Abstract
Depression and illness-specific distress are more common among adults with Type 1 diabetes (T1DM) than the general population and have been associated with poorer control of blood glucose and increased risk for serious diabetes-related complications. Treatment nonadherence has also been associated with depressive symptoms and diabetes-related distress, and has repeatedly been suggested as an important modifiable behavioral pathway linking depression and diabetes outcomes. The present study reports on the feasibility and acceptability of a pilot intervention using group-based cognitive-behavioral therapy to improve treatment adherence among adults with T1DM and elevated levels of diabetes-related distress or depressive symptoms. We describe the components of the intervention and utilize qualitative data along with descriptive outcome data. Our findings suggest that participation in the group was acceptable and associated with reductions in depressive symptoms and diabetes-specific distress. Challenges to feasibility and future directions are discussed.
Keywords: Type 1 diabetes, depression, diabetes-related distress, cognitive behavioral therapy, peer support
Type 1 diabetes (T1DM) is an autoimmune disease marked by an inability to produce insulin resulting in chronic hyperglycemia; it is usually diagnosed in childhood or early adolescence, and accounts for approximately 5% of all diabetes cases (Centers for Disease Control [CDC], 2011). Although T1DM is a controllable illness, it requires a lifetime of adhering to a demanding and complex daily medical and behavioral self-management regimen (Ingersoll & Cohen, 2008; Snoek, van der Ven, & Lubach, 1999). The American Diabetes Association (ADA) recommends patients seek to maintain a glycosylated hemoglobin (HbA1c), an indicator of glycemic control over time, below 7%. Uncontrolled T1DM can lead to serious medical complications including nerve damage (neuropathy), kidney disease (nephropathy), vision loss (retinopathy), vascular damage and increased risk for heart attack and stroke, amputation, increased morbidity and mortality, and reduced quality of life (ADA, 2012; CDC, 2011; Watson & Logan, 1998). Despite these risks, about half of patients with diabetes are able to meet goals for glycemic control and less than 20% are able to achieve optimal control over blood glucose and other cardiovascular risk factors (Stark, Fradkin, Saydah, Rust, & Cowie, 2013).
Difficulties with diabetes self-management and risk for diabetes complications have been consistently linked to depression and emotional distress. Adults with diabetes are twice as likely to suffer from depression as those without (Anderson, Freedland, Clouse, & Lustman, 2001). A systematic review found that adults with T1DM had four times the rates of depression as control groups (12% vs. 3%; Barnard, Skinner, & Peveler, 2006). Additionally, depression is associated with poorer diabetes self-management, hyperglycemia, diabetes-related complications, and increased mortality (de Groot, Anderson, Freedland, Clouse, & Lustman, 2001; Gonzalez, Peyrot, et al., 2008; Rustad, Musselman, & Nemeroff, 2011; Van Tilburg et al., 2001; Zhang et al., 2005). Nonpsychiatric emotional distress, indexed by measures of depressive symptoms and diabetes-related distress, is consistently associated with poorer diabetes self-management, suboptimal glycemic control, and risk for negative health outcomes (Gonzalez, Delahanty, Safren, Meigs, & Grant, 2008; Lloyd, Pambianco, & Orchard, 2010). Importantly, these subclinical presentations of emotional distress are two to three times as common as psychiatric mood disorders, such as major depressive disorder (Fisher et al., 2008).
In light of the impact of depressive symptoms and emotional distress on both self-management and diabetes outcomes, treatment adherence has been suggested as a possible pathway between negative mood states, glycemic control and diabetes complications (Alam, Sturt, Lall, & Winkley, 2009; Fisher et al., 2008; Gonzalez et al., 2007; Gonzalez et al., 2008; McKellar, Humphrys, & Piette, 2004). Reviews of behavioral health interventions in diabetes management suggest that interventions that address diabetes self-management concurrently with depression may have a greater impact on glycemic outcomes than those that focus on depression (Markowitz, Gonzalez, Wilkinson, & Safren, 2011; Plack, Herpertz, & Petrack, 2010). Thus, addressing the personal and psychological burden of living with T1DM may be an important part of overall diabetes management (Esbitt, Tanenbaum, & Gonzalez, 2013).
Despite the linkages between emotional distress and problems with diabetes self-management, depression and diabetes control are often targeted individually; few intervention approaches have been evaluated addressing both problems in managing T1DM and concurrent diabetes-related distress or depression. Available findings for adult T1DM-specific interventions geared towards reducing psychological distress and increasing glycemic control have been few and mixed. Van der Ven's (2003) systematic review of diabetes group interventions offered support for group-based interventions regarding improvements in psychosocial functioning and glycemic control, but methodological issues made it difficult to generalize study findings. In a systematic review and meta-analysis of randomized controlled trials assessing the effectiveness of psychological therapies for improving glycemic control among individuals with T1DM, one study specifically targeted both distress and diabetes in adults; however, no significant reductions in psychological distress or HbA1c were found (Winkley, Landau, Eisler, & Ismail, 2006). Reductions in distress and HbA1c were found among children, and Winkley and colleagues (2006) suggested that child and adolescent interventions might incorporate a higher level of support than most adult interventions.
CBT for Depression and Adherence in T1DM
CBT for Depression and Adherence in T1DM (CBT-AD) is an approach rooted in traditional CBT for the treatment of depression combined with techniques adapted for people with chronic illness (Safren, Gonzalez, & Soroudi, 2008a, 2008b; Soroudi et al., 2008). The theoretical basis for CBT-AD is grounded in the idea that there is often an iterative relationship between difficulties managing chronic illness and depression: by improving self-management, patients engage in targeted behavioral activation and gain increased feelings of mastery and self-efficacy. To this end, the cognitive and behavioral strategies used in CBT treatment of depression have applicability for adherence training and coping with chronic illness (Gonzalez et al., 2010; Safren et al., 2009; Safren et al., 2004; Safren, Otto, & Worth, 1999). CBT for Depression and Adherence in Individuals with Chronic Illness is available in therapist guide and patient workbook formats, making it an easily accessible tool for clinicians wishing to address problems in T1DM management and emotional distress using evidence-based techniques within their own patient population (Safren et al., 2008a, 2008b).
CBT-AD has had significant effects on medication adherence and depression outcomes, as well as indicators of illness control, relative to wait-list control or enhanced usual care in two randomized trials of depressed adults with HIV/AIDS (Safren et al., 2009; Safren et al., 2012). Gonzalez and colleagues reported on the adaptation of CBT-AD for depressed individuals with T2DM in an open phase pilot and feasibility study (Gonzalez et al., 2010). Subsequent results from a randomized trial demonstrated significant benefits for CBT-AD treatment in terms of improved adherence, reduced depression, and lower HbA1c among treated adults with Type 2 diabetes, as compared to controls (Safren et al., 2014). Markowitz and colleagues adapted CBT-AD for use with adults struggling with both depression and T1DM. Participants in this pilot study received 10 to 12 individually based sessions of CBT-AD. Clinically meaningful decreases in depression as well as improvements in treatment adherence, glucose monitoring and HbA1c were found (Markowitz, Carper, Gonzalez, Delahanty, & Safren, 2012). CBT-AD, then, is a promising approach to addressing comorbid problems in depression or psychological distress and diabetes self-management among adults with diabetes. However, individually delivered interventions do not also address the isolation adults with T1DM, who can face more serious repercussions of poor diabetes outcomes while comprising a small portion of adults with diabetes, may experience socially and within the health care system; this isolation can contribute to diabetes-related distress and depressive symptoms (Savin, 2012). Therefore, a group format may be especially appropriate to address the support needs of adults with T1DM (Due-Christensen, Zoffmann, Hommel, & Lau, 2012; Savin, 2012; Weinger, 2003). The adaptation of evidence-based interventions to group settings is also important in light of the need for cost-effective treatment in an era of managed care and health care reform.
