SUMMARY
Emerging evidence demonstrates that the DNA repair kinase DNA-PKcs exerts divergent roles in transcriptional regulation of unsolved consequence. Here, in vitro and in vivo interrogation demonstrate that DNA-PKcs functions as a selective modulator of transcriptional networks that induce cell migration, invasion, and metastasis. Accordingly, suppression of DNA-PKcs inhibits tumor metastases. Clinical assessment revealed that DNA-PKcs is significantly elevated in advanced disease, and independently predicts for metastases, recurrence, and reduced overall survival. Further investigation demonstrated that DNA-PKcs in advanced tumors is highly activated, independent of DNA damage indicators. Combined, these findings reveal unexpected DNA-PKcs functions, identify DNA-PKcs as a potent driver of tumor progression and metastases, and nominate DNA-PKcs as a therapeutic target for advanced malignancies.
INTRODUCTION
The DNA-dependent protein kinase (DNA-PK) is a serine/threonine protein kinase complex composed of a Ku heterodimer (Ku70/Ku80) and a catalytic subunit (DNA-PKcs) that plays an important role in the DNA damage response (DDR) and maintenance of genomic stability. In this context, DNA-PK primarily mediates ligation of DNA double-strand breaks (DSBs) through nonhomologous end joining (NHEJ), wherein the Ku heterodimer recognizes and binds broken DNA ends, facilitating recruitment and activation of DNA-PKcs (Yoo and Dynan, 1999). Activated DNA-PKcs phosphorylates and alters the function of factors that mediate NHEJ, including DNA-PKcs itself and histone H2AX (γH2AX) (An et al., 2010; Chan et al., 2002). While mechanisms governing DNA-PKcs activity are incompletely defined, it is clear that DNA-PKcs activation is critical for DNA DSB repair (Kurimasa et al., 1999; Zhao et al., 2006).
DNA-PKcs expression has been shown to correlate with decreased therapeutic response to DNA-damaging agents in multiple cancers, implicating DNA-PKcs-mediated DNA repair as a mechanism for tumor cell survival (Beskow et al., 2009; Bouchaert et al., 2012). However, DNA-PKcs has also been linked to poor prognosis in the absence of DNA damaging therapies (Evert et al., 2013; Willmore et al., 2008), suggesting a DDR-independent role for DNA-PKcs in human malignancies. Studies further identified DNA-PKcs as a modulator of cancer-associated pathways distinct from DNA repair, including hypoxia, metabolism, inflammatory response, and transcriptional regulation (Goodwin and Knudsen, 2014). Notably, DNA-PKcs was originally discovered and characterized as part of Sp1 transcriptional complexes (Jackson et al., 1990) and as a regulatory component of transcriptionally poised RNA polymerase II (RNAPII) (Dvir et al., 1992); accordingly, recent studies revealed that DNA-PKcs is recruited to active sites of transcription (Ju et al., 2006). DNA-PKcs can interact with the basal transcriptional machinery (Maldonado et al., 1996) and both binds and modulates the function of multiple sequence specific transcription factors (e.g. AIRE, p53, and ERG) as well as select nuclear receptors (including the glucocorticoid (GR), progesterone (PR), estrogen (ER), and androgen receptors (AR)) (Goodwin and Knudsen, 2014). Recently, a critical link was identified between AR signaling and DNA-PKcs that underlies the capacity of this steroid hormone receptor to promote DSB repair (Goodwin et al., 2013; Polkinghorn et al., 2013). Briefly, it was shown that AR binds to the regulatory locus of PRKDC (the gene encoding DNA-PKcs) in response to androgen stimulation and DNA damage, thereby inducing PRKDC expression and subsequent DNA-PKcs activity. This induction proved essential for AR-mediated DSB repair and cell survival in the presence of genomic insult, and elevated levels of DNA-PKcs were shown to create a positive feedback loop by virtue of the established ability of DNA-PKcs to serve as an AR comodulator. These findings provided the mechanistic basis for clinical observations demonstrating that suppression of AR activity enhances the response to radiotherapy (Al-Ubaidi et al., 2013; Warde et al., 2011), concordant with reports showing that AR suppression dampens expression of repair factors in prostatic adenocarcinoma (PCa) (Al-Ubaidi et al., 2013; Warde et al., 2011), and illustrated the significance of AR-DNA-PKcs interplay in PCa. Given the potential implications of DNA-PKcs-mediated transcriptional activity in human malignancies, it was imperative to discern the molecular basis of DNA-PKcs function and the contribution of DNA-PKcs-mediated transcriptional regulation on tumor phenotypes.
