chromosomes, their segregation is unlinked in segregating populations. To determine whether the PHYB mutation in abp1-5 is associated with the long hypocotyl phenotype observed in abp1-5, we examined the phenotypes and genotypes of segregating F2 seedlings from abp1-5 crossed to the wild type (Columbia-0 [Col-0]). The 3:1 segregation of short hypocotyls to long hypocotyls within this segregating backcross suggested that this phenotype was caused by a single, recessive mutation (Figure 2B). We then genotyped these segregating individuals and found that the long-hypocotyl phenotype was associated with the mutation in PHYB, but not the mutation in ABP1 (Figure 2B).
abp1-5 CONTAINS NUMEROUS BACKGROUND MUTATIONS
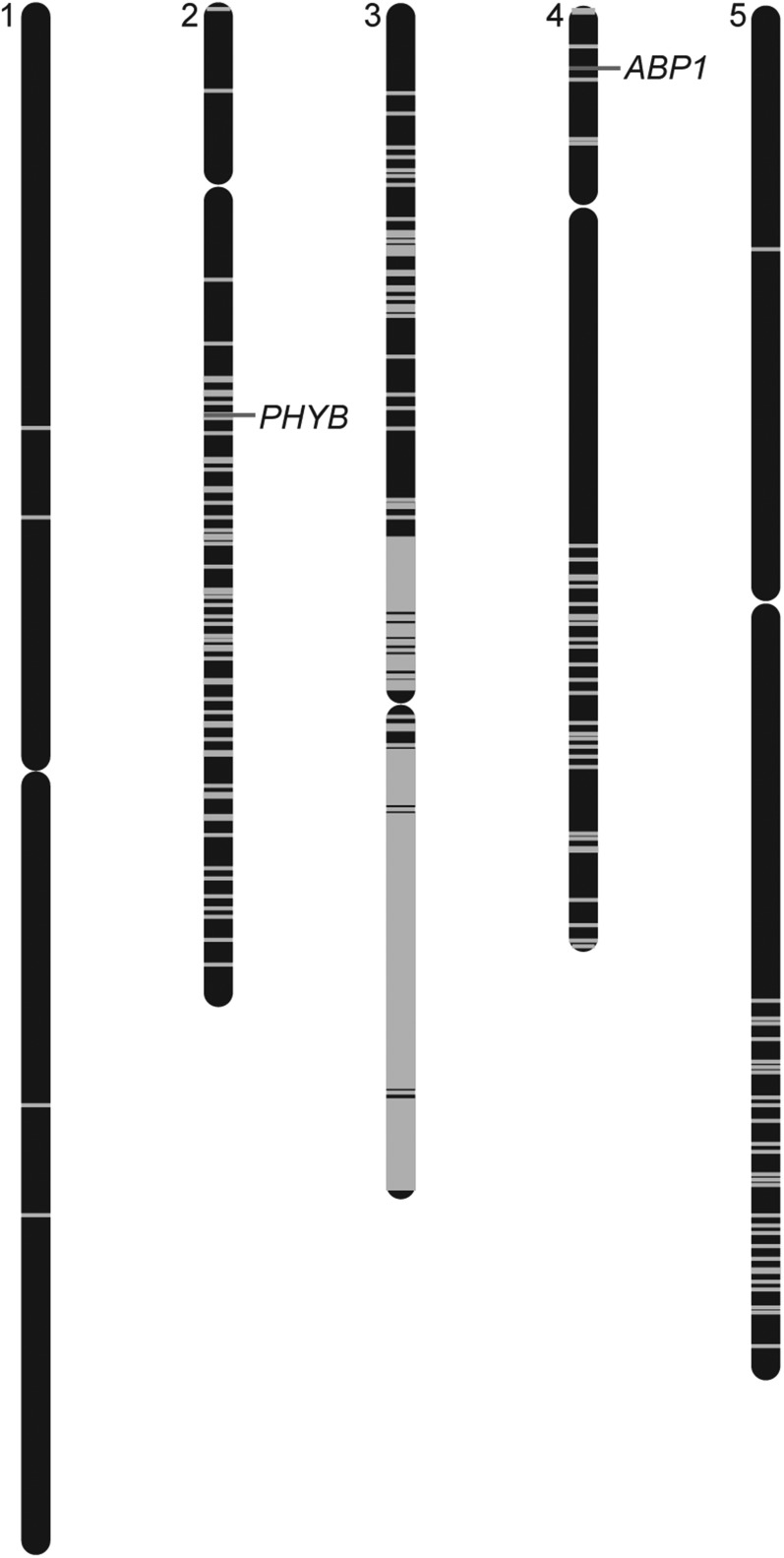
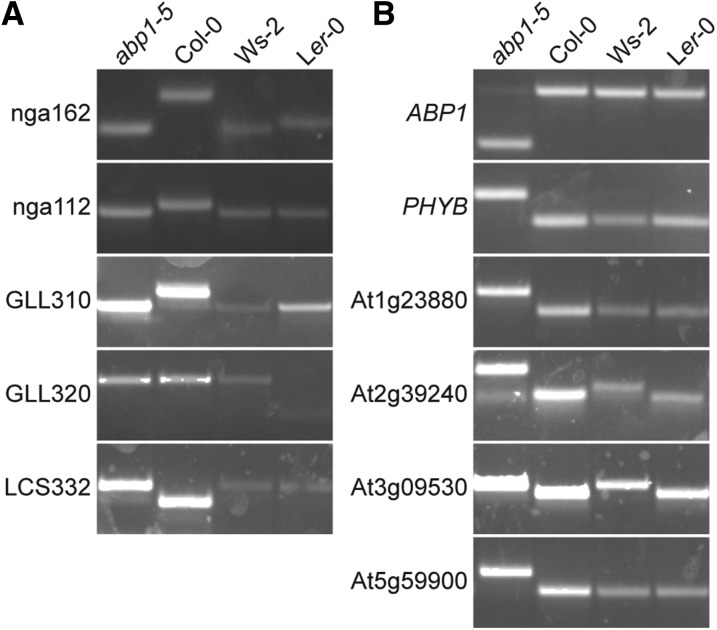
After discovering a PHYB mutation in the abp1-5 background, we decided to sequence the genome of the original abp1-5 line (Xu et al., 2010) to discover whether it carried additional background mutations. Whole-genome sequencing of this abp1-5 line revealed over 8500 single nucleotide polymorphisms (SNPs) (2627 of which are consistent with EMS mutagenesis) in the exome (Table 1), with a concentration of mutations on Chromosome 3, even though ABP1 is located on the north arm of Chromosome 4 (Figure 3). Of these SNPs, 4034 