Abstract
The metabolism of many anaerobes relies on [NiFe]-hydrogenases, whose characterization when bound to substrates has proven non-trivial. Presented here is direct evidence for a hydride bridge in the active site of the 57Fe-labelled fully reduced Ni-R form of Desulfovibrio vulgaris Miyazaki F [NiFe]-hydrogenase. A unique ‘wagging' mode involving H− motion perpendicular to the Ni(μ-H)57Fe plane was studied using 57Fe-specific nuclear resonance vibrational spectroscopy and density functional theory (DFT) calculations. On Ni(μ-D)57Fe deuteride substitution, this wagging causes a characteristic perturbation of Fe–CO/CN bands. Spectra have been interpreted by comparison with Ni(μ-H/D)57Fe enzyme mimics [(dppe)Ni(μ-pdt)(μ-H/D)57Fe(CO)3]+ and DFT calculations, which collectively indicate a low-spin Ni(II)(μ-H)Fe(II) core for Ni-R, with H− binding Ni more tightly than Fe. The present methodology is also relevant to characterizing Fe–H moieties in other important natural and synthetic catalysts.
 Understanding the catalytic mechanism of redox-active hydrogenases is a key to efficient hydrogen production and consumption. Here, the authors use nuclear resonance vibrational spectroscopy to study [NiFe]-hydrogenase, and observe a bridging hydride structure in an EPR silent intermediate.
Understanding the catalytic mechanism of redox-active hydrogenases is a key to efficient hydrogen production and consumption. Here, the authors use nuclear resonance vibrational spectroscopy to study [NiFe]-hydrogenase, and observe a bridging hydride structure in an EPR silent intermediate.
A central goal of the hydrogen economy is to forestall a continual buildup of CO2 and the threat of global climate change1. However, achieving independence of carbon-based fuels necessitates the development of better H2-processing catalysts from earth-abundant materials. In this regard, bidirectional hydrogenase enzymes, which catalyse both production and consumption of H2 (refs 2, 3), have attracted interest either for direct utilization4 or as aspirational targets for biomimetic catalysts5,6,7,8,9,10,11.
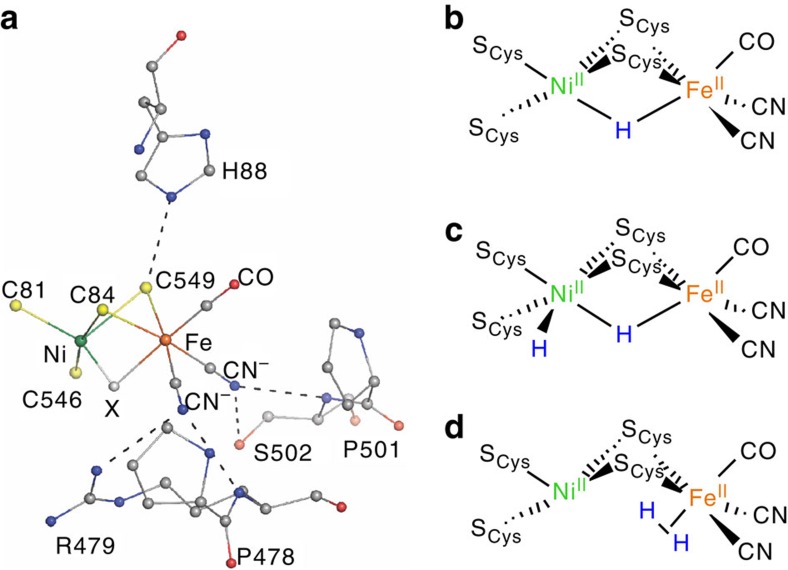
The redox-active hydrogenases are classified as either [FeFe]- or [NiFe]-hydrogenase, according to the metals present at their active sites. While both types possess high catalytic activity12,13,14, the latter are attractive practically in that they are more O2-tolerant12,15. The [NiFe]-hydrogenase active site features Ni and Fe centres bridged by two Cys residues (Fig. 1a), with two further Cys ligands binding Ni terminally, and the Fe coordination sphere being completed by one CO and two CN− groups. The [NiFe] site functions in concert with an electron transport chain comprising three Fe–S clusters. The catalytic cycle is generally thought to involve three key redox states of the bimetallic centre: electron spin (paramagnetic) resonance (EPR)-silent Ni-SIa, EPR-active Ni-C and another EPR-silent species known as Ni-R16,17,18. Despite progress in characterizing hydrogenases by crystallography, spectroscopy, and theory16, questions remain about the molecular and electronic structure of various intermediates and inhibited species. This particularly applies to the electronic structure of Ni-R.
Figure 1. The Ni-R reduced state of [NiFe]-hydrogenase.
(a) X-ray structure of the Desulfovibrio vulgaris Miyazaki F (DvMF) [NiFe]-hydrogenase active site from PDB entry 1WUI (ref. 62). The bridging ligand X is oxygenic for deactivated states, with catalytically active states having either a hydride or a vacant site. (b–d) Some of the structures proposed for isoelectronic Ni-R forms19,20,21,22,23,24,25,26.
Up to three isoelectronic Ni(II)Fe(II) forms of Ni-R are characterized by their pH-dependent Fourier transform infrared (FT-IR) signatures18. The structures proposed for these Ni-R subspecies (Fig. 1b–d) most commonly have a bridging hydride at the active site (Ni(μ-H)Fe)19,20,21,22, with some even featuring an additional (terminal) hydride at Ni (HNi(μ-H)Fe)23,24. Another suggested form has an Fe-bound dihydrogen ligand (NiFe(η2-H2))25,26. A recent high-resolution crystallographic analysis of Ni-R1, a subspecies of Ni-R, indicates a Ni(μ-H)Fe core with a protonated terminal cysteinate27. Among proposals supporting the bridging hydride, there is further debate as to whether H− is bound more strongly to Fe or Ni (ref. 28) and whether Ni(II) is high29 or low spin30 or whether both spin configurations coexist in the bulk31,32.
The Ni-R state represents a special challenge for spectroscopy in that it is EPR-silent, photolabile (and thus difficult to study with Raman spectroscopy) and expected to feature an active site M–H moiety (being notoriously difficult to observe using infrared methods)33. The present study instead employs a synchrotron radiation technique called nuclear resonance vibrational spectroscopy (NRVS), which involves X-ray excitation of a Mössbauer-active nuclide34,35,36,37. The raw NRVS data are commonly translated into partial vibrational density of states (PVDOS) spectra38, which show vibrational energy contributions specifically from the Mössbauer-active nuclei, such as 57Fe. PVDOS can also be predicted using density functional theory (DFT) or empirical force field calculations, assisting in confident spectral assignments.