RESULTS
DNA-PKcs interacts with AR and is recruited to sites of AR action
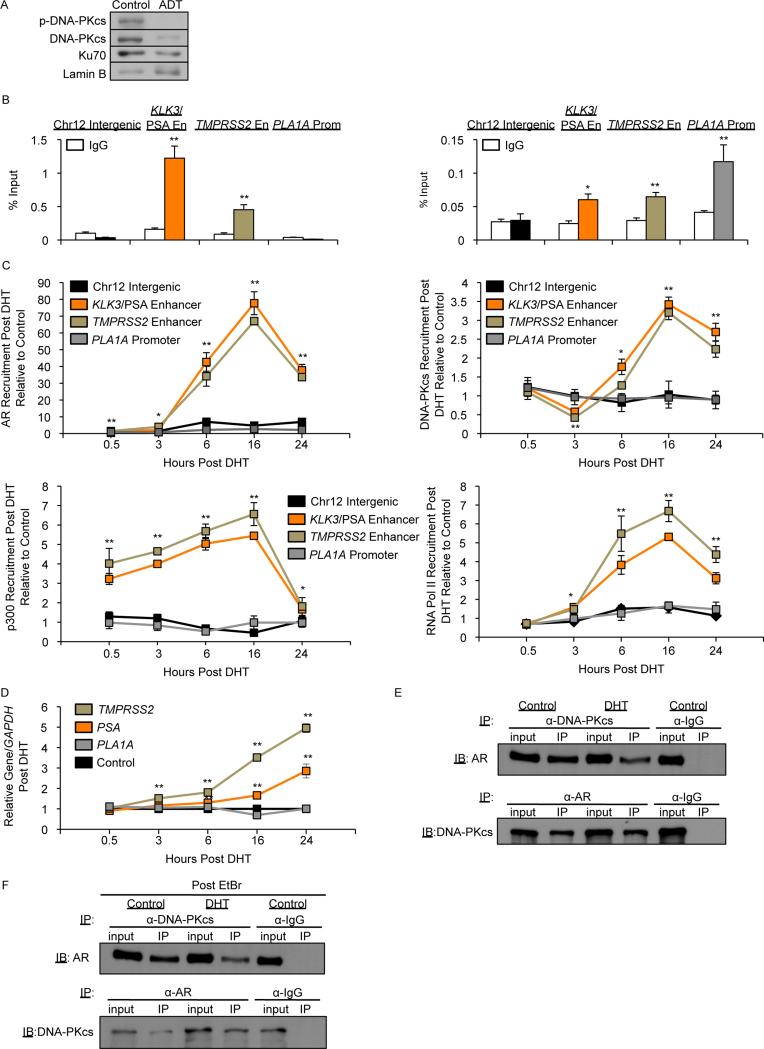
Since DNA-PKcs is induced by AR activity and functions as an AR coactivator in advanced PCa that can bypass anti-androgen therapy (castration-resistant PCa, CRPC), CRPC models were selected to interrogate DNA-PKcs-mediated transcriptional regulation. PCa is dependent on AR activity for growth and progression, and therapies that suppress AR activity through ligand deprivation are the first line of intervention for metastatic disease. While effective, tumors ultimately recur, almost invariably through restoration of AR activity (Knudsen and Scher, 2009). Thus, discerning the impact of DNA-PKcs on AR function in CRPC is of translational relevance. Consistent with identification of PRKDC and XRCC6 as androgen-regulated genes in CRPC (Al-Ubaidi et al., 2013; Goodwin et al., 2013), hormone deprivation decreased DNA-PKcs S2056 phosphorylation (indicative of decreased activity (Chen et al., 2005)) along with total DNA-PKcs and Ku70 levels (Fig 1A). As such, studies assessing the function of DNA-PKcs as a transcriptional regulator were performed in hormone-proficient conditions. Loci explored initially focused on gene regulatory elements governed by AR and ERG in PCa cells, as DNA-PKcs was implicated as a modulator of both factors. As predicted, chromatin immunoprecipitation (ChIP) analysis revealed AR occupancy at two well-characterized loci (KLK3/PSA and TMPRSS2 enhancers), but not at the promoter of the ERG-regulated gene PLA1A (Fig 1B, left). DNA-PKcs was detected at all three regions (Fig 1B, right), but not in the control region, showing specificity of DNA-PKcs binding (Brenner et al., 2011). In response to DHT, AR was recruited to each AR regulatory site within 30 min, with maximum occupancy at 16 hrs post-treatment (Fig 1C, top left, Fig S1A). In contrast, DNA-PKcs recruitment was delayed (6 hrs post-treatment) at AR regulatory regions with maximum occupancy at 16 hrs (Fig 1C, top right, Fig S1A), and unchanged at PLA1A, demonstrating specificity of the DNA-PKcs response to hormone stimulation (Fig 1C, top). The AR coregulator p300 was enriched 30 min post-DHT, followed by RNAPII binding (3-6 hrs) at the AR regulatory loci, while neither was enriched at the PLA1A promoter in response to DHT (Fig 1C, bottom, Fig S1A), suggesting that DNA-PKcs binding facilitates coactivator function and potentiates transcriptional activation. DNA-PKcs levels were not significantly enriched after DHT treatment at these early timepoints (Fig S1B). Notably, DNA-PKcs detection was abrogated at all 3 loci by