result in nonsynonymous amino acid changes in the encoded proteins and 66 of the mutations result in the creation of premature stop codons (Supplemental Data Set 1). Although most of the identified SNPs on Chromosomes 1, 2, 4, and 5 are consistent with EMS mutagenesis (G-to-A or C-to-T), a majority of the SNPs found on Chromosome 3 are not EMS related, suggesting that the origin of these polymorphisms may be more complicated, such as a cross to a non-Columbia ecotype during the reported six cycles of backcrossing (Xu et al., 2010). Indeed, the abp1-5 polymorphisms identified on the south arm of Chromosome 3 are also present in the Wassilewskija (Ws-2) accession (Figure 4A), consistent with the possibility that a cross to the Ws accession at some point in the history of abp1-5 was the origin of these polymorphisms. Conversely, examined abp1-5 SNPs on Chromosomes 1, 2, 4, and 5 do not appear to be present in either the Ws-2 or Landsberg erecta (Ler-0) ecotypes (Figure 4B). The abp1-5 lines characterized in other publications (Robert et al., 2010; Baster et al., 2013; Effendi et al., 2013; Chen et al., 2014; Paque et al., 2014; Xu et al., 2014) likely carry a distinct subset of background mutations, depending on mutation segregation as a result of crosses made to create abp1-5 carrying molecular reporters.