NRVS is uniquely suited to detailed investigations of 57Fe-labelled enzyme active sites, avoiding interference from the thousands of protein modes present in a typical infrared or Raman spectrum. NRVS has enabled the observation of Fe–CN and Fe–CO bending and stretching modes for the active sites in [NiFe]-hydrogenases39,40, despite the presence of 11 (or more) Fe centres in clusters of the electron transport chain. This is because Fe–CN and Fe–CO modes are strongest in the region from 440 to 640 cm−1, while Fe–S cluster modes only make significant contributions below 440 cm−1 (refs 41, 42, 43). Another recent application of NRVS to a trans-H/D-57Fe-(CO) compound shed light on coupling of Fe–H/D and Fe–CO bending modes33.
The present study discloses the first spectroscopic evidence for the bridging hydride in the Ni-R active site and the unprecedented NRVS observation of a Fe–H stretching mode in a synthetic Ni–H–Fe system. A combined experimental/theoretical analysis of both Ni-R and its synthetic mimics is presented here, an approach that we anticipate to be of broad utility for the characterization of (bio)inorganic Fe-hydride catalysts.
Results
NRVS of model complexes
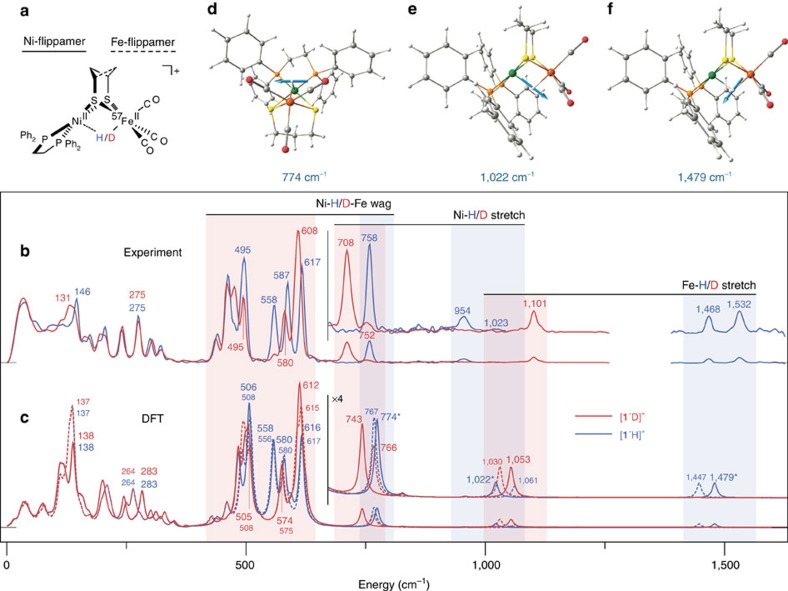
The Ni(II)(μ-thiolate)2(μ-H)Fe(II) core proposed for Ni-R is reproduced by the diamagnetic H2-evolving catalyst [(dppe)Ni(μ-pdt)(μ-H)Fe(CO)3]+ ([1H]+, dppe=1,2-bis(diphenylphosphino)ethane=1,2-Ph2PCH2CH2P Ph2, pdt2−=1,3-propanedithiolate=−SCH2CH2CH2S−)44,45 shown in Fig. 2a, which has recently been studied using resonance Raman spectroscopy46. Synthetic methodology allowed the preparation of the labelled analogue [(dppe)Ni(μ-pdt)(μ-H/D)57Fe(CO)3]+ in both hydride [1′H]+ and deuteride [1′D]+ forms for 57Fe NRVS analysis (see Supplementary Figs 1–16 and Supplementary Note 1). Complementary 13CO-labelled species [1′′H/D]+ were prepared as well.
Figure 2. Metal-hydride bands for complexes [1′H/D]+.
(a) Structure of [1′H]+ showing ‘flippamer' conformations of the pdt2− ligand. (b,c) Full-range 57Fe PVDOS for [1′H]+ (blue trace) and [1′D]+ (red trace) from NRVS experiments (b) and DFT calculations (c). In c, the plain/broken traces are spectra calculated for the dominant/alternative Ni-/Fe-flippamer, respectively. Spectra are repeated in the region >700 cm−1 with their intensities × 4 amplified. The colour bars highlight specific M–H/D bands, as well as their shifts on isotopic substitution. (d–f) Scaled-arrow representations of the M–H normal modes calculated for the Ni-flippamer of [1′H]+ are shown, with the corresponding bands indicated (*) in c. Unscaled-arrow and animated representations of these M–H modes can be found in Supplementary Fig. 22 and Supplementary Movies 1,2,3, respectively.
The NRVS spectra for [1′H/D]+ are presented in Fig. 2b. Apart from low-frequency (180–340 cm−1) Fe–S modes, there are strong bands in the 440–630 cm−1 region assigned to νFe–CO stretches and δFe–CO bends (Supplementary Table 1). On the high-energy side, NRVS analysis of [1′H]+ also reveals peaks at 1,532 and 1,468 cm−1 that have previously been assigned to νFe–H modes of two different conformations of the pdt2− ligand (‘Ni/Fe-flippamers'47,48,49,50, see Fig. 2a and Supplementary Fig. 17 using Raman spectroscopy on [1H]+ (ref. 46, Supplementary Table 2). As expected, these features red-shift to ∼1,101 cm−1 on D substitution in the bridge. While NRVS has uncovered δFe–H/D (ref. 33) and νFe–D modes51 for other species, the 1,532 and 1,468 cm−1 bands for [1′H]+ are the first Fe–H stretching modes detected using this technique, as well as being the highest-frequency bands observed using NRVS to date. The band at 954 cm−1 for [1′H]+, previously assigned to a νNi–H mode46, is evidently also Fe-coupled, given its detection using NRVS. In [1′D]+, the corresponding Ni-D stretch is at 708 cm−1. As expected from a harmonic oscillator in which H/D binds a much heavier nucleus, the νFe–H/νFe–D and νNi–H/νNi-D frequency ratios are ∼2½. This difference in the H/D and 57Fe nuclear masses also results in pure hydride bands having low NRVS intensities, as such vibrations involve only small displacements of the 57Fe centre. In addition, our successful use of NRVS to detect the well-defined νNi-D stretch in 57Fe-labelled [1′D]+ contrasts Raman studies of natural Fe abundance [1D]+, in which the Ni-D band was obscured by solvent modes46.
On the basis of previous studies, one would expect δOC–Fe–H/D modes to appear in the 530–750/410–640 cm−1 regions, respectively33,46. The hydride [1′H]+ exhibits a well-defined NRVS feature at 758 cm−1 consistent with a δFe–H mode, this region being obscured by solvent bands in Raman data46. As will become clear, this 758 cm−1 mode observed for [1′H]+ is of particular relevance to the interpretation of NRVS data for [NiFe]-hydrogenase. A distinct assignment for the corresponding δFe-D mode in [1′D]+ is prevented by its mixing with the Fe–CO bending modes, such that the NRVS intensity is redistributed throughout the 440–630 cm−1 region.