siRNA-mediated depletion (Fig S1C), but was specifically undetected at AR-regulated loci after treatment with the AR antagonist MDV3100 (Fig S1D). Combined, these findings suggest that DNA-PKcs is recruited to sites of AR function in response to AR and initiating p300 occupancy, facilitating active transcription. The impact of DNA-PKcs recruitment was determined in parallel. PLA1A was not induced in response to DHT, and while significant induction of both KLK3/PSA and TMPRSS2 was observed 3 hrs post-DHT (Fig 1D), maximum induction was not observed until after peak recruitment of AR and DNA-PKcs. Further analyses revealed that AR and DNA-PKcs are found in complex, and that the interaction is not further enriched by exogenous DHT (Fig 1E). The AR-DNA-PKcs interaction is not dependent on DNA binding, as pre-addition of ethidium bromide did not disrupt the complex (Fig 1F), but did result in dismissal of Ku70, as expected (Brenner et al., 2011) (Fig S1E). Further, coimmunoprecipitation in 22Rv1 cells (which contain full length AR and an AR splice variant, AR-V7, lacking the ligand binding domain (LBD) (Guo et al., 2009)) revealed DNA-PKcs interaction with AR-V7 (Fig S1F), suggesting that DNA-PKcs can bind AR-V7 containing complexes; by contrast, in vitro interaction between Ku70 and AR was mapped to the AR LBD (Mayeur et al., 2005). Finally, DNA-PKcs activity was not required for AR interaction but is important for AR function, as a highly selective DNA-PKcs inhibitor, NU7441 (Zhao et al., 2006) (Fig S1G,H) did not suppress complex formation but decreased DHT-stimulated AR activity (Fig S1I). In sum, these findings reveal that DNA-PKcs is found in complex with AR and facilitates AR-dependent transcriptional transactivation.
Figure 1. DNA-PKcs binds AR and is recruited to sites of AR action.
(A) C4-2 cells were treated with ADT (CSS) for 24 hrs and immunoblot analysis for phospho-S2056 DNA-PKcs, total DNA-PKcs, and Ku70, performed. (B,C) C4-2 cells in hormone proficient media were (B) harvested for ChIP-qPCR analysis and percent (input) occupancy of AR (left) or DNA-PKcs (right) reported or (C) treated with 10nM DHT and harvested for ChIP-qPCR analysis with percent (input) occupancy of AR, DNA-PKcs, p300, or RNPII set relative to control at each time point. (D) C4-2 cells were treated with 10nM DHT and relative transcript expression analyzed as normalized to GAPDH mRNA at each timepoint. (E,F) C4-2 cells were treated with 10nM DHT for 6 hrs and co-immunoprecipitation performed in the absence (E) or presence (F) of 50μg/mL ethidium bromide. Data are reported as mean +/− SD. *p<0.05 **p<0.01. See also Fig S1.