Table 1. SNPs in the abp1-5 Exome.
| Chromosome | Synonymous | Nonsynonymous | Stop Gained | ncRNAa | Start Lost | Total |
|---|---|---|---|---|---|---|
| 1 | 0 | 4 (2) | 0 | 0 | 0 | 4 (2) |
| 2 | 23 (20) | 48 (46) | 2 (2) | 0 | 0 | 73 (68) |
| 3 | 4388 (1469) | 3936 (984) | 61 (22) | 51 (19) | 8 (1) | 8444 (2495) |
| 4 | 12 (10) | 22 (21) | 2 (2) | 0 | 0 | 36 (33) |
| 5 | 8 (8) | 20 (20) | 1 (1) | 0 | 0 | 29 (29) |
| Mitochondria | 4 (0) | 4 (0) | 0 | 0 | 0 | 8 (0) |
| Chloroplast | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 4435 (1507) | 4034 (1073) | 66 (27) | 51 (19) | 8 (1) | 8594 (2627) |
Numbers in parentheses indicate the number of SNPs consistent with EMS mutagenesis (C to T or G to A).
ncRNA, noncoding RNA.
Figure 3.
Whole-Genome Sequencing of abp1-5 Revealed Numerous SNPs.
Map positions of homozygous SNPs (see Supplemental Data Set 1 for a list of mutations identified) are identified by a gray line on each of the five Arabidopsis chromosomes.
Figure 4.
Genotyping Reveals Outcrossing in the History of abp1-5.
(A) PCR-based genotyping of abp1-5, Col-0, Ws-2, and Ler-0 using mapping markers nga162 (Bell and Ecker, 1994), nga112 (Bell and Ecker, 1994), GLL310 (Strader et al., 2010), GLL320, and LCS332. See Table 2 for genotyping information.
(B) PCR-based genotyping of abp1-5, and the Col-0, Ws-2, and Ler-0 ecotypes for polymorphisms in the ABP1, PHYB, At1g23880, At2g39240, At3g09530, and At5g5990 genes identified by abp1-5 whole-genome sequencing (see Supplemental Data Set for a list of mutations identified). See Table 2 for genotyping information.
The extent to which these abp1-5 background mutations contribute to abp1-5 phenotypes is unknown; in addition, these mutations are likely to have segregated independently and variably as labs have made additional crosses with abp1-5, increasing the complexity of understanding abp1-5 phenotypes for which no complementation line was used.
REASSESSING abp1-5 PHENOTYPES
For decades, identification of ABP1 roles in signaling and development was elusive to researchers because of the lack of genetic resources. Identification of the abp1-5 TILLING allele provided a useful tool to examine ABP1 function and allowed identification of downstream ABP1 signaling components. However, our data revealing numerous background mutations in an abp1-5 line (Figure 3), combined with recent characterization of two independent new abp1 null alleles with no discernible phenotypes (Gao et al., 2015), suggest that our community will need to reassess abp1-5 phenotypes in cases where no complementation line was included in the analysis. Additionally, using the recently identified ABP1 null alleles (Gao et al., 2015) to reexamine phenotypes may aid in understanding phenotype differences among abp1 alleles.
Phenotypic inconsistencies among abp1 alleles have led to confusion regarding ABP1 function. Unlike the recently identified ABP1 null alleles that display no discernible phenotype (Gao et al., 2015), the inducible repression alleles and abp1-5 display a wide variety of cell expansion and cell division defects throughout plant development (Braun et al., 2008; Tromas et al., 2009; Robert et al., 2010; Xu et al., 2010; Chen et al., 2012, 2014; Paque et al., 2014). In addition, a recent report demonstrating that expression of wild-type ABP1 in the abp1-1 insertion mutant fails to rescue its embryo-lethal phenotype (Grones et al., 2015) suggests the possibility that the abp1-1 embryo lethality is caused by a defect in a gene other than ABP1. Off-target effects in inducible repression lines, background mutations in abp1-5 or the T-DNA lines, or compensatory systems in the CRISPR and T-DNA insertional knockout lines could contribute to the distinct phenotypes (or lack thereof) observed in different abp1 alleles. Alternatively, these phenotypic differences might arise from differing plant growth responses under chronic and acute lack of ABP1 activity.