Analysis of [1′H]+ revealed several intense Fe–CO bands in the 440–630 cm−1 region (Fig. 2b), the energies of which are almost identical to those of the conjugate base (dppe)Ni(μ-pdt)57Fe(CO)3 (1′)47, which lacks the hydride bridge. This is exemplified by the δFe–CO NRVS triplets for [1′H]+ (558, 587 and 617 cm−1) and 1′ (557, 588 and 613 cm−1) being virtually coincident (Supplementary Table 1), suggesting that the presence of H− does not significantly perturb the δFe–CO dynamics. In contrast, the δFe–CO region for [1′D]+ collapses to a pair of bands at 580 and 608 cm−1, consistent with significant coupling to the δFe-D bending motion. Thus, although Fe–D modes have intrinsically low NRVS intensity, the Fe–D/Fe–CO coupling allows for the high-intensity δFe–CO region to serve as an indicator of whether or not a Fe–D moiety is present33.
DFT of model complexes
DFT calculations were undertaken to better understand the dynamics of H/D-coupled motions in the model complexes. The DFT-simulated 57Fe PVDOS of [1′H/D]+ Ni/Fe-flippamers were compared with NRVS data over the range 0–1,600 cm−1 (Fig. 2c, see also Supplementary Fig. 18 and Supplementary Tables 1 and 2). As with our recently reported spectra for [1′]0/+ (ref. 52), the observed and calculated band positions and intensities are in good agreement below 700 cm−1. Given that the broad >700 cm−1 region is solely populated by Ni/Fe–H/D bands, DFT also allows for a confident assignment of these NRVS features (Fig. 2b,c) despite their low intensities and the difficulties recognized in the accurate theoretical prediction of M–H vibrational frequencies46.
In line with previous DFT calculations and Raman analyses46, the key distinction between calculated NRVS data for the two [1′H]+ Ni/Fe-flippamers results from splitting of the νFe–H=1,479/1,447 cm−1 and νNi–H=1,022/1,061 cm−1 modes, respectively, as indicated in Fig. 2c. For the Ni-flippamer, the calculated νFe/Ni–H modes are shown correspondingly in Fig. 2f,e and Supplementary Movies 1 and 2. As expounded in the ‘Further Discussion on Model Complex' section of the Supplementary Discussion, a fine yet noticeable interplay of the optimized Ni/Fe–H distances (Supplementary Table 3) gives rise to the inverted character of these ∼30–40 cm−1 flippamer-dependent splittings.
One of the most interesting and useful results from the present calculations on [1′H]+ is the prediction of a 57Fe PVDOS band at 774/767 cm−1 for the Ni/Fe-flippamers, respectively. With only a small flippamer-dependent splitting of 7 cm−1, this mode gives rise to the most intense feature above 700 cm−1 and aligns well with the NRVS band observed at 758 cm−1 (Fig. 2b,c). Inspection of the DFT-calculated nuclear displacements (see Fig. 2d and Supplementary Movie 3 for the mode animation) shows a H nucleus motion normal to the Ni(μ-H)Fe plane, in what is a unique ‘wagging' mode. While the Ni–H–Fe wag is relatively isolated in [1′H]+ (H motion accounts for ∼80% of the kinetic energy), the corresponding Ni-D-Fe motion in [1′D]+ is predicted to be heavily mixed with Fe–CO modes (Supplementary Table 1). The results of such mixing are evident in the intense 440–630 cm−1 Fe–CO region (Fig. 2b,c). Thus, the NRVS signatures of the Ni–H–Fe moiety in the enzyme mimic are the weak wag band observed for [1′H]+ and the change in the amplified features in the Fe–CO region when comparing spectra of [1′H]+ and [1′D]+.
Hydrogenase NRVS results
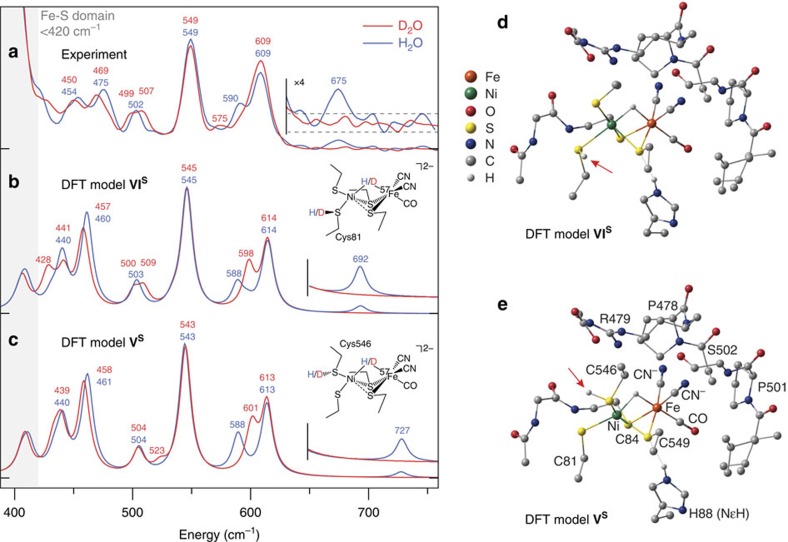
NRVS data for Ni-R in H2/H2O and in D2/D2O are compared in Fig. 3a. While vibrations of the three electron transport Fe–S clusters exclusively populate the <420 cm−1 region39,40,43, this work instead focuses on the Fe–CO/CN region and higher-energy NRVS features to assign spectroscopic markers characteristic of the bridging hydride. Analysis of Ni-R in H2O revealed a sharp band at 549 cm−1 previously assigned to a νFe–CO mode39, with additional features at 454, 475 and 502 cm−1 arising from a mixture of Fe–CO and Fe–CN modes. Compared with our previous results39, the absence of shoulders and additional features around the νFe–CO band indicates a higher level of sample purity. The NRVS band positions are similar to but nevertheless distinct from Raman peaks for the Ni-L Ni(I)Fe(II) state of [NiFe]-hydrogenase, for which νFe–CO was observed at 559 cm−1 (ref. 53). Samples of Ni-R in H2O exhibit bands at 590 and 609 cm−1 that collapse to a single peak at 609 cm−1 when D2O is instead used. Qualitatively, one can attribute the differences in this Fe–CO/CN region to a different coupling to Fe–H and Fe–D motion in the respective samples, as discussed above for [1′H/D]+. Other details about the active site, such as whether cysteine ligands are unprotonated or protonated, cannot be addressed on the basis of the NRVS data alone.
Figure 3. Ni–H–Fe-hydride wag exposed in the reduced state Ni-R of [NiFe]-hydrogenase.