The aim of this study was to develop and pilot a group-based CBT intervention for CBT-AD in T1DM that addressed self-management, depression, and diabetes-related distress in an integrative manner. We report on the development of the intervention as well as its feasibility and acceptability. Descriptive outcome data from two pilot groups and qualitative feedback are presented. We also collected qualitative data on feasibility and acceptability via a structured exit interview of participants post treatment.
Methods
Participants
Adults between the ages of 18–70 diagnosed with T1DM, who were able to read and write English, were eligible to participate in the initial baseline assessment. A total of 35 participants completed the baseline assessment (described in greater detail; see Tanenbaum & Gonzalez, 2012), of which 23 qualified for the intervention. Ten failed to meet the inclusion criteria for depression or diabetes-related distress and 2 had missing HbA1c values. Participant, site, and provider schedules limited the times available to hold the group, and not all interested and eligible individuals were able to participate as a result. Eleven of the 23 eligible participants were available to take part in the intervention. Nine attended one or more sessions with 8 completing both baseline and follow-up assessment. All participants provided informed consent, and all study procedures were approved by the Institutional Review Board at the Albert Einstein College of Medicine, Yeshiva University.
Exclusion criteria included serious psychological symptoms or other factors that would likely interfere with participation, suicidality, or inability to consent or participate in English. Multiple recruitment strategies were used, including large mailings to hospital-affiliated diabetes clinic patients, outreach to endocrinology practices, and print advertising in local clinics. Participants were eligible to participate in the pilot intervention group if they scored 3 or greater on the Diabetes Distress Scale (DDS), indicating a high level of distress (Fisher, Hessler, Polonsky, & Mullan, 2012); 16 or higher on the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977); or 4 or higher on the Montgomery Asberg Depression Rating Scale (MADRS; Montgomery & Asberg, 1979; Zimmerman, Posternak, & Chelminski, 2004), indicating at least mild depression.
Measures
Clinician-Administered Assessment of Depression
Depressive symptoms over the past week were assessed via the MADRS, a 10-item semistructured clinical interview measuring severity of cardinal depression symptoms over the past week (Montgomery & Asberg, 1979). Each symptom was explored with general questions and specific probes to assess particular aspects of symptomology. The interviewer also rated the individual's apparent sadness. The presence of each symptom was rated on a scale from 0 to 6. These scores were summed to obtain an overall score. Scores below 6 indicate an absence of depression; 7 to 19 indicate mild depression; 20 to 34 moderate depression; and 35 to 60 severe depression. The MADRS was chosen as a better fit than many other assessments of depression for working with a medical population as it does not emphasize the somatic symptoms of depression. Additionally, the MADRS has acceptable reliability, validity, and sensitivity to change over time (Montgomery & Asberg, 1979). In the current study, Cronbach's alpha was .81.
Self-Report Measures
Depression
Participants completed the Patient Health Questionnaire-9 (PHQ-9), a self-report measure that assesses the nine diagnostic criteria of depression, based on the DSM-IV TR (Spitzer, Kroenke, & Williams, 1999). It comprises 9 items, rated along a Likert-type scale, ranging from 0 (not at all) to 3 (nearly every day), with scores of 10 or above indicating at least mild depression. The PHQ-9 has good reliability and validity in medical and nonmedical settings (Kroenke, Spitzer, & Williams, 2001; Martin, Rief, Klaiberg, & Braehler, 2006; Spitzer et al., 1999). In the current study, the Cronbach's alpha coefficient was .84.
Diabetes-related distress
Diabetes-related distress was measured via the DDS, a 17-item measure to facilitate patient-provider communication (Polonsky et al., 1995). The DDS has four subscales: Emotional Burden, Physician-Related Distress, Regimen-Related Distress, and Interpersonal Distress. Items are rated along a Likert-type scale ranging from 1 (not a problem) to 6 (a very serious problem). Items are summed and averaged, to arrive at a mean total scale score and can be analyzed according to discrete levels of severity or along a continuum. As per Fisher, mean scores under 2 indicate “little or no” diabetes distress, mean scores between 2 and 2.9 indicate “moderate” diabetes distress, and mean scores of 3 or higher indicate “high” diabetes distress (Fisher et al., 2012). The DDS has adequate internal reliability and validity across the four subscales and a consistent, generalizable factor structure (Polonsky et al., 2005). In the current study, the Cronbach's alpha coefficient was .90.
Diabetes treatment adherence
Diabetes self-management was assessed by the Self-Care Inventory-Revised (SCI-R), a brief, psychometrically valid 15-item measure of adherence to diabetes self-care activities (e.g., “check blood glucose with monitor” and “eat the correct food portions”) over the past 1 to 2 months (La Greca, 2004; Weinger, Butler, Welch, & La Greca, 2005). Participants rate the frequency with which they engaged in self-care activities on a Likert scale ranging from 1 (never) to 5 (always). Scores range from 0–100, with the total score calculated by averaging the items and then converting the average to a scale (Weinger et al., 2005). Higher scores reflect greater diabetes self-care. The SCI-R has been validated in adults with T1DM and T2DM, and has been found to have high internal consistency and adequate reliability and validity (La Greca; Weinger et al.). In the current study, the Cronbach's alpha coefficient was .88.
Glycemic control
Glycemic control was assessed via glycosylated hemoglobin (HbA1C), a measure of blood glucose control over approximately 3 months, obtained via blood draw by a registered nurse at baseline visit and 3-months post-intervention. To minimize risk of attrition and reduce participant burden, a recent HbA1c test (within 1 month of follow-up assessment) was allowed to substitute for in-clinic test.
Procedures
Baseline assessments lasted approximately 3–4 hours and were conducted by psychology graduate students in a research clinic on the campus of a major medical center in the Albert Einstein College of Medicine, Yeshiva University. After providing informed consent, participants completed an assessment battery consisting of two structured clinical interviews to assess psychiatric history and current depressive symptoms as well as an extensive packet of self-report measures. Eligible participants were later contacted and invited to participate in the CBT-AD groups. Following the intervention, all participants returned for individual follow-up visits approximately 3-months post-intervention, which included the MADRS; a selection of baseline measures; a blood draw to obtain HbA1c; a measurement of height/weight; and a structured qualitative exit interview. The purpose of the exit interview was to obtain participants’ feedback, thoughts and reactions about the intervention overall, as well as their experience participating in the specific modules, and to gather suggestions for areas of improvement. Exit interviews lasted from 1–2 hours and were audio recorded.
Intervention
Two pilot CBT-AD groups were conducted with a total of 9 participants. The first group was cofacilitated by the principal investigator, a licensed clinical psychologist, and one psychology graduate research assistant. The second group was cofacilitated by two psychology graduate research assistants. The principal investigator provided supervision for both groups. All intervention sessions were video-recorded. In order to maximize attendance, facilitators made weekly reminder calls and transportation was reimbursed, if needed.