This cautionary tale of background mutations in abp1-5 is not the first of its kind. Other examples include a pen2 mutation in the coi1-16 allele (Westphal et al., 2008) and an are1 mutation in ctr1-1 (Shin et al., 2013). Recently, whole-genome sequencing has been used to reveal an unexpected pedigree for a classic “trisomic” line (Salomé and Weigel, 2015). These findings underscore the importance of examining multiple alleles whenever possible and keeping careful records of crossing history. Additionally, as in the case for abp1-5, complementation tests are required to ensure that any new phenotypes attributed to a mutation are rescued in a complementation line, preferably using the native promoter to drive expression.
Our findings described here are meant to inform other researchers using the abp1-5 allele. We encourage the use of a rescue line alongside any future uses of this allele, so that identified phenotypes can be justifiably attributed to the abp1-5 mutation. Moreover, the newly described abp1 null alleles (Gao et al., 2015) may provide excellent resources for ABP1 functional studies using reverse genetics, provided they are free of off-target effects, insertion position effects, or background mutations. The numerous background mutations in the abp1-5 line described here may help explain some of the phenotypic differences between the abp1 CRISPR and T-DNA insertion null alleles (Gao et al., 2015) and abp1-5 (Robert et al., 2010; Xu et al., 2010, 2014; Baster et al., 2013; Effendi et al., 2013; Chen et al., 2014; Paque et al., 2014).
METHODS
Phenotypic Assays
Arabidopsis thaliana mutants were in the Col-0 background, which was used as the reference sequence and the wild type in all experiments. For phenotypic assays, seeds were surface sterilized (Last and Fink, 1988), stratified for 2 d at 4°C, and plated on plant nutrient media (Haughn and Somerville, 1986) supplemented with 0.5% sucrose (w/v) and solidified with 0.6% (w/v) agar. To examine hypocotyl elongation, seedlings were grown at 22°C under continuous illumination. Seedlings were imaged after 7 d of growth.
For measurement of hypocotyls length under monochromatic illumination, surface-sterilized seeds were cold-stratified at 4°C for 4 d in darkness and germinated on Murashige and Skoog media containing 1% (w/v) sucrose adjusted to pH 5.7 with KOH and 0.7% (w/v) Phytoblend agar (Caisson Labs). Seedlings were grown at 22°C for 7 d under continuous far-red (FRc; λmax∼735 nm), red (Rc; λmax∼670 nm), blue (Bc; λmax∼470 nm), or white (Wc) light using light sources previously described (Warnasooriya and Montgomery, 2009). Fluence rate of FR was measured using a StellarNet EPP2000 spectroradiometer (Apogee Instruments), and fluence rates of Rc, Bc, and Wc were measured using a LI-250A light meter (Li-Cor). Hypocotyl lengths were measured using Image J software (NIH).
Genetic Analyses
The abp1-5 mutant used in this study was provided by the lab of Zhenbiao Yang (University of California-Riverside) and was initially described by Xu et al. (2010). The abp1-5 mutant, originally in the Col-0 background (Robert et al., 2010; Xu et al., 2010), was backcrossed to Col-0 and resultant F2 examined for segregation of the long hypocotyl phenotype. Individual F2 were genotyped using PCR analysis (Table 2).