(a–c) High-frequency NRVS for [NiFe]-hydrogenase reduced in H2O (blue trace) and D2O (red trace; a) and the corresponding 57Fe PVDOS simulations given for the representative DFT models VIS (b) and VS (c). The higher regions of spectra containing the Ni–H–Fe wag band (in H2O samples) are repeated with their intensities × 4 amplified. The low-energy region of the Ni-R spectrum in H2O reveals a triplet of bands (454, 475 and 502 cm−1) that correspond to those located at 440, 461 and 504 cm−1 in the calculated spectrum of the model VS. Further, two intense bands seen at 549 and 609 cm−1 in Ni-R map on calculated bands at 543 and 613 cm−1, with an additional weak band observed at 590 cm−1 that can be correlated with the calculated band appearing at 588 cm−1. (d,e) Representative DFT-optimized models VIS (d) and VS (e) for the Ni-R active site. Arrows indicate the position of CysSH. Non-substrate H atoms have been omitted for clarity (excluding HNɛ of His88).
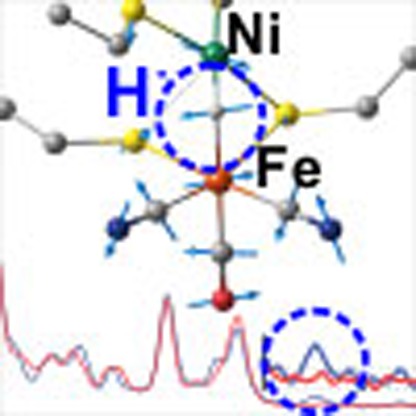
NRVS analysis of [NiFe]-hydrogenase in H2O also revealed a weak but well-resolved band at 675 cm−1 not observed for other samples. This band is presumably related to the Ni–H–Fe wag exposed for [1′H]+ at 758 cm−1 (Fig. 2b,d), making this the first assignment of a Fe–H-related mode in any enzyme by NRVS and the first direct spectroscopic evidence for a Ni(μ-H)Fe core in Ni-R. Deuteration of Ni-R is expected to red-shift this mode into the 420–620 cm−1 Fe–CO/CN region, in agreement with the changes observed and calculated for [1′D]+. Analogous to the model complex, the Ni-D-Fe wag is strongly mixed with Fe–CO/CN modes, which, in the case of Ni-R, makes its unique assignment very difficult.
Hydrogenase DFT results
To interpret NRVS measurements in terms of suitable structural candidates for Ni-R, we performed DFT calculations on a series of active site models featuring different binding modes of the H2 substrate or its heterolysis products (see Supplementary Fig. 19). Limiting our models to the [NiFe] site is appropriate in that Fe–S clusters do not feature NRVS bands in the >420 cm−1 region of interest39,40. Two main structures were considered: one in which substrate is present in the form of a dihydrogen ligand ((η2-H2)NiFe, I or NiFe(η2-H2), II) and another where a bridging hydride is present (Ni(μ-H)Fe, III or HNi(μ-H)Fe, IV). In addition, variants of III, in which a terminal Cys ligand is protonated ((Cys546)SHNi(μ-H)Fe, V and (Cys81)SHNi(μ-H)Fe, VI), were also studied. Taking into account the Ni(II)Fe(II) Ni-R active site16,18, and assuming Fe(II) remains low-spin, each model may exist in electronic singlet (S=0) or triplet (S=1) Ni(II) states, both of which were evaluated computationally. The DFT-calculated 57Fe PVDOS for selected models were compared with the NRVS data for Ni-R over the range 400–750 cm−1 (Fig. 3a–c, see ‘Further Discussion of Enzyme Cluster Models' of the Supplementary Discussion and Supplementary Figs 20–30). DFT-calculated NRVS for models VS and VIS (Fig. 3d,e, superscript S denotes singlet Ni(II)) match experimental data remarkably well, with the number and positions of absorption bands being in accordance. Relative intensities of the calculated peaks are also in good agreement with our measurements.
According to the normal mode analysis of model VS (Supplementary Figs 28 and 29; animated representations of vibrational modes for models VS and VIS are provided in Supplementary Movies 4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34), vibrations in the 440–504 cm−1 region predominantly involve Fe–CN bending and stretching, while higher-energy bands (543–613 cm−1) are derived from Fe–CO vibrations. The feature calculated at 543 cm−1 has significant νFe–CO character, while that at 613 cm−1 is assigned to a δFe–CO mode. Likewise, the 588 cm−1 band can be assigned to an H–Fe–CO bend in which H, Fe and C remain nearly collinear. The above normal modes are highly mixed, which make the assignment of individual fragments complicated.
Both bridging hydride models VS and VIS are predicted to exhibit a weak Ni–H–Fe out-of-plane wagging band (at 727 and 692 cm−1, respectively, see Fig. 3; Supplementary Movies 11 and 28 for mode animations) whose intensity is comparable to that of the 675 cm−1 feature observed for Ni-R. When compared with the Fe–CO/CN region, both theory and experiment predict lower NRVS intensity of the wag in Ni-R than that observed and calculated for [1′H]+ (at 758 and 774/767 cm−1, respectively, see Fig. 2). Moreover, simulations for VS and VIS accurately reproduce the disappearance of the 675 cm−1 band in data for Ni-R in D2O. The difference between the experimental and calculated frequencies of the wagging mode are likely due to limitations in our model, which does not take into account direct contacts between the protonated cysteines and surrounding residues, as well as anharmonicity effects. Moreover, one would expect an intrinsic error of the chosen functional/basis set combination. While hydride bands are extremely sensitive to (electronic) structure, we note that the observed error is still well within normal limits54,55,56,57,58 expected for this methodology. However, since the full spectral information is considered in the interpretation, the present conclusions can be made with confidence. Finally, calculated H/D isotope shifts for the two representative models (Supplementary Tables 4 and 5) in the low-energy region are also fully consistent with the observed data. The overall analysis here identifies the 675 cm−1 feature in the Ni-R NRVS spectrum as the Ni–H–Fe wag mode.
Discussion
Our NRVS measurements on synthetic bridging hydrides and Ni-R, combined with DFT calculations, provide new constraints on the structure of this key catalytic state of [NiFe]-hydrogenase. Spectra of [1′H/D]+ feature characteristic Fe–H/D stretches whose energies (1,532/1,468 cm−1 for the Ni/Fe-flippamer, respectively) are comparable to those for bridging hydrides in other structures, including another recently reported Ni(μ-thiolate)2(μ-H)Fe species for which infrared spectroscopy revealed a νFe–H band at 1,687 cm−1 (ref. 28). Symmetrical μ2-H− bridges, such as those in Fe4H4+ clusters, give rise to symmetric stretches at around 1,400 cm−1 (ref. 59), while purely terminal hydrides have νFe–H∼1,700–2,300 cm−1 (refs 33, 60). The sensitivity of hydride vibrations to structural perturbations underscores their enormous diagnostic value in understanding catalyst structure and function.