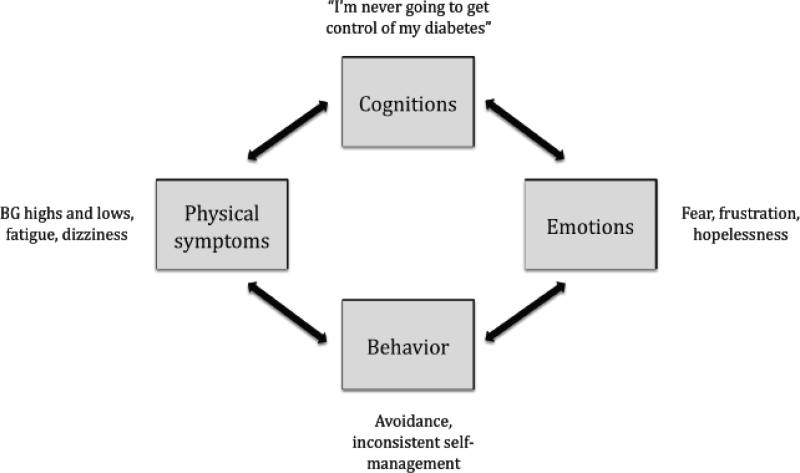
The group intervention consisted of seven modules spread across 10 sessions (Table 1), with content designed to address the intersection of maladaptive cognitions, negative emotions, self-defeating behaviors, and worsening diabetes symptoms theorized to underlie problems in diabetes self-management among adults with co-occurring depression and/or diabetes-related distress (Figure 1). Each session incorporated self-relaxation exercises (i.e., progressive muscle relaxation, diaphragmatic breathing, guided imagery, autogenic training, and meditation), a review of the sessions’ topic and discussion, and homework to be completed over the course of the upcoming week. The behavioral components of the intervention included strategies to improve self-management, motivational interviewing to discuss change, medication regimen, glucose monitoring logs, and activity scheduling. The emotional components emphasized mood-monitoring while attending to the impact of daily activities such as diabetes self-management. The cognitive component included exercises such as using thought records, reviewing problem-solving strategies and cognitive restructuring. Psychoeducational components focused on the CBT model of depression; the relationship between depression, diabetes-related distress, and implications for self-management; and health outcomes. To reinforce learning, the content of prior sessions, including any assigned homework, was reviewed at the start of each session before beginning new module content.
Table 1.
Intervention Modules
| Module | Module Content |
|---|---|
| Psychoeducation about the CBT Model of Depression and Motivational Interviewing | Understand the relationship between thoughts, behaviors, and physical sensations, and how each can contribute to depression and diabetes self-management. |
| Self-management of Diabetes (Life-Steps) | Identify components of diabetes self-care that contribute to glycemic control and managing the risk of complications. |
| Activity Scheduling | Use activity logs to plan ways to increase pleasurable activities and positive mood. |
| Thinking Adaptively (Cognitive Restructuring) | Learn cognitive restructuring techniques, including evaluating thoughts and beliefs that lead to feeling depressed, and examining maladaptive thinking patterns. |
| Problem Solving | Create an action plan from a list of possible solutions and break down bigger tasks into smaller steps. |
| Relaxation Training and Diaphragmatic Breathing | Practice breathing and muscle relaxation exercises to be able to relax in stressful situations. |
| Review, Maintenance, and Relapse Prevention | Reviews treatment strategies learned in prior sessions. Addresses the goal of maintianing change, and internalizing these lessons so clients become ther own therapists. |
Figure 1.
Cognitive Behavior Theory, (CBT), Model, of, Depression and Treatment Adherence in Type 1 Diabetes
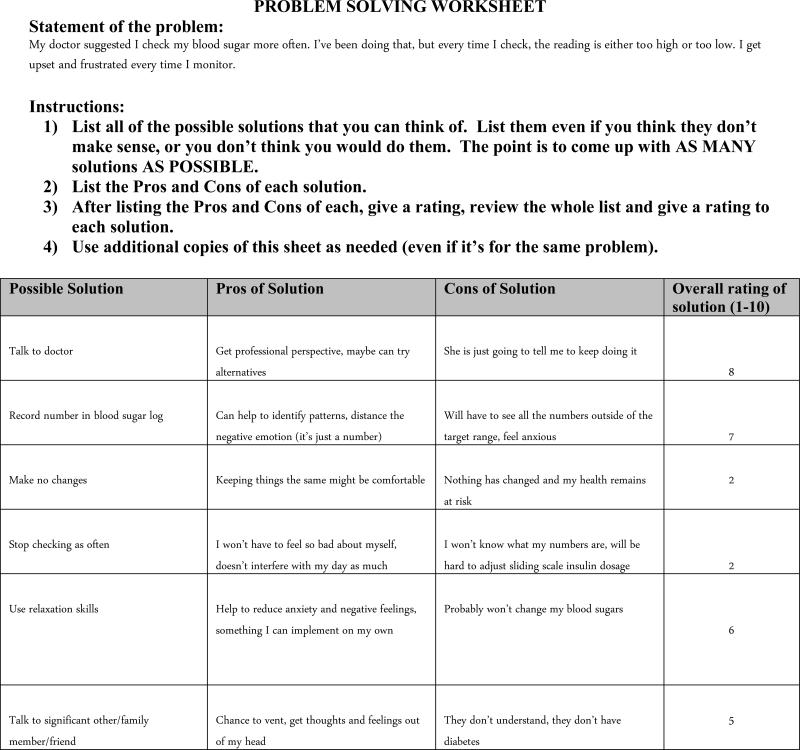
Several adaptations were made to CBT-AD in order to maximize the benefit in a group-based format, increase session flow, and integrate participant feedback. The primary adaptation was the inclusion of peer feedback in every module. For example, in the problem-solving module one participant described difficulty utilizing a problem-solving worksheet given to the group (Figure 2). This became a springboard for facilitators to review the cognitive-behavioral model and the connections among thoughts, feelings, and behavior, and for participants to actively utilize the CBT-AD tools in session. In this example, a participant identified frustration and upset after checking their blood sugar; this was a common experience among participants and group discussion revealed the association between negative thoughts about themselves, such as “I'm not good enough” and “I'm never going to get this monitoring right,” and these negative feelings. During this exercise, cofacilitators emphasized the participants’ capacity to intervene by identifying and challenging these maladaptive thoughts, thereby affecting their negative emotions. Facilitators actively engaged members in this process, soliciting from participants a variety of options available to cope with this challenge, while concurrently validating the difficult emotions evoked by difficulties in diabetes management. Assessing the pros and cons of the solutions helped participants better understand how automatic thoughts and feelings can drive maladaptive reactions to problems in diabetes management. Last, utilizing tools such as the Problem Solving Worksheet with the group provided an in-vivo opportunity to recognize, normalize, and challenge common cognitive distortions, such as catastrophizing and black-and-white thinking. In this way the exercise helped to build the participants’ self-efficacy, as they were able to assist others, as well as themselves, in identifying skills that can be implemented when negative thoughts and feelings come up (i.e., utilizing social support, relaxation techniques).
Figure 2.
Other adaptations included extending sessions from 1 hour to 1.5 hours to ensure that the sessions ran smoothly and that time remained for peer feedback, within the context of modules. Participants were also encouraged to share strategies with one another outside of session. Additionally, participants were asked for feedback throughout the intervention, resulting in several alterations. For example, participants in the second group expressed a desire to decrease the length of the relaxation strategies in order to increase other group activities (e.g., psychoeducation and sharing personal strategies for diabetes management). Overall, the group adaptation of the CBT-AD intervention involved time to share and provide support with one another in addition to covering the material in each module.
Data Analysis
Means and standard deviations of baseline- and post-intervention scores across measures were calculated, as was Cohen's d to reflect the magnitude of the treatment effect using the pooled mean divided by the SD of the paired differences, correcting for dependence among means (Howell, 2007). Due to the small sample size and in keeping with our focus on feasibility and acceptability, tests of statistical significance were not conducted. The a priori goals of this project were to (a) explore how addressing depression and diabetes adherence in an integrated group-based protocol impacts diabetes-related distress, diabetes self-care behaviors, diabetes outcomes, and depressive symptoms; (b) highlight possible mechanisms by which the group intervention may impact diabetes management; (c) understand if other factors not addressed in the intervention may positively or negatively influence diabetes management, and (d) become aware of any issues regarding the group intervention that could help improve the feasibility and efficacy. Exit interviews were recorded, transcribed, and analyzed using the methodology of thematic analysis, a qualitative approach to data analysis used to elucidate patterns across data (Braun & Clarke, 2006). Two graduate student coders (SE and MT) read each transcript independently to highlight repeating ideas that emerged throughout the interviews. The two coders then met to compare their codes, discuss and resolve any differences in coding, and group the codes into major themes.