Table 2. Genotyping Primers and Markers.
| Size of Products (bp) |
|||||||
|---|---|---|---|---|---|---|---|
| Marker | Gene | Enzyme | abp1-5 | Col-0 | Ws-2 | Ler-0 | Oligonucleotidesa |
| ABP1 | At4g02980 | RsaI | 107+43 | 150 | 150 | 150 | CATTGGTATCCGCTCGGCTCTTATCTGTG |
| GTACCACTGCCCTTTAGGACAAC | |||||||
| PHYB | At2g18790 | PstI | 155 | 130+25 | 130+25 | 130+25 | AAGGTATGCTTGTGAGTTTTTGCTG |
| GTGTAACAATTCCAGCAGGCGAGTC | |||||||
| At1g23880 | At1g23880 | AccI | 190 | 169+21 | 169+21 | 169+21 | GTATTCAGTGGAAACGGTGGTA |
| TGAAATCCACCAAGTCTGACGCAAAAT | |||||||
| At2g39240 | At2g39240 | XhoI | 150 | 130+20 | 130+20 | 130+20 | GAGAGACTCCTGAAGATGCCCTCGA |
| TGCATACAAAATCAAGTCCAATAAT | |||||||
| At3g09530 | At3g09530 | HinfI | 199 | 178+21 | 199 | 178+21 | CCAACAATCTCCAACACGTTGACT |
| GCTTCTTCGGGCGACATCTCCAC | |||||||
| At5g59900 | At5g59900 | AccI | 200 | 176+24 | 176+24 | 176+24 | GTGAGCTAGTCTCGAGAAGATTGTAGA |
| CAACAGTGATGAAGCAGGCCAGAAGAGATTA | |||||||
| nga162 | At3g13960 | – | 85 | 107 | 85 | 89 | CATGCAATTTGCATCTGAGG |
| CTCTGTCACTCTTTTCCTCTGG | |||||||
| nga112 | At3g62650 | – | 189 | 197 | 189 | 189 | CGTGTATGCAGCTGCATAGACAGTGG |
| GGCGTTATCTCCATCACTCCCTATAGC | |||||||
| GLL310 | At3g51380 | – | 204 | 246 | 204 | 204 | AGAAGAGACAGTGACAGAATCGGGTAATAAG |
| GTCTATCTCCCCCACTTGTTCATC | |||||||
| GLL320 | At3g50550 | ApoI | 191 | 191 | 191 | 114+77 | GAATGGCTAGCCCCAAAGAC |
| TATTGCGTAAAGAAGCGAAAAC | |||||||
| LCS332 | At3g17850 | DpnII | 148 | 118+30 | 148 | 148 | CTGATGGCGGCAAAGTAGGGCTGAG |
| CGAAGATGGGTGCTGATGGTGGTGAC | |||||||
Underlined nucleotide is the introduced mutation for the derived cleaved amplified polymorphic sequence marker (Neff et al., 2002).
Whole-Genome Sequencing
Arabidopsis abp1-5 seeds were surface sterilized and plated on media on top of sterile filter paper. After 7 d of growth, abp1-5 tissue was collected and genomic DNA extracted (Thole et al., 2014). A library was prepared from the genomic DNA using an Illumina Genomic DNA kit. Libraries were then sequenced at the Washington University Genome Technology Assistance Center (https://gtac.wustl.edu) on an Illumina HiSequation 2000 using multiplexing in a 100-bp paired end run. Using Novoalign (Novo-craft), reads were aligned to the Arabidopsis Col-0 reference genome with Arabidopsis Information Resource 10 gene annotations (Arabidopsis Genome Initiative, 2000). SNPs were identified using SAMtools (Li et al., 2009) and annotated using snpEFF (Cingolani et al., 2012).
Accession Numbers
Accession numbers for each gene are listed in Table 2 and Supplemental Data Set 1.
Supplemental Data
Supplemental Data Set 1. abp1-5 whole-genome sequencing data.
Supplementary Material
Acknowledgments
We thank Ram Dixit, Joseph Jez, Dmitri Nusinow, and Petra Levin for valuable discussion as well as David Korasick, Samantha Powers, and Elizabeth Frick for critical comments on the article. This research was supported by the National Science Foundation (DGE-1143954 to T.A.E. and IOS-1453750 to L.C.S.), by the U.S. Department of Energy, Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science (DE-FG02-91ER20021 to B.L.M.), and by the National Institutes of Health (R00 GM089987 to L.C.S.). We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The center is partially supported by the National Cancer Institute Cancer Center Support Grant (P30 CA91842) to the Siteman Cancer Center and by ITCTS/CTSA (UL1 TR000448) from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research.