Unfortunately, even the strongest of these relatively pure stretches, νFe-D, is predicted to have much lower NRVS intensity than the νFe–CO modes. Thus, while [1′H/D]+ allowed for direct observation of νFe–H/D and νNi–H/D modes, the resolution of similar bands for [NiFe]-hydrogenase is beyond our current capabilities.
Of special significance is the DFT prediction of a Ni–H–Fe wag (Fig. 2d), this vibration being assigned to an observed NRVS band at 758 cm−1 for [1′H]+, a mode likely obscured by solvent bands in Raman spectra46. A key advantage of NRVS is thus demonstrated in that its sole detection of modes coupled to the Mössbauer-active 57Fe nucleus makes it unaffected by solvent or matrix modes. The NRVS intensity of the Ni–H–Fe wag is at least four times greater than those of the Ni–H/Fe–H stretches. The Ni–H–Fe wag is a valuable diagnostic probe of the Ni-R structure, with NRVS data for Ni-R in H2O featuring a weak but reproducible band at 675 cm−1 that is absent when a D2O medium was used (Fig. 3). Given that 57Fe NRVS-active modes necessarily involve motion of this metal centre, observation of an H/D isotopically sensitive band at 675 cm−1 is strong evidence for the presence of a Fe–H moiety in Ni-R.
A second observable indicating the presence of a bridging H−/D− in both Ni-R and its mimics [1′H/D]+ stems from the coupling of Fe–CO and Fe–CN stretches and bends with the Ni-D-Fe wag. While coupling to the Fe–CO/CN modes makes resolution of the Ni-D-Fe wag impossible, it also results in marked changes in band position and intensity in the 440–630 cm−1 region on H/D substitution (Figs 2 and 3). In line with the marked isotope effects described above for [1′H/D]+, NRVS spectra of Ni-R display similar shifts in position and intensity around 450–480 cm−1, splitting of a band at 502 cm−1, and disappearance of a shoulder peak at 590 cm−1. This coupling provides an indirect but powerful method for characterizing theFe–H/D-binding geometry. The NRVS experiments were complemented by DFT simulations, which further pointed to the presence of an active-site bridging hydride. Spectra predicted for the singlet (S=0) models (Cys81)SHNi(μ-H)Fe (VIS) and (Cys546)SHNi(μ-H)Fe (VS) reproduce the data exceptionally well, in particular with respect to the Ni–H–Fe wag in each H/D isotopologue. The model VS is also supported by the recent high-resolution crystallographic analysis of Ni-R1 (ref. 27). As detailed in the ‘Further Discussion of Enzyme Cluster Models' section of the Supplementary Discussion, the NRVS data are best reproduced assuming a low-spin Ni(II). DFT also indicated asymmetric binding of H− to the Ni(II)Fe(II) core, with the ligand more strongly bound to Ni than to Fe (see ‘Electronic Structure of Model V' of the Supplementary Discussion).
Taken together, NRVS analysis of Ni-R, in combination with NRVS and DFT data for [1′H/D]+, indicates that models VIS and VS provide a consistent and detailed picture of Ni-R. This cohesive study thus represents the first evidence from vibrational spectroscopy for the presence of a bridging H− in the active site of Ni-R, a state central to the function of [NiFe]-hydrogenase. The combined experimental and theoretical approach described here has applicability far beyond the Ni-R and [NiFe]-hydrogenase. Ideal for the detailed study of Fe–H fragments, we envisage that such methods will also be of importance for unravelling the mechanisms of [FeFe]-hydrogenase and nitrogenase61, as well as for the development of synthetic catalysts inspired by these metalloenzymes.
Methods
General
Protocols employed for chemical and biochemical synthesis, as well as NRVS measurements and DFT calculations, are outlined here. Full procedures, including associated references, are given in the Supplementary Methods.
Model complex preparation
Metallic 57Fe was converted to the complex (dppe)Ni(μ-pdt)57Fe(CO)3 (1′) via the intermediates 57Fe2I4(2-propanol)4, (dppe)Ni(μ-pdt)57FeI2 and [(dppe)Ni(μ-pdt)(μ-I)57Fe(CO)3]BF4 ([1′I]BF4) (ref. 52). Species 1′ underwent facile exchange with 13CO (1 atm), allowing access to isotopologue (dppe)Ni(μ-pdt)57Fe(13CO)3 (1″). The two Ni(I)Fe(I) derivatives 1′ and 1″were then subjected to protonation and deuteronation (effected with excess HBF4 and HBF4/CD3OD, respectively), affording the Ni(II)Fe(II) salts [1′H]BF4, [1′D]BF4, [1′H]BF4 and [1″D]BF4 as crystalline solids. The isotopic purity of the hydrides and deuterides was confirmed using multinuclear (1H, 2H, 13C and 31P) NMR spectroscopy, infrared spectroscopy and ESI mass spectrometry. NRVS analysis was conducted on a solid sample of each of the four model complexes (vide infra).
D. vulgaris Miyazaki F [NiFe]-hydrogenase preparation
[NiFe]-hydrogenase expressed in D. vulgaris was isolated and purified as described earlier62. The as-isolated protein was transferred from 25 mM Tris-HCl (pH=7.4) buffer to 100 mM MES (pH=5.0). The solution was placed in a tube, which was sealed, degassed and then purged with H2 (1.2 bar) for 8 h. In the case of the sample in D2O, the buffer was replaced by 100 mM MES (pD=5.0) in D2O and the mixture placed under D2 (1.3 bar) for 8 h. Samples were transferred to an anaerobic chamber and loaded into NRVS cells. FT-IR spectra were recorded on a Bruker IFS66v/S FT-IR spectrometer with a 2 cm−1 spectral resolution at 293 K (Supplementary Fig. 31).
NRVS measurements and data analysis
The NRVS data were collected according to a published procedure39 at SPring-8 BL09XU (with flux ∼1.4 × 109 photons s−1) and BL19LXU (∼6 × 109 photons s−1) using 14.4 keV radiation at 0.8 meV resolution. The spectral maximum counts/second (cts s−1) at BL19 is ∼2.6–3 times of that at BL09. To compare the data from different beamlines, we rescale the BL19 counting time on the basis of its max cts s−1 versus the max cts s−1 at BL09 and create BL09 equivalent seconds, for example, the 10 (s) per point (s/pt) at BL19 is corresponding to 26 or 30 equivalent s/pt at BL09. Delayed nuclear and Fe K fluorescence (from internal conversion) were recorded with a 2 × 2 APD (avalanche photodiode) array in either beamline, and raw NRVS data were converted to single-phonon 57Fe PVDOS using the PHOENIX software39. Sample temperatures were maintained at 30–50 K during analysis.