Results
Feasibility and Attendance
Of the nine individuals who attended one or more sessions, one dropped out after attending three out of four sessions and was lost to follow-up. The remaining eight completed both baseline and follow-up assessments.
Quantitative Results
Reductions in depressive symptoms and diabetes-related distress, as well as improvements in diabetes self-care were found (see Table 2). MADRS scores decreased from moderate at baseline to mild depressive level post-intervention (Cohen's d = 0.91). PHQ-9 scores decreased from a moderate at baseline to mild depressive level post-intervention (Cohen's d = 0.82). DDS scores dropped from a high level of diabetes-related distress at baseline to a moderate level at follow-up (Cohen's d = 0.34). SCI-R scores dropped slightly from baseline to follow-up (Cohen's d = 0.16). A small negative effect was also found between baseline and follow up for HbA1c (Cohen's d = −0.08).
Table 2.
Individual Demographics and Pre- and Post- Intervention Scores
| ID | Age | Gender | Race/Ethnicity | Education | Income | Marital status | Years with T1DM | Number of sessions | HbA1c | MADRS | PHQ-9 | DDS | SCI | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | Pretest | Posttest | |||||||||
| 1 | 44 | F | Black | Some college |
25k>50k | Single | 11 | 9 | 8.40% | 13.60% | 17 | 13 | 9 | 6 | 2.17 | 2.12 | 46.75 | 52.5 |
| 2 | 40 | M | Hispanic/ Asian |
Some high school |
15k>25k | Single | 35 | 6 | 7.80% | 7.90% | 21 | 17 | 12 | 9 | 4.71 | 4.18 | 73.25 | 56.75 |
| 3 | 41 | F | White | Less than high school |
50k>100k | Married | 37 | 9 | 10% | 11% | 23 | 12 | 13 | 6 | 4.65 | 3.89 | 53.25 | 46.75 |
| 4 | 46 | M | Black | Some college |
>10k | Single | 29 | 10 | 8.90% | 6.90% | 44 | 2 | 20 | 0 | 3.94 | 1.53 | 46.75 | 50.00 |
| 5 | 41 | M | Black | Some college |
25k>50k | Single | 20 | 8 | 8.60% | 8.50% | 12 | 6 | 1 | 2 | 2.24 | 3.65 | 73.25 | 56.75 |
| 6 | 36 | F | Hispanic | College | 50k>100k | Single | 10 | 10 | 7.20% | 7.20% | 18 | 15 | 12 | 12 | 3.4 | 2.8 | 60.00 | 45.00 |
| 7 | 33 | M | White | College | >10k | Single | 8 | 10 | 7.60% | 7.60% | 12 | 5 | 3 | 2 | 2.24 | 2.65 | 57.75 | 61.00 |
| 8 | 42 | F | White | Graduate school |
100k>150k | Married | 29 | 10 | 10.00% | 8.00% | 28 | 6 | 18 | 2 | 3.29 | 2.65 | 40.00 | 53.25 |
| Mean | 40.38 | 22.38 | 9 | 8.56% | 8.73% | 21.88 | 9.63 | 11.25 | 4.88 | 3.19 | 2.82 | 50.98 | 49.22 | |||||
| SD | 4.173 | 11.69 | 1.41 | 1.04% | 2.27% | 10.44 | 5.32 | 6.61 | 4.12 | 0.97 | 0.98 | 12.83 | 7.19 | |||||
Qualitative Results
Feedback on the CBT-AD Intervention Overall
Participants initially expected that diabetes-specific education would be the primary benefit of CBT-AD, but reported that the group introduced them to topics that they had not previously discussed in the context of diabetes management, such as counseling, stress management and relaxation techniques.
Control of diabetes, you hear it all the time...it also was cool that you had new things [in the group], the breathing and the therapy and the stress management to add on to that. (Participant 4)
Increased support, motivation to change, personal empowerment and agency to improve self-care, and the value of an integrative approach to diabetes and depression control were key themes that emerged from the analysis of the exit interview texts. Participants shared how the experience of not being alone, feeling supported, and having one's struggles normalized often lead to increased motivation and a sense of empowerment.
“I'm not alone”: Increased social support
The most salient theme was the value of creating a group specifically geared toward adults with T1DM. For many participants, CBT-AD was their first experience interacting with other adults with T1DM. Participants described the benefits of both having a forum to share their own experience and hearing the problems and successes of others. They repeatedly described a therapeutic benefit from simply being with others who had gone through similar experiences. The peer environment allowed participants to learn from each other, to have a positive social experience surrounding their diabetes, and to feel less isolated.
Having something in common with people and knowing that you're not the only one with this condition that is going through the difficulties — that was my therapy. (Participant 5)
Outside of a shared diagnosis of T1DM, participants in the intervention were highly heterogeneous. One female participant mentioned feeling uncomfortable hearing about the sexual complications of male participants, such as erectile dysfunction. However, no participants mentioned any barriers to benefiting from the group based on the racial, ethnic, educational, or social differences. Participants noted benefits from being in groups where the participants had differing levels of diabetes control. For example, several participants described learning strategies to improve their diabetes self-care from hearing about the self-care behaviors of other participants whose diabetes was better controlled. Similarly, several participants who were already performing some of the self-care activities addressed in the group found these positive behaviors reinforced from participating alongside others whose diabetes was more advanced. Ultimately, hearing other adults discuss struggling with their own problems had a powerful normalizing effect.
Motivation to change
Group participants expressed increased motivation to manage their diabetes and commit to their health.
I was in a rut with my own self-care...like “Why bother, it's not going to make any sort of a difference”... Now I feel like I'm being better about those things. (Participant 7)
Motivation to change was described in relation to several aspects of the group. Learning the value of specific self-care activities and increasing awareness of how psychological factors affect T1DM were highlighted as increasing motivation. Many participants struggled to balance the demands of T1DM with their busy, complex lives. Participants with a shorter duration of diabetes described the effects of seeing others further down the road with their diabetes as motivation to prioritize their own physical and mental health. They also reported that frank discussions about self-care activities alongside the emotional and practical barriers to engaging in self-management helped them to gain insight into their personal self-management challenges and to develop personalized approaches to behavioral change.
“I can do something about it”: Empowerment for self-care and self-advocacy
Virtually all participants experienced an increased sense of empowerment, including the ability to advocate for themselves and communicate more effectively with family, significant others, friends, and providers about their needs. One participant had a long history of severe hypoglycemic episodes, which affected his ability to work and to fully engage in family life. This participant reported struggling to identify warning signs and situations that increased his risk for hypoglycemic episodes. Through participation, he developed an increased awareness and a greater ability to problem-solve when confronting a hypoglycemic episode.
[The group] told me how to diagnose myself...what is making me feel bad and what I can do to correct it. So it made me become a doctor of myself. ...so I could manage the condition better. (Participant 4)
The benefits of addressing depression and diabetes together
Participants expressed benefiting from an intervention that addressed both diabetes and depression at the same time. Group participants expressed that through CBT training, implemented by trained providers, they became better able to identify their feelings, the impact depression had on their lives, and the links between their emotional and physical health:
We are all here because we were not taking care of ourselves and we put everything before ourselves. Pour depression on that, and we got a cycle of [a] bunch of us committing passive suicide. (Participant 1)
In the interviews, participants explained that the new perspective on the relationship between their thoughts, feelings, and diabetes management they gained from participating in the group was important for taking ownership of their illness.