AUTHOR CONTRIBUTIONS
T.A.E., Z.Y., B.L.M., and L.C.S. planned the experiments. T.A.E. and S.O. conducted experiments. T.A.E., S.O., Z.Y., B.L.M., and L.C.S. wrote the article.
References
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- Baster P., Robert S., Kleine-Vehn J., Vanneste S., Kania U., Grunewald W., De Rybel B., Beeckman T., Friml J. (2013). SCF(TIR1/AFB)-auxin signalling regulates PIN vacuolar trafficking and auxin fluxes during root gravitropism. EMBO J. 32: 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C.J., Ecker J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19: 137–144. [DOI] [PubMed] [Google Scholar]
- Bradley J.M., Murphy G.P., Whitelam G.C., Harberd N.P. (1996). Identification of phytochrome B amino acid residues mutated in three new phyB mutants of Arabidopsis thaliana. J. Exp. Bot. 47: 1449–1455. [Google Scholar]
- Braun N., Wyrzykowska J., Muller P., David K., Couch D., Perrot-Rechenmann C., Fleming A.J. (2008). Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 20: 2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman E.J., Estelle M. (2009). Mechanism of auxin-regulated gene expression in plants. Annu. Rev. Genet. 43: 265–285. [DOI] [PubMed] [Google Scholar]
- Chen J.-G., Ullah H., Young J.C., Sussman M.R., Jones A.M. (2001). ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev. 15: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Schwab R., Chory J. (2003). Characterization of the requirements for localization of phytochrome B to nuclear bodies. Proc. Natl. Acad. Sci. USA 100: 14493–14498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Grandont L., Li H., Hauschild R., Paque S., Abuzeineh A., Rakusová H., Benkova E., Perrot-Rechenmann C., Friml J. (2014). Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 516: 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Naramoto S., Robert S., Tejos R., Löfke C., Lin D., Yang Z., Friml J. (2012). ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr. Biol. 22: 1326–1332. [DOI] [PubMed] [Google Scholar]
- Cingolani P., Platts A., Wang L., Coon M., Nguyen T., Wang L., Land S.J., Lu X., Ruden D.M. (2012). A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6: 80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David K.M., Couch D., Braun N., Brown S., Grosclaude J., Perrot-Rechenmann C. (2007). The auxin-binding protein 1 is essential for the control of cell cycle. Plant J. 50: 197–206. [DOI] [PubMed] [Google Scholar]
- Effendi Y., Ferro N., Labusch C., Geisler M., Scherer G.F. (2015). Complementation of the embryo-lethal T-DNA insertion mutant of AUXIN-BINDING-PROTEIN 1 (ABP1) with abp1 point mutated versions reveals crosstalk of ABP1 and phytochromes. J. Exp. Bot. 66: 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effendi Y., Jones A.M., Scherer G.F. (2013). AUXIN-BINDING-PROTEIN1 (ABP1) in phytochrome-B-controlled responses. J. Exp. Bot. 64: 5065–5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders T.A., Strader L.C. (2015). Auxin activity: Past, present, and future. Am. J. Bot. 102: 180–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y., Zhang, Y., Zhang, D., Dai, X., Estelle, M., and Zhao, Y. (2015). Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc. Natl. Acad. Sci. USA 112: 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grones P., Chen X., Simon S., Kaufmann W.A., De Rycke R., Nodzyński T., Zažímalová E., Friml J. (2015). Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J. Exp. Bot., in press. [DOI] [PubMed] [Google Scholar]
- Haughn G.W., Somerville C. (1986). Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol. Gen. Genet. 204: 430–434. [Google Scholar]
- Henikoff S., Till B.J., Comai L. (2004). TILLING. Traditional mutagenesis meets functional genomics. Plant Physiol. 135: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.M. (1994). Auxin-binding proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 393–420. [Google Scholar]
- Last R.L., Fink G.R. (1988). Tryptophan-requiring mutants of the plant Arabidopsis thaliana. Science 240: 305–310. [DOI] [PubMed] [Google Scholar]