The average cts s−1 is 0.6–0.8 at the Fe–CO peak (609 cm−1) and 0.10–0.12 at X–Fe–H peak (675 cm−1), while the measured dark background cts are ∼0.03 cts s−1. To improve weak features, we use 1–3 s/pt from −240 to 400 cm−1, 5–10 s/pt for the Fe–CN and Fe–CO regions, and 10–30 s/pt for the candidate X–Fe–H bending region (at 620–770 cm−1).
Model complex calculations
Initial coordinates for the DFT calculations on [1′H/D]+ were extracted from the X-ray structure of [1H]BF4·3THF (ref. 44). The methodology applied was mostly equivalent to our earlier set-up on [1′]0/+ (ref. 52). Structural optimizations and subsequent normal mode analyses were performed using GAUSSIAN 09 on the basis of the densities exported from single point calculations using JAGUAR 7.9. The BP86 functional and the LACV3P** basis set were employed. The environment was considered using a self-consistent reaction field model. 57Fe PVDOS spectra were generated using Q-SPECTOR, successfully applied earlier33. Simulated spectra were broadened by convolution with a full-width at half-maximum=12 cm−1 Lorentzian.
Enzyme cluster model calculations
DFT calculations on active-site cluster models were performed using ORCA 3.0. The initial geometry was prepared from the crystal structure of reduced [NiFe]-hydrogenase from Desulfovibrio vulgaris Miyazaki F (PDB 1H2R)63. Constraint geometry optimizations and vibrational frequency calculations at the scalar relativistic level (ZORA) employed B3LYP(-D3) hybrid-GGA with RIJCOSx and the COSMO model (ɛ=4). Segmented all-electron relativistically contracted basis sets with corresponding auxiliary basis sets were used (def2-TZVPP: Ni, Fe, CN−, CO, Sγ, H−, H+, H2.; def2-SV(P): remaining atoms). Vibrational frequencies and normal mode compositions were utilized to simulate NRVS data (Lorentzian fitting, linewidth 12 cm−1).
Additional information
How to cite this article: Ogata, H. et al. Hydride bridge in [NiFe]-hydrogenase observed by nuclear resonance vibrational spectroscopy. Nat. Commun. 6:7890 doi: 10.1038/ncomms8890 (2015).
Supplementary Material
Supplementary Figures 1-31, Supplementary Tables 1-4, Supplementary Note 1, Supplementary Discussion, Supplementary Methods and Supplementary References
Model complex [1'H]+ (H isotopomer), Fe-H stretching mode, v = 1479 cm-1
Model complex [1'H]+ (H isotopomer), Ni-H stretching mode, v = 1022 cm-1
Model complex [1'H]+ (H isotopomer), Ni-H-Fe wagging mode, v = 774 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 411.00 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 439.93 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 460.91 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 504.68 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 543.53 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 588.69 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 613.20 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 726.92 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 408.20 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 432.27 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 438.72 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 458.01 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 504.14 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 522.60 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 544.16 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 600.64 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 613.26 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 409.49 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 439.68 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 460.61 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 502.78 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 545.14 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 588.10 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 614.86 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 692.53 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 406.20 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 427.43 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 440.82 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 457.11 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 500.43 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 508.69 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 545.50 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 597.65 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 613.77 cm-1
Acknowledgments
We thank Patricia Malkowski (MPI-CEC) for her assistance with the 57Fe [NiFe]-hydrogenase sample preparation. This work was supported by the DOE Office of Biological and Environmental Research (S.P.C.), NIH grant GM-65440 (S.P.C.), DOE grant DEFG02-90ER14146 (T.B.R.), BMBF (03SF0355C), EU/Energy Network project SOLAR-H2 (FP7 contract 212508), DFG-funded Cluster of Excellence RESOLV (EXC1069), Max Planck Society (W.L., T.K., M.v.G., F.N. and H.O.) and the DFG-funded ‘Unifying Concepts in Catalysis' (UniCat) initiative (V.P.). Use of SPring-8 is supported by JASRI (proposals 2012A0032-2014B1032) and RIKEN (proposals 20120107, 20130022 and 20140033).
Footnotes
Author contributions H.O. prepared the 57Fe-labelled [NiFe]-hydrogenase samples. H.W., L.B.G., A.D.S., Y.Y., Y.T. and S.P.C. measured and analysed the NRVS spectra. T.B.R. and D.S. prepared and characterized the model complexes. V.P. performed the DFT calculations on the model complexes. T.K., M.v.G., F.N. performed the DFT calculations on [NiFe]-hydrogenase. H.O., T.K., H.W., D.S., V.P., M.v.G., W.L. and S.P.C. wrote the manuscript. S.P.C. designed and coordinated the project.
References
- Bockris J. O. M. The hydrogen economy: its history. Int. J. Hydrogen Energy 38, 2579–2588 (2013). [Google Scholar]
- Jugder B. E., Welch J., Aguey-Zinsou K. F. & Marquis C. P. Fundamentals and electrochemical applications of Ni-Fe uptake hydrogenases. RSC Adv. 3, 8142–8159 (2013). [Google Scholar]
- Kim J. Y. H. & Cha H. J. Recent progress in hydrogenase and its biotechnological application for viable hydrogen technology. Korean J. Chem. Eng. 30, 1–10 (2013). [Google Scholar]
- Mertens R. & Liese A. Biotechnological applications of hydrogenases. Curr. Opin. Biotechnol. 15, 343–348 (2004). [DOI] [PubMed] [Google Scholar]
- Cammack R., Frey M., Robson R. (Eds.) Hydrogen as a Fuel: Learning from Nature Taylor & Francis (2001). [Google Scholar]
- Heinekey D. M. Hydrogenase enzymes: recent structural studies and active site models. J. Organomet. Chem. 694, 2671–2680 (2009). [Google Scholar]
- Tard C. & Pickett C. J. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev. 109, 2245–2274 (2009). [DOI] [PubMed] [Google Scholar]
- Yang J. Y., Bullock M., Rakowski DuBois M. & DuBois D. L. Fast and efficient molecular electrocatalysts for H2 production: Using hydrogenase enzymes as guides. MRS Bull. 36, 39–47 (2011). [Google Scholar]
- Fritsch J., Lenz O. & Friedrich B. Structure, function and biosynthesis of O2-tolerant hydrogenases. Nat. Rev. Microbiol. 11, 106–114 (2013). [DOI] [PubMed] [Google Scholar]
- Simmons T. R. & Artero V. Catalytic hydrogen oxidation: Dawn of a new iron age. Angew. Chem. Int. Ed. 52, 6143–6145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T., Kim K., Nakai H., Hibino T. & Ogo S. Organometallic catalysts for use in a fuel cell. ChemCatChem 5, 1368–1373 (2013). [Google Scholar]
- Evans R. M. et al. Principles of sustained enzymatic hydrogen oxidation in the presence of oxygen—the crucial influence of high potential Fe-S clusters in the electron relay of [NiFe]-hydrogenases. J. Am. Chem. Soc. 135, 2694–2707 (2013). [DOI] [PubMed] [Google Scholar]
- Armstrong F. A. Dynamic electrochemical experiments on hydrogenases. Photosynth. Res. 102, 541–550 (2009). [DOI] [PubMed] [Google Scholar]
- Shafaat H. S., Rüdiger O., Ogata H. & Lubitz W. [NiFe] hydrogenases: a common active site for hydrogen metabolism under diverse conditions. Biochim. Biophys. Acta 1827, 986–1002 (2013). [DOI] [PubMed] [Google Scholar]
- Guiral M. et al. Hyperthermostable and oxygen resistant hydrogenases from a hyperthermophilic bacterium Aquifex aeolicus: physicochemical properties. Int. J. Hydrogen Energy 31, 1424–1431 (2006). [Google Scholar]
- Lubitz W., Ogata H., Rüdiger O. & Reijerse E. Hydrogenases. Chem. Rev. 114, 4081–4148 (2014). [DOI] [PubMed] [Google Scholar]
- Ogata H., Lubitz W. & Higuchi Y. [NiFe] hydrogenases: structural and spectroscopic studies of the reaction mechanism. Dalton Trans. 7577–7587 (2009). [DOI] [PubMed] [Google Scholar]
- Pandelia M.-E., Ogata H. & Lubitz W. Intermediates in the catalytic cycle of [NiFe] hydrogenase: functional spectroscopy of the active site. ChemPhysChem 11, 1127–1140 (2010). [DOI] [PubMed] [Google Scholar]
- Dole F. et al. Nature and electronic structure of the Ni-X dinuclear center of Desulfovibrio gigas hydrogenase. Implications for the enzymatic mechanism. Biochemistry 36, 7847–7854 (1997). [DOI] [PubMed] [Google Scholar]
- Bruschi M. et al. A theoretical study of spin states in Ni-S4 complexes and models of the [NiFe] hydrogenase active site. J. Biol. Inorg. Chem. 9, 873–884 (2004). [DOI] [PubMed] [Google Scholar]
- Lill S. O. N. & Siegbahn P. E. M. An autocatalytic mechanism for NiFe-hydrogenase: reduction to Ni(I) followed by oxidative addition. Biochemistry 48, 1056–1066 (2009). [DOI] [PubMed] [Google Scholar]
- Zampella G., Bruschi M., Fantucci P. & De Gioia L. DFT investigation of H2 activation by [M(NHPnPr3)(‘S3')] (M=Ni, Pd). Insight into key factors relevant to the design of hydrogenase functional models. J. Am. Chem. Soc. 127, 13180–13189 (2005). [DOI] [PubMed] [Google Scholar]
- Amara P., Volbeda A., Fontecilla-Camps J. C. & Field M. J. A hybrid density functional theory/molecular mechanics study of nickel-iron hydrogenase: investigation of the active site redox states. J. Am. Chem. Soc. 121, 4468–4477 (1999). [Google Scholar]
- Stein M. & Lubitz W. Relativistic DFT calculation of the reaction cycle intermediates of [NiFe] hydrogenase: a contribution to understanding the enzymatic mechanism. J. Inorg. Biochem. 98, 862–877 (2004). [DOI] [PubMed] [Google Scholar]
- Siegbahn P. E. M., Blomberg M. R. A., Pavlov M. W. N. & Crabtree R. H. The mechanism of the Ni-Fe hydrogenases: a quantum chemical perspective. J. Biol. Inorg. Chem. 6, 460–466 (2001). [DOI] [PubMed] [Google Scholar]
- Niu S., Thomson L. M. & Hall M. B. Theoretical characterization of the reaction intermediates in a model of the nickel-iron hydrogenase of Desulfovibrio gigas. J. Am. Chem. Soc. 121, 4000–4007 (1999). [Google Scholar]
- Ogata H., Nishikawa K. & Lubitz W. Hydrogens detected by subatomic resolution protein crystallography in a [NiFe] hydrogenase. Nature 520, 571–574 (2015). [DOI] [PubMed] [Google Scholar]
- Ogo S. et al. A functional [NiFe] hydrogenase mimic that catalyzes electron and hydride transfer from H2. Science 339, 682–684 (2013). [DOI] [PubMed] [Google Scholar]
- Fan H. J. & Hall M. B. High-spin Ni(II), a surprisingly good structural model for [NiFe] hydrogenase. J. Am. Chem. Soc. 124, 394–395 (2002). [DOI] [PubMed] [Google Scholar]
- Jayapal P., Robinson D., Sundararajan M., Hillier I. H. & McDouall J. J. W. High level ab initio and DFT calculations of models of the catalytically active Ni-Fe hydrogenases. Phys. Chem. Chem. Phys. 10, 1734–1738 (2008). [DOI] [PubMed] [Google Scholar]
- Bruschi M., Zampella G., Fantucci P. & De Gioia L. DFT investigations of models related to the active site of [NiFe] and [Fe] hydrogenases. Coord. Chem. Rev. 249, 1620–1640 (2005). [Google Scholar]
- Yson R. L., Gilgor J. L., Guberman B. A. & Varganov S. A. Protein induced singlet-triplet quasidegeneracy in the active site of [NiFe]-hydrogenase. Chem. Phys. Lett. 577, 138–141 (2013). [Google Scholar]
- Pelmenschikov V., Guo Y., Wang H., Cramer S. P. & Case D. A. Fe-H/D stretching and bending modes in nuclear resonant vibrational, Raman and infrared spectroscopies: comparisons of density functional theory and experiment. Faraday Discuss. 148, 409–420 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champeney D. C. The scattering of Mössbauer radiation by condensed matter. J. Rep. Prog. Phys. 42, 1017–1054 (1979). [Google Scholar]
- Seto M., Yoda Y., Kikuta S., Zhang X. W. & Ando M. Observation of nuclear resonant scattering accompanied by phonon excitation using synchrotron radiation. Phys. Rev. Lett. 74, 3828–2831 (1995). [DOI] [PubMed] [Google Scholar]
- Alp E. E. et al. Vibrational dynamics studies by nuclear resonant inelastic X-ray scattering. Hyperfine Interact. 144/145, 3–20 (2002). [Google Scholar]
- Sturhahn W. Nuclear resonant spectroscopy. J. Phys. Condens. Matter 16, S497–S530 (2004). [Google Scholar]
- Sturhahn W. et al. Phonon density of states measured by inelastic nuclear resonant scattering. Phys. Rev. Lett. 74, 3832–3835 (1995). [DOI] [PubMed] [Google Scholar]
- Kamali S. et al. Observation of the Fe-CN and Fe-CO vibrations in the active site of [NiFe] hydrogenase by nuclear resonance vibrational spectroscopy. Angew. Chem. Int. Ed. 52, 724–728 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach L. et al. Nuclear resonance vibrational spectroscopy reveals the FeS cluster composition and active site vibrational properties of an O2-tolerant NAD(+)-reducing [NiFe] hydrogenase. Chem. Sci. 6, 1055–1060 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. et al. Dynamics of Rhodobacter capsulatus [2Fe-2S] ferredoxin VI and Aquifex aeolicus ferredoxin 5 via nuclear resonance vibrational spectroscopy (NRVS) and resonance raman spectroscopy. Biochemistry 47, 6612–6627 (2008). [DOI] [PubMed] [Google Scholar]