“Let me participate again”: The need for continued support
Participants strongly expressed the desire to continue the connections made in the group. Several participants connected their positive experience with the group to a new desire to reach out and help others with T1DM. Many reported exchanging contact information and making efforts to stay in contact with fellow group participants after the intervention ended.
Feedback on Group Facilitators
The facilitators received strong positive feedback across both groups. Participants appreciated the understanding stance of facilitators, and noted their lack of judgment or blame. Facilitators used layman's terms as opposed to medical jargon, which was appreciated by participants. Participants also reported feeling a real sense of caring, often in contrast to other diabetes-related experiences with health care providers; this caring came cross during sessions (e.g., support and validation when disclosing difficult personal content) as well as from the structure of the program itself (e.g., feeling welcome and appreciated due to weekly reminder calls).
Challenges With the Group Format
Many of the participants noted that a 90-minute session did not feel long enough for covering the content, practicing it in session, and having adequate time for each participant to share and contribute. Some participants also described feeling frustrated when certain participants shared more than others, and had to be redirected by facilitators, in light of the limited length of the sessions.
Feedback on CBT-AD Modules
On the whole, participants provided positive feedback about the modules as well as some suggestions for improvement. Many participants noted that learning about specific CBT-techniques, such as problem solving or identifying maladaptive cognitions, helped them gain new perspectives on how to handle their problems (e.g., de-escalating anxiety, reducing anger towards others or reducing negative emotions towards oneself). Some commented on the initial challenges of adopting CBT techniques:
You're so used to [managing diabetes] this way that when someone comes and tells you a better way of doing it...trying a new and different thing and accepting it is the hard thing about it. (Participant 6)
The relaxation exercises received strong positive feedback across participants:
[The relaxation exercises] gave me the feeling of being calm without having to introduce a foreign substance into my body to get the same feeling. (Participant 1)
Participants spoke positively about the concrete, step-by-step techniques offered through the modules. Several participants commented that log-keeping was an important tool, which brought habits into consciousness and facilitated change. Participant 4 tried keeping a glucose log to better understand how his blood sugar fluctuated. He was encouraged to do so with an attitude of curiosity about the relationships between his day-to-day life and his blood sugar readings, a markedly less-self-critical stance than his prior attitude. This participant explained that this change helped him reduce his avoidance of glucose testing and improve his ability to better control his diabetes. Several participants noted ongoing difficulty keeping a food diary, despite awareness of potential benefits on their diet. Others described positive effects of sharing their glucose or food logs with their doctors, as they were able to make helpful adjustments to their treatment regimens. Increased awareness of moods and thoughts through use of logs was noted as helpful for managing emotions during stressful situations.
Despite considerable variability among participants in terms of years managing diabetes and diabetes knowledge, many participants described that the introduction to the CBT model and the integration of it into diabetes self-management and depression was novel.
I refer to [the CBT model] as the circuit breaker section because that's what that really is. It is a way to short circuit those negative thought patterns...” (Participant 1)
The activity scheduling module, while receiving positive feedback overall, was one of the only areas where participants noted that the group format presented a challenge. Since each participant had very different interests and lifestyles, some found the group format too general for activity scheduling. Additionally, multiple participants described difficulty managing diabetes while juggling multiple responsibilities. Among those that made time for activity scheduling, multiple participants found it helpful for reducing stress and improving mood.
Participants reflected that a problem-solving approach (e.g., breaking down problems, investigated pros and cons of potential solutions, and rated outcomes) made problems more manageable and helped them to think more critically about their own problem-solving approaches. Several participants mentioned that just by sharing their problems with the group, they were better able to see them clearly and arrive at solutions. One participant offered that simply hearing the challenges of others and how they think about their issues influenced her approach to her own problems in a new way.
Discussion and Conclusions
This first pilot group adaptation of the more intensive, individually oriented CBT-AD intervention (Safren et al., 2008a, 2008b) was well received by participants and considered highly acceptable. In exit interviews, participants described experiencing increased agency, peer support, and insight regarding their illness-related behavior and the impact of depression on their T1DM. After participating in the CBT-AD group, participants showed a reduction in depression, from moderate levels to mild, as well as a small reduction in diabetes-related distress from severe to moderate. Several challenges to feasibility were noted and are discussed in detail, in the hopes of informing future interventions.
Although our quantitative analysis is limited by our small sample size, these findings reflect clinically meaningful psychological effects of the intervention and large to moderate effect sizes. Despite meaningful reductions in depression and diabetes-related distress, however, the intervention did not improve self-care or glycemic control. This is in contrast to the qualitative data, in which participants provided uniformly positive feedback about the impact of the intervention on their own self-care behaviors, their sense of self-efficacy for managing their diabetes, and their understanding of appropriate diabetes self-management behaviors. Notably, diabetes self-care and glycemic control are difficult to measure, due to the various interrelated components (e.g., an individual may effectively change one component and not another). Consequently, we suggest several possible explanations of these findings.
First, in line with previous applications of CBT-AD in diabetes (Gonzalez et al., 2010; Safren et al., 2014), it may be beneficial to involve other health care professionals (e.g., nurse or diabetes educators) in the delivery of the intervention content in order to impact health outcomes. Also, as HbA1c is a summary measure of the past 3 months of glucose control, the final HbA1c may not have captured the full effect of behavior change. Future work would do well to incorporate multiple follow-up measures of HbA1c, such as immediately post-intervention, 3 months out (as we did), and again at 6 months. This could allow us to better capture the full effect of behavior change, as well as the sustainability of gains made. Another factor may be that baseline HbA1c also ranged broadly among participants, from 10% (well above the ADA recommended target of 7%) to 7.2%. The behavioral changes necessary for patients whose HbA1c is significantly above the ADA recommendation to lower their blood glucose may be qualitatively different than those needed for a commensurate drop for patients whose HbA1c is closer to the target value, resulting in a floor effect.
In the exit interviews, participants reported increased knowledge about self-care, as well as reductions in their previous avoidance-based approaches to self-care. It is plausible that the increased knowledge and awareness surrounding diabetes self-care acquired from the intervention may have affected how participants approached and answered the SCI-R, resulting in a more negative—but realistic—assessment of their self-care behaviors. Additionally, as the SCI-R asks participants to report on how often they engaged in specific self-care behaviors over the past 1 to 2 months, follow-up responses may not fully capture the impact of a 10-week intervention. Further work would do well to expand follow-up to incorporate multiple assessments across time. Finally, we did not collect data on the use of the insulin pump. Insulin pumps have been found to improve glycemic control (Jeitler et al., 2008) and may reduce or eliminate the need for manual injections of insulin and manual blood glucose testing, influencing how participants may have endorsed their self-care on the SCI-R.
Nine participants began the intervention with eight completing the intervention as well as the baseline and follow up assessments. While the consistently high attendance rate and positive qualitative feedback results suggest that group-based CBT-AD for T1DM and depression is acceptable, a number of factors posed challenges to the feasibility of the intervention. Even with strong support from specialist and primary care providers treating adults with T1DM, who reported a need for this type of intervention, recruitment presented the single largest barrier to expansion of the current study. As T1DM accounts for a small minority of all diabetes cases, even major metropolitan hospital networks and specialty diabetes practices, such as the one we recruited from, have a relatively small number of adult patients, limiting the efficacy of recruitment techniques such as in-clinic screenings. Due to the geographic location of the study itself, recruitment across multiple hospital systems and endocrinology practices was not feasible. Scheduling the intervention as a research pilot study, away from centers for clinical care, may have presented challenges above and beyond those facing clinicians implementing CBT-AD with known patients at a clinic or community site. Additionally, the length of the baseline visit (3–4 hours), which also required daytime attendance to collect a blood sample if recent HbA1c was not otherwise available, may have reduced our participant pool. Potential participants who may have been open to a 2-hour after-work support group may not have been able to set aside the block of time required for inclusion. Keeping baseline assessment brief or utilizing telephone or online assessment may increase participation in future interventions.