- Leblanc N., David K., Grosclaude J., Pradier J.-M., Barbier-Brygoo H., Labiau S., Perrot-Rechenmann C. (1999). A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J. Biol. Chem. 274: 28314–28320. [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R.; 1000 Genome Project Data Processing Subgroup (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke D., Muralla R., Sweeney C., Dickerman A. (2008). Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci. 13: 483–491. [DOI] [PubMed] [Google Scholar]
- Neff M.M., Turk E., Kalishman M. (2002). Web-based primer design for single nucleotide polymorphism analysis. Trends Genet. 18: 613–615. [DOI] [PubMed] [Google Scholar]
- Paque, S., Mouille, G., Grandont, L., Alabadi, D., Gaertner, C., Goyallon, A., Muller, P., Primard-Brisset, C., Sormani, R., Blázquez, M.A., and Perrot-Rechenmann, C. (2014). AUXIN BINDING PROTEIN1 Links Cell Wall Remodeling, Auxin Signaling, and Cell Expansion in Arabidopsis. Plant Cell 26: 280–295. [DOI] [PMC free article] [PubMed]
- Perrot-Rechenmann C. (2010). Cellular responses to auxin: division versus expansion. Cold Spring Harb. Perspect. Biol. 2: a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.W., Nagpal P., Poole D.S., Furuya M., Chory J. (1993). Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell 5: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S., et al. (2010). ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 143: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehin M., Bagchi R., Estelle M. (2015). SCFTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27: 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé P.A., Weigel D. (2015). Plant genetic archaeology: whole-genome sequencing reveals the pedigree of a classical trisomic line. G3 (Bethesda) 5: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi M., et al. (2014). An auxin-mediated shift toward growth isotropy promotes organ formation at the shoot meristem in Arabidopsis. Curr. Biol. 24: 2335–2342. [DOI] [PubMed] [Google Scholar]
- Sauer M., Robert S., Kleine-Vehn J. (2013). Auxin: simply complicated. J. Exp. Bot. 64: 2565–2577. [DOI] [PubMed] [Google Scholar]
- Shi J.H., Yang Z.B. (2011). Is ABP1 an auxin receptor yet? Mol. Plant 4: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Lee R.A., Lee I., Lee S., Park S.K., Soh M.S. (2013). Genetic identification of a second site modifier of ctr1-1 that controls ethylene-responsive and gravitropic root growth in Arabidopsis thaliana. Mol. Cells 36: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strader L.C., Chen G.L., Bartel B. (2010). Ethylene directs auxin to control root cell expansion. Plant J. 64: 874–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thole J.M., Beisner E.R., Liu J., Venkova S.V., Strader L.C. (2014). Abscisic acid regulates root elongation through the activities of auxin and ethylene in Arabidopsis thaliana. G3 (Bethesda) 4: 1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromas A., Braun N., Muller P., Khodus T., Paponov I.A., Palme K., Ljung K., Lee J.-Y., Benfey P., Murray J.A.H., Scheres B., Perrot-Rechenmann C. (2009). The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE 4: e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzafrir I., Pena-Muralla R., Dickerman A., Berg M., Rogers R., Hutchens S., Sweeney T.C., McElver J., Aux G., Patton D., Meinke D. (2004). Identification of genes required for embryo development in Arabidopsis. Plant Physiol. 135: 1206–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnasooriya S.N., Montgomery B.L. (2009). Detection of spatial-specific phytochrome responses using targeted expression of biliverdin reductase in Arabidopsis. Plant Physiol. 149: 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal L., Scheel D., Rosahl S. (2008). The coi1-16 mutant harbors a second site mutation rendering PEN2 nonfunctional. Plant Cell 20: 824–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., et al. (2014). Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 343: 1025–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Wen M., Nagawa S., Fu Y., Chen J.G., Wu M.J., Perrot-Rechenmann C., Friml J., Jones A.M., Yang Z. (2010). Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 143: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.