DNA-PKcs is a selective effector of transcriptional networks
Given the impact of DNA-PKcs on AR, subsequent studies were directed at identifying the totality of DNA-PKcs mediated transcriptional networks. Initial gene expression analyses were performed in CRPC cells either depleted of DNA-PKcs or treated with NU7441 (Fig 2A, left); as shown, the siPRKDC pool suppressed DNA-PKcs expression, whereas NU7441 had no effect on DNA-PKcs levels, and neither impacted Ku70 expression (Fig 2A, right). Genes up- or downregulated by >1.5 fold were selected for further analysis (Fig 2B). For both manipulations, the number of genes downregulated far exceeded those that were upregulated, suggesting that DNA-PKcs primarily positively regulates transcriptional events. Comparison between groups demonstrated that DNA-PKcs depletion results in overlapping but distinct effects as compared to enzymatic inhibition. To minimize potential off-target effects of NU7441, subsequent analyses primarily focused on transcriptional alterations induced by DNA-PKcs knockdown. Gene Set Enrichment Analysis (GSEA) and associated motif analysis revealed significant enrichment of genes regulated by MAZ, MYC and the known DNA-PKcs-interacting partner Sp1, validating the concept that DNA-PKcs modulates a select subset of transcriptional networks (Fig 2C). Gene ontology (GO) analysis demonstrated that genes sensitive to DNA-PKcs associate with distinct biological processes including transcription and regulation of gene expression, further supporting a role for DNA-PKcs in gene regulation (Fig 2D). Combined, these findings begin to define the cellular consequence of DNA-PKcs mediated transcriptional regulation, and demonstrate that DNA-PKcs selectively governs transcriptional networks.
Figure 2. DNA-PKcs selectively impacts gene expression in CRPC.
(A) RNA harvested from C4-2 cells depleted of DNA-PKcs or treated with 1μM NU7441 (DNA-PKcsi) for 24 hrs was analyzed by microarray analysis (left). Immunoblot of phospho-S2056 DNA-PKcs, total DNA-PKcs, and Ku70 after knockdown or NU7441 treatment (right). (B) Genes identified as upregulated (left) or downregulated (right) by ≥1.5 fold compared to untreated. (C,D) GSEA motif (left) or gene ontology (right) analyses of all genes altered at least 1.5-fold after DNA-PKcs knockdown.
DNA-PKcs and AR cooperate to suppress UGT enzyme expression in CRPC
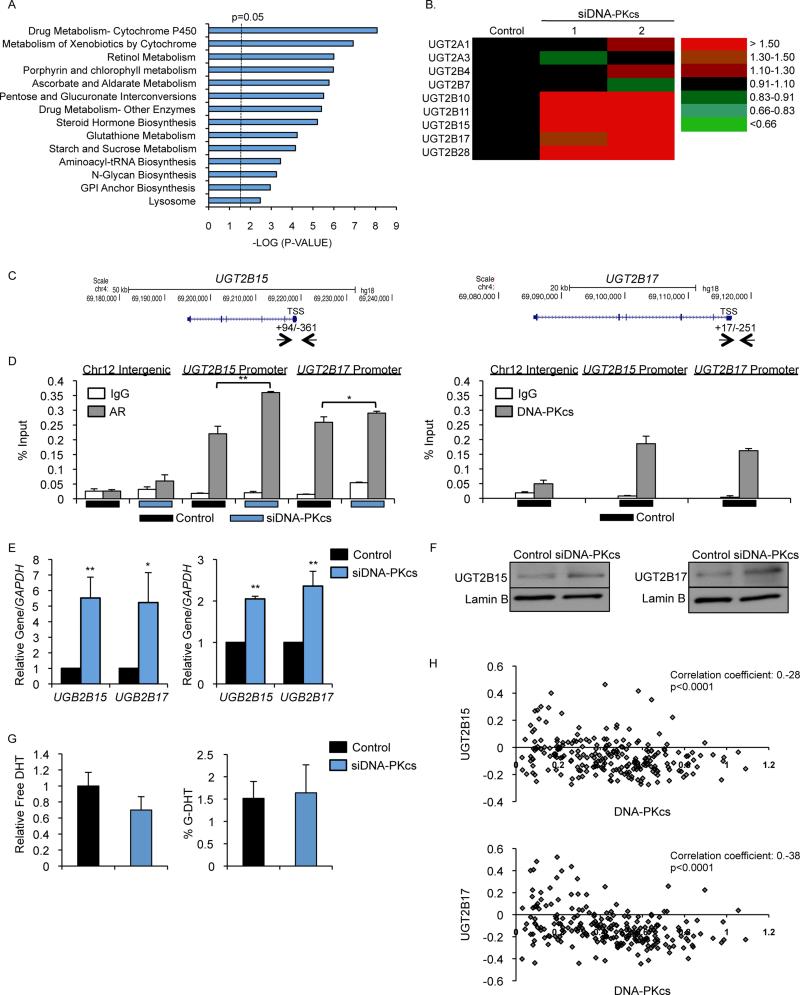