- Mitra D. et al. Dynamics of the [4Fe-4S] cluster in Pyrococcus furiosus D14C ferredoxin via nuclear resonance vibrational and resonance raman spectroscopies, force field simulations, and density functional theory calculations. Biochemistry 50, 5220–5235 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra D. et al. Characterization of [4Fe-4S] cluster dynamics and structure in nitrogenase Fe Protein at three oxidation levels via combined NRVS, EXAFS and DFT analyses. J. Am. Chem. Soc. 135, 2530–2543 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. E., Whaley C. M., Rauchfuss T. B. & Gray D. L. Nickel-iron dithiolato hydrides relevant to the [NiFe]-hydrogenase active site. J. Am. Chem. Soc. 131, 6942–6943 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton B. E. & Rauchfuss T. B. Hydride-containing models for the active site of the nickel-iron hydrogenases. J. Am. Chem. Soc. 132, 14877–14885 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaat H. S., Weber K., Petrenko T., Neese F. & Lubitz W. Key hydride vibrational modes in [NiFe] hydrogenase model compounds studied by resonance Raman spectroscopy and density functional calculations. Inorg. Chem. 51, 11787–11797 (2012). [DOI] [PubMed] [Google Scholar]
- Bertini L., Greco C., Bruschi M., Fantucci P. & De Gioia L. CO affinity and bonding properties of [FeFe] hydrogenase active site models. A DFT study. Organometallics 29, 2013–2025 (2010). [Google Scholar]
- Liu T. B., Li B., Singleton M. L., Hall M. B. & Darensbourg M. Y. Sulfur oxygenates of biomimetics of the diiron subsite of the [FeFe]-hydrogenase active site: Properties and oxygen damage repair possibilities. J. Am. Chem. Soc. 131, 8296–8307 (2009). [DOI] [PubMed] [Google Scholar]
- Schilter D. et al. Mixed-valence nickel-iron dithiolate models of the [NiFe]-hydrogenase active site. Inorg. Chem. 51, 2338–2348 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice A. K. et al. Redox and structural properties of mixed-valence models for the active site of the [FeFe]-hydrogenase: progress and challenges. Inorg. Chem. 47, 7405–7414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann U. et al. Observation of Fe-H/D modes by nuclear resonant vibrational spectroscopy. J. Am. Chem. Soc. 125, 4016–4017 (2003). [DOI] [PubMed] [Google Scholar]
- Schilter D. et al. Synthesis and vibrational spectroscopy of 57Fe-labeled models of [NiFe] hydrogenase: first direct observation of a nickel-iron interaction. Chem. Commun. 50, 13469–13472 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert E. et al. Resonance Raman spectroscopy as a tool to monitor the active site of hydrogenases. Angew. Chem. Int. Ed. 52, 5162–5165 (2013). [DOI] [PubMed] [Google Scholar]
- Petrenko T. et al. Characterization of a genuine iron(V)-nitrido species by nuclear resonant vibrational spectroscopy coupled to density functional calculations. J. Am. Chem. Soc. 129, 11053–11060 (2007). [DOI] [PubMed] [Google Scholar]
- Peng Q., Pavlik J. W., Scheidt W. R. & Wiest O. Predicting nuclear resonance vibrational spectra of [Fe(OEP)(NO)]. J. Chem. Theory Comput. 8, 214–223 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. D. et al. Elucidation of the Fe(IV)=O intermediate in the catalytic cycle of the halogenase SyrB2. Nature 499, 320–323 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. et al. Nuclear resonance vibrational spectroscopic and computational study of high-valent diiron complexes relevant to enzyme intermediates. Proc. Natl Acad. Sci. USA 110, 6275–6280 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. F. et al. Comprehensive Fe-ligand vibration identification in {FeNO}6 hemes. J. Am. Chem. Soc. 136, 18100–18110 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart I. et al. H2 adsorption on 3d transition metal clusters: a combined infrared spectroscopy and density functional study. J. Phys. Chem. A 112, 1139–1149 (2008). [DOI] [PubMed] [Google Scholar]
- Kubas G. J. Fundamentals of H2 binding and reactivity on transition metals underlying hydrogenase function and H2 production and storage. Chem. Rev. 107, 4152–4205 (2007). [DOI] [PubMed] [Google Scholar]
- Hoffman B. M., Lukoyanov D., Yang Z. Y., Dean D. R. & Seefeldt L. C. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114, 4041–4062 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H. et al. Activation process of [NiFe] hydrogenase elucidated by high-resolution X-ray analyses: conversion of the ready to the unready state. Structure 13, 1–8 (2005). [DOI] [PubMed] [Google Scholar]
- Higuchi Y., Ogata H., Miki K., Yasuoka N. & Yagi T. Removal of the bridging ligand atom at the Ni-Fe active site of [NiFe] hydrogenase upon reduction with H2, as revealed by X-ray structure analysis at 1.4 Å resolution. Structure 7, 549–556 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-31, Supplementary Tables 1-4, Supplementary Note 1, Supplementary Discussion, Supplementary Methods and Supplementary References
Model complex [1'H]+ (H isotopomer), Fe-H stretching mode, v = 1479 cm-1
Model complex [1'H]+ (H isotopomer), Ni-H stretching mode, v = 1022 cm-1
Model complex [1'H]+ (H isotopomer), Ni-H-Fe wagging mode, v = 774 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 411.00 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 439.93 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 460.91 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 504.68 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 543.53 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 588.69 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 613.20 cm-1
Enzyme cluster model V (H isotopomer), Singlet state, v = 726.92 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 408.20 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 432.27 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 438.72 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 458.01 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 504.14 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 522.60 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 544.16 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 600.64 cm-1
Enzyme cluster model V (D isotopomer), Singlet state, v = 613.26 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 409.49 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 439.68 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 460.61 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 502.78 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 545.14 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 588.10 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 614.86 cm-1
Enzyme cluster model VI (H isotopomer), Singlet state, v = 692.53 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 406.20 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 427.43 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 440.82 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 457.11 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 500.43 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 508.69 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 545.50 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 597.65 cm-1
Enzyme cluster model VI (D isotopomer), Singlet state, v = 613.77 cm-1