Finally, the group format of the intervention itself also presented a scheduling challenge absent from individually delivered interventions; while individual sessions could be rescheduled, providing multiple opportunities for participants to fully participate in all modules, in a group-based intervention such as ours all 10 sessions were scheduled beforehand. Identification of a more centrally located partner site or the identification of multiple sites may allow future recruitment efforts to cast a wider net in order to fulfill this unmet need. Assessing and addressing barriers to feasibility key; the barriers to delivering these kinds of interventions may be reflected in the lack of research with this population. The incorporation of technology, such as video conferencing, may also reduce this significant logistical barrier and improve accessibility for a larger number of potential participants. Remote video technology has been gaining in popularity as a tool for delivering individual and group-based medical and mental health interventions to individuals who would otherwise not have access to services (Richardson, Frueh, Grubaugh, Egede, & Elhai, 2009).
It is noteworthy how limited our participants' prior experience had been with other adults with T1DM before attending the group. For many, this was their first experience in a T1DM group. Given that T1DM makes up only 5% of all diabetes cases (CDC, 2011), there is clearly a greater challenge to accessing peer support with this patient population as compared with T2DM. However, in our sample of adults with T1DM, a group intervention appeared to be particularly appropriate to address issues related to isolation and emotional distress (Due-Christensen et al., 2012; Savin, 2012; Weinger, 2003). As participants in the current study noted, they felt more at home in a room with others with the same illness; those with experience attending general diabetes groups for T1 and T2DM strongly preferred being in a homogeneous T1DM group. Given participants' limited prior experience with others with T1DM, our intervention provided the optimal context and shared experience for delivering an intervention geared at improving depressive symptoms and treatment adherence.
The acceptability of a group format, despite the barriers presented by location and scheduling, may be due in part to the nonhierarchical reciprocal relationships created by sharing similar lived experiences (Dennis, 2003). Heisler, Vijan, Makki, and Piette (2010) found that reciprocal peer support among individuals with diabetes resulted in improved HbA1c over time compared to nurse care management in a randomized control trial. For adults living with the unique challenges of T1DM, group interventions may be especially relevant; virtually all participants reported seeking or wishing for more T1DM-specific peer support. These findings are important given the health benefits associated with receiving and giving social support and the efficiency of providing interventions in a group format (Dennis; Joseph, Griffin, Hall, Sullivan, 2001; Schwartz & Sendor, 1999).
The development of a strong therapeutic alliance is pivotal for engagement and progress in psychotherapies such as CBT (Imel & Wampold, 2008; Lambert & Barley, 2001). Gaps in the transition between pediatric and adult care in T1DM have been frequently noted in the literature, suggesting that many adults with T1DM have had at least one rupture in the patient-provider relationships and continuity of care; these ruptures can have meaningful implications for continued engagement in medical care and diabetes outcomes (Garvey et al., 2012; Hilliard et al., 2013). As reflected in the qualitative findings, our participants provided extremely positive feedback regarding facilitators. This is important as both qualitative and quantitative investigations suggest patient-provider factors such as communication and alliance are associated with patient engagement, satisfaction, and outcomes in T1 and T2DM (Ciechanowski, Katon, Russo, & Walker, 2001; Ciechanowski et al., 2004; Heisler, Bouknight, Hayward, Smith, & Kerr, 2002; Matthews, Peden, & Rowles, 2009). Utilizing behavioral mental health providers who are trained to deliver evidence-based interventions from the foundation of a solid therapeutic alliance may have contributed to the attendance and acceptability of our intervention. Future interventions would do well to incorporate assessments of patient-provider alliance and communication, as well as provider attributes to better operationalize the impact of group facilitators on treatment outcomes. Future investigations may also wish to explore the relative contributions of behavioral mental health providers compared to traditional medical providers of diabetes education or diabetes peer mentors to treatment outcomes.
Future interventions may benefit from the addition of a structured maintenance program. Amsberg et al.'s (2009a, 2009b) 8-week group CBT-based intervention for adults with poorly controlled T1DM found significant improvements in HbA1c, self-reported well-being, diabetes-related distress, frequency of blood glucose testing, hypoglycemic events, perceived stress, anxiety, and depression in the intervention group compared with the control group. This may reflect the extended maintenance program, which consisted of two group sessions and two individual sessions at 12 and 24 weeks, in addition to five phone calls from the provider between 10 and 42 weeks, targeted towards behavioral change and problem-solving challenges in maintenance. This allowed participants to continue to collaborate with the facilitators and group when relapses or challenges to self-care arose (Amsberg et al., 2009a, 2009b). Van Bastelaar et al. (2011) found an online CBT-based intervention effective for reducing diabetes-related distress and depression, suggesting one possible future approach for sustaining connections and integrating maintenance and relapse prevention.
In conclusion, despite the limitations of a small pilot study, group CBT-AD for T1DM was shown to be potentially effective and acceptable for treating depression and diabetes-related distress within a socioculturally and medically diverse urban adult population. As adults with chronic illness are at elevated risk of depression, interventions targeting this population make sense from a larger public health standpoint. The qualitative findings emphasize a real need for the integration of psychosocial treatment with the care of adults with T1DM. Interview data revealed feelings of isolation and desire for peer support, and reflected meaningful changes in the way participants thought about, felt towards, and approached their diabetes. In the context of frequent social and medical isolation and in an era of 15-minute medical visits, participants also reported feeling heard and understood by treatment providers. These aspects of the adult experience of T1DM have been minimally addressed in the literature and may contribute to the depression and diabetes-related distress reported by our participants.
Given the difficulties we faced recruiting participants for this project, further efforts towards collaboration with medical practitioners and co-location in clinical settings, or the incorporation of remote technologies, may improve feasibility in future research. More research locating groups in endocrinology practices serving adults with T1DM may improve the accessibility of group CBT-AD for T1DM by utilizing the natural resource of multiple adults with the illness as a home for peer-based interventions. Further research may better address the feasibility of the intervention alongside the diverse needs of the population.
Research Highlights.
A group intervention for adults with T1DM to address self-management and depression was developed
Adapting individual CBT-AD for T1DM to a group format was found highly acceptable
Postintervention reductions in depressive symptoms and diabetes-specific distress were found.
Peer support and the CBT model were key components of the intervention, per qualitative findings
Improvements in blood glucose control and self-care behavior were not found
Participant recruitment posed a significant challenge to feasibility
Acknowledgments
This study was supported by a pilot and feasibility grant from the Einstein Diabetes Research Center, which receives support from NIDDK (P60 DK020541). Dr. Gonzalez's effort is also partially supported by R18 DK098742.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Contributor Information
Sabrina A. Esbitt, Ferkauf Graduate School of Psychology, Yeshiva University
Abigail W. Batchelder, Ferkauf Graduate School of Psychology, Yeshiva University
Molly L. Tanenbaum, Ferkauf Graduate School of Psychology, Yeshiva University
Erica Shreck, Ferkauf Graduate School of Psychology, Yeshiva University.
Jeffrey S. Gonzalez, Ferkauf Graduate School of Psychology, Yeshiva University, and Albert Einstein College of Medicine, Yeshiva University
References
- Alam R, Sturt J, Lall R, Winkley K. An updated meta-analysis to assess the effectiveness of psychological interventions delivered by psychological specialist and generalist clinicians on glycaemic control and on psychological status. Patient Education and Counseling. 2009;75(1):25–36. doi: 10.1016/j.pec.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Amsberg S, Anderbro T, Wredling R, Lisspers J, Lins PE, Adamson U, Johansson UB. A cognitive behavior therapy-based intervention among poorly controlled adult type 1 diabetes patients--a randomized controlled trial. Patient Education and Counseling. 2009a;77(1):72–80. doi: 10.1016/j.pec.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Amsberg S, Anderbro T, Wredling R, Lisspers J, Lins PE, Adamson U, Johansson UB. Experience from a behavioural medicine intervention among poorly controlled adult type 1 diabetes patients. Diabetes Research and Clinical Practice. 2009b;84(1):76–83. doi: 10.1016/j.diabres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care. 2012;34:S11–61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Balfe M, Doyle F, Smith D, Sreenan S, Brugha R, Hevey D, Conroy R. What's distressing about having type 1 diabetes? A qualitative study of young adults' perspectives. BMC Endocrine Disorders. 2013;13(25) doi: 10.1186/1472-6823-13-25. doi: 10.1186/1472-6823-13-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with Type 1 diabetes: systematic literature review. Diabetic Medicine. 2006;23(4):445–451. doi: 10.1111/j.1464-5491.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- Beck JS. Cognitive behavior therapy: Basics and beyond. 2nd ed. Guilford; New York: 2011. [Google Scholar]
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3(2):77–101. [Google Scholar]
- Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. American Journal of Psychiatry. 2001;158:29–35. doi: 10.1176/appi.ajp.158.1.29. [DOI] [PubMed] [Google Scholar]
- Ciechanowski PS, Russo J, Katon W, Von Korff M, Ludman E, Lin E, Bush T. Influence of patient attachment style on self-care and outcomes in diabetes. Psychosomatic Medicine. 2004;66(5):720–728. doi: 10.1097/01.psy.0000138125.59122.23. [DOI] [PubMed] [Google Scholar]
- de Groot M, Anderson R, Freedland K, Clouse R, Lustman P. Association of depression and diabetes complications: A meta-analysis. Psychosomatic Medicine. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Dennis CL. Peer support within a health care context: A concept analysis. International Journal of Nursing Studies. 2003;40(3):321–332. doi: 10.1016/s0020-7489(02)00092-5. [DOI] [PubMed] [Google Scholar]
- Due-Christensen M, Zoffmann V, Hommel E, Lau HM. Can sharing experiences in groups reduce the burden of living with diabetes, regardless of glycaemic control? Diabetic Medicine. 2012;29(2):251–256. doi: 10.1111/j.1464-5491.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- Esbitt SA, Tanenbaum ML, Gonzalez JS. Disentangling clinical depression from diabetes-specific distress: Making sense of the mess we've made. In: Lloyd C, Pouwer F, Hermanns N, editors. Screening for depression and other psychological problems in diabetes. Springer; London, UK: 2013. pp. 27–46. [Google Scholar]
- Fisher L, Skaff M, Mullan J, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabetic Medicine. 2008;25:1096–1101. doi: 10.1111/j.1464-5491.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress meaningful?: Establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35(2):259–264. doi: 10.2337/dc11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey KC, Wolpert HA, Rhodes ET, Laffel LM, Kleinman K, Beste MG, Finkelstein JA. Health care transition in patients with type 1 diabetes: young adult experiences and relationship to glycemic control. Diabetes Care. 2012;35(8):1716–1722. doi: 10.2337/dc11-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman N, Snell-Bergeon JK, McFann K, , Kinney G, Paul Wadwa R, Bishop F, Maahs DM. Prevalence and correlates of depression in individuals with and without type 1 diabetes. Diabetes Care. 2009;32(4):575–579. doi: 10.2337/dc08-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Delahanty L, Safren S, Meigs J, Grant R. Differentiating symptoms of depression from diabetes-specific distress: Relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:1822–1825. doi: 10.1007/s00125-008-1113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales J, McCarl L, Wexler D, Cagliero E, Delehanty L, Soper T, Safren S. Cognitive-behavioral therapy for adherence and depression in type 1 diabetes. Journal of Cognitive Psychotherapy. 2010;24(4):329–343. doi: 10.1891/0889-8391.24.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Peyrot M, McCarl L, Collins E, Serpa L, Mimiaga M, Safren S. Depression and diabetes treatment nonadherence: A meta-analysis. Diabetes Care. 2008;31:2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J, Safren S, Cagliero E, Wexler D, Delahanty L, Wittenberg E, Grant R. Depression, self-care, and medication adherence in type 2 diabetes: Relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. Journal of General Internal Medicine. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Annals of Internal Medicine. 2010;153(8):507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard ME, Perlu JG, Clark LM, Haynie DL, Plotnik LP, Guttmann-Bauman I, Iannotti RJ. Perspectives from before and after the pediatric to adult care transition: a mixed-methods study in type 1 diabetes. Diabetes Care. 2013 doi: 10.2337/dc13-1346. doi: 10.2337/dc13-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DC. Statistical Methods in Psychology. 6th ed. Thomson Wadsworth; Belmont, CA: 2007. [Google Scholar]
- Imel Z, Wampold B. Handbook of Counseling Psychology. 4th ed. John Wiley & Sons; New York: 2008. The importance of treatment and the science of common factors in psychotherapy. pp. 249–262. [Google Scholar]
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: A review of literature. Journal of Behavioral Medicine. 2008;31(3):213–224. doi: 10.1007/s10865-007-9147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, Siebenhofer A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: Systematic review and meta-analysis. Diabetologia. 2008;51:941–951. doi: 10.1007/s00125-008-0974-3. [DOI] [PubMed] [Google Scholar]
- Joseph DH, Griffin M, Hall RF, Sullivan ED. Peer coaching: An intervention for individuals struggling with diabetes. Diabetes Educator. 2001;27(5):703–710. doi: 10.1177/014572170102700511. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca A. Manual for the Self Care Inventory. Author; Coral Gables, FL: 2004. [Google Scholar]
- Laing SP, Jones ME, Swerdlow AJ, Burden AC, Gatling W. Psychosocial and socioeconomic risk factors for premature death in young people with type 1 diabetes. Diabetes Care. 2013;28(7):1618–1623. doi: 10.2337/diacare.28.7.1618. [DOI] [PubMed] [Google Scholar]
- Lambert MJ, Barley DE. Research summary on the therapeutic relationship and psychotherapy outcome. Psychotherapy: Theory, Research, Practice, Training. 2001;38(4):357–361. [Google Scholar]
- Lloyd CE, Pambianco G, Orchard TJ. Does diabetes-related distress explain the presence of depressive symptoms and/or poor self-care in individuals with Type 1 diabetes? Diabetes Medicine. 2010;27(2):234–237. doi: 10.1111/j.1464-5491.2009.02896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz SM, Carper MM, Gonzalez JS, Delahanty LM, Safren SA. Cognitive-behavioral therapy for the treatment of depression and adherence in patients with type 1 diabetes: Pilot data and feasibility. Primary Care Companion CNS Disorders. 2012;14(2) doi: 10.4088/PCC.11m01220. doi: 10.4088/PCC.11m01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz S, Gonzalez J, Wilkinson J, Safren S. Treating depression in diabetes: Emerging findings. Psychosomatics. 2011;52(1):1–18. doi: 10.1016/j.psym.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Rief W, Klaiberg A, Braehler E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. General Hospital Psychiatry. 2006;28(1):71–77. doi: 10.1016/j.genhosppsych.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Matthews SM, Peden AR, Rowles GD. Patient-provider communication: understanding diabetes management among adult females. Patient Education and Counseling. 2009;76(1):31–37. doi: 10.1016/j.pec.2008.11.022. [DOI] [PubMed] [Google Scholar]
- McKellar JD, Humphreys K, Piette JD. Depression increases diabetes symptoms by complicating patients’ self-care adherence. Diabetes Educator. 2004;30:485–492. doi: 10.1177/014572170403000320. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: Results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabetic Medicine. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- Plack K, Herpertz S, Petrak F. Behavioral medicine interventions in diabetes. Current Opinion in Psychiatry. 2010;23(2):131–138. doi: 10.1097/YCO.0b013e3283366555. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, Schwartz CE. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, Jackson RA. Assessing psychosocial stress in diabetes: development of the Diabetes Distress Scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- Pyatak EA, Sequeira P, Peters AL, Montoya L, Weigensberg MJ. Disclosure of psychosocial stressors affecting diabetes care among uninsured young adults with Type 1 diabetes. Diabetic Medicine. 2013;30(9):1140–1144. doi: 10.1111/dme.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Richardson LK, Frueh BC, Grubaugh AL, Egede L, Elhai JD. Current directions in videoconferencing tele-mental health research. Clinical Psychology: Science and Practice. 2009;16(3):323–338. doi: 10.1111/j.1468-2850.2009.01170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy MS, Peng B, Roy A. Risk factors for coronary disease and stroke in previously hospitalized African-Americans with Type 1 diabetes: A 6-year follow-up. Diabetic Medicine. 2007;24(12):1361–1368. doi: 10.1111/j.1464-5491.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- Roy MS, Roy A, Affouf M. Depression is a risk factor for poor glycemic control and retinopathy in African-Americans with type 1 diabetes. Psychosomatic Medicine. 2007;69(6):537–542. doi: 10.1097/PSY.0b013e3180df84e2. [DOI] [PubMed] [Google Scholar]
- Rustad JK, Musselman DL, Nemeroff CB. The relationship of depression and diabetes: Pathophysiological and treatment implications. Psychoneuroendocrinology. 2011;36(9):1276–1286. doi: 10.1016/j.psyneuen.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Safren S, Gonzalez J, Soroudi N. CBT for Depression and Adherence in Individuals with Chronic Illness: Client Workbook (Treatments That Work) Oxford University Press; New York: 2008a. [Google Scholar]
- Safren S, Gonzalez J, Soroudi N. CBT for Depression and Adherence in Individuals with Chronic Illness: Therapist Guide (Treatments That Work) Oxford University Press; New York: 2008b. [Google Scholar]
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, Margolina AI, Cagliero E. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in patients with uncontrolled type 2 diabetes. Diabetes Care. 2014 doi: 10.2337/dc13-0816. [published ahead of print, October 29, 2013]. doi:10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Hendriksen ES, Mayer KH, Mimiaga MJ, Pickard R, Otto MW. Cognitive-behavioral therapy for HIV medication adherence and depression. Cognitive and Behavioral Practice. 2004;11:415–424. [Google Scholar]
- Safren SA, O'Cleirigh C, Tan J, Raminani S, Reilly LC, Otto MW, Mayer KH. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychology. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, O'Cleirigh CM, Bullis JR, Otto MW, Stein MD, Pollack MH. Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology. 2012;80(3):404–415. doi: 10.1037/a0028208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth J. Life-Steps: Applying cognitive-behavioral therapy to participant adherence to HIV medication treatment. Cognitive and Behavioral Practice. 1999;6:332–341. [Google Scholar]
- Savin K. Empowering the chronic patient: Evaluating a peer-led diabetes support group. 2012 Unpublished manuscript. [Google Scholar]
- Schwartz CE, Sendor M. Helping others helps oneself: Response shift effects in peer support. Social Science Medicine. 1999;48(11):1563–1575. doi: 10.1016/s0277-9536(99)00049-0. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;20:22–33. [PubMed] [Google Scholar]
- Skinner TC, Hampson SE. Personal models of diabetes in relation to self-care, well-being and glycemic control: A prospective study in adolescence. Diabetes Care. 2001;24:828–833. doi: 10.2337/diacare.24.5.828. [DOI] [PubMed] [Google Scholar]
- Snoek FJ, van der Ven N, Lubach C. Cognitive behavioral group training for poorly controlled type 1 diabetes patients: a psychoeducational approach. Diabetes Spectrum. 1999;12(3):147–157. [Google Scholar]
- Soroudi N, Perez G, Gonzalez J, Greer J, Pollack M, Otto M, Safren S. CBT for medication adherence and depression (CBT-AD) in HIV-infected patients receiving methadone maintenance therapy. Cognitive and Behavioral Practice. 2008;15:93–106. [Google Scholar]
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Stark CS, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36(8):2271–2279. doi: 10.2337/dc12-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ML, Gonzalez JS. The influence of diabetes on a clinician-rated assessment of depression in adults with type 1 diabetes. Diabetes Educator. 2012;38(5):695–704. doi: 10.1177/0145721712452795. [DOI] [PubMed] [Google Scholar]
- Trief PM, Sandberg JG, Dimmock JA, Forken PJ, Weinstock RS. Personal and relationship challenges of adults with Type 1 diabetes: A qualitative focus group study. Diabetes Care. 36(9):2483–2488. doi: 10.2337/dc12-1718. 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bastelaar K, Pouwer F, Cuijpers P, Riper H, Snoek F. Web-based depression treatment for type 1 and type 2 diabetic patients: A randomized, controlled trial. Diabetes Care. 2011;34(2):320–325. doi: 10.2337/dc10-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ven N. Psychosocial group interventions in diabetes care. Diabetes Spectrum. 2003;16(2):88–95. [Google Scholar]
- Van Tilburg M, McCaskill C, Lane J, Edwards C, Bethel A, Feinglos M, Surwit R. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosomatic Medicine. 2001;63:551–555. doi: 10.1097/00006842-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Watson T, Logan P. Diabetes mellitus (insulin dependent). In: Phelps L, editor. Health-related disorders in children and adolescents: A guidebook for understanding and educating. American Psychological Association; Washington, DC: 1998. pp. 238–247. [Google Scholar]
- Weinger K. Group Interventions: Emerging applications for diabetes care: preface. Diabetes Spectrum. 2003;9(2):86–87. [Google Scholar]
- Weinger K, Butler HA, Welch GW, La Greca AM. Measuring diabetes self-care: a psychometric analysis of the self-care inventory-revised with adults. Diabetes Care. 2005;11:1346–1352. doi: 10.2337/diacare.28.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education and Counseling. 2001;42:123–131. doi: 10.1016/s0738-3991(00)00098-7. [DOI] [PubMed] [Google Scholar]
- Weinger K, Lee J. Psychosocial and psychiatric challenges of diabetes mellitus. The Nursing Clinics of North America. 2006;41(4):667–680. doi: 10.1016/j.cnur.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Winkley K, Landau S, Eisler I, Ismail K. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: Systematic review and meta-analysis of randomized controlled trials. British Medical Journal. 2006;333(7558):65–70. doi: 10.1136/bmj.38874.652569.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Norris S, Gregg E, Cheng Y, Beckles G, Kahn H. Depressive symptoms and mortality among persons with and without diabetes. American Journal of Epidemiology. 2005;161:652–660. doi: 10.1093/aje/kwi089. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, Chelminski I. Defining remission on the Montgomery-Asberg Depression Rating Scale. Journal of Clinical Psychiatry. 2004;65(2):163–168. doi: 10.4088/jcp.v65n0204. [DOI] [PubMed] [Google Scholar]