Numerous metabolic and hormone pathways of potential clinical impact in PCa were upregulated by DNA-PKcs depletion (Fig 3A), including steroid hormone biosynthesis, which exhibited upregulation of UGT glycosyltransferases (Fig 3B). UGT enzymes catalyze transfer of glucuronic acid to small molecules (including androgens), facilitating metabolism and excretion (Rowland et al., 2013). In the prostate, local androgen inactivation occurs when DHT is directly modified by glucuronidation or is metabolized to 5α-androstane-3α-diol (3α-diol) and androsterone (AST), which are then glucuronidated by UGT2B15 and UGT2B17. Consistent with previous reports suggesting that these genes are also AR regulated (Bao et al., 2008), AR occupied the proximal promoters of both UGT2B15 and 2B17, with a modest but significant increase observed upon DNA-PKcs depletion (Fig 3C, D left). DNA-PKcs co-occupied these sites (Fig 3D, right), suggesting that negative regulation by DNA-PKcs is direct. DNA-PKcs depletion resulted in increased UGT2B15 and 2B17 expression, underscoring the impact of DNA-PKcs on this pathway (Fig 3E). Previous studies showed that DNA-PKcs negative transcriptional regulation can be mediated through NCoR and SMRT (Jeyakumar et al., 2007; Yu et al., 2006), and both were both enriched at the UGT2B15 and 2B17 promoters. Corepressor binding was significantly reduced by DNA-PKcs depletion (Fig S2A) but not after kinase inhibition (Fig S2B), suggesting that DNA-PKcs occupancy (but not activity) is needed for NCoR and SMRT residence. As expected, DHT stimulation decreased UGT2B15 and 2B17, which was partially reversed by DNA-PKcs depletion (Fig S2C), consistent with a role for DNA-PKcs in negative regulation. As UGT2B15 and 2B17 protein accumulation was also enhanced after DNA-PKcs depletion (Fig 3F), the impact of DNA-PKcs depletion on DHT metabolites was quantified by HPLC (Fig S2D). Cells depleted of DNA-PKcs trended towards decreased overall levels of free DHT, but did not reach statistical significance (Fig 3G, left) and there was no impact on G-DHT (Fig 3G, right) or G-AST (Fig S2E), suggesting that elevated UGT2B15 and 2B17 is not sufficient to independently alter hormone metabolism. Similar regulation of other UGT enzymes after DNA-PKcs depletion (Fig 3B) argues against functional redundancy impacting DHT levels. The overall findings are of translational significance, as UGT2B15 and 2B17 are being developed as prognostic markers and therapeutic targets in PCa (Grosse et al., 2013), and the mechanisms of regulation are not well understood. To assess clinical relevance, a cohort of 232 patients with high-risk localized PCa was examined, wherein it was observed that both UGT2B15 (correlation coefficient −0.28, p<0.0001) and UGT2B17 (correlation coefficient −0.38, p<0.0001) expression strongly negatively correlated with DNA-PKcs (Fig 3H), supporting the concept that DNA-PKcs suppresses expression of UGT enzymes in human tumors. Further analysis in response to NU7441 confirmed the function of DNA-PKcs as a selective negative regulator of transcription (Fig S2F). On balance, these findings identify gene networks that are negatively regulated by DNA-PKcs, and identify DNA-PKcs as a key modulator of the UGT enzyme cancer-associated pathway.
Figure 3. DNA-PKcs and AR cooperate to suppress UGT enzyme expression in CRPC.
(A) GSEA KEGG pathway analysis of genes upregulated by ≥1.5 fold compared to control after DNA-PKcs knockdown. (B) Heat map of transcript change of UGT enzymes in the DNA-PKcs knockdown groups. (C,D) C4-2 cells depleted of DNA-PKcs were harvested for ChIP-qPCR analysis and percent (input) occupancy of AR (D, left) or DNA-PKcs (D, right) at indicated loci reported, TSS= transcriptional start site. (E,F) CRPC cells depleted of DNA-PKcs were subject to either qPCR (E, C4-2 left, 22Rv1 right) or immunoblot (F, C4-2) analysis. (G) Free (left) and G-DHT (right) levels in C4-2 cells depleted of DNA-PKcs were determined by HPLC. (H) Tumor samples were profiled for mRNA expression of DNA-PKcs, UGT2B15, and UGT2B17 and correlation coefficients determined. Data are reported as mean +/− SD. *p<0.05 **p<0.01. See also Fig S2.
DNA-PKcs promotes pro-metastatic signaling
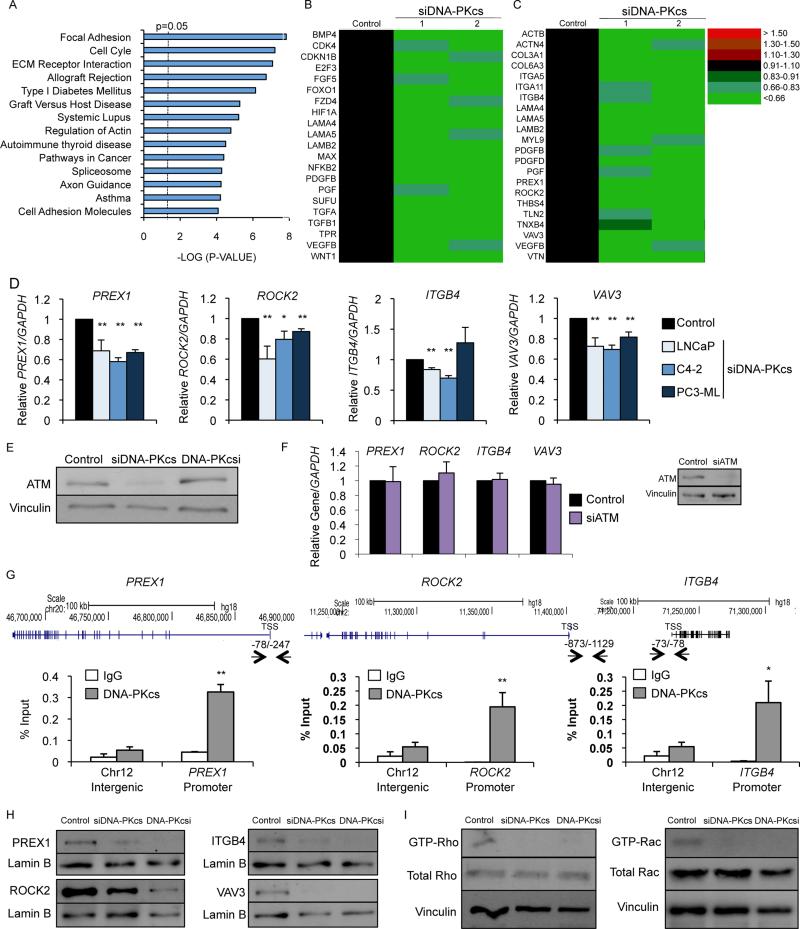
Whereas DNA-PKcs negatively regulates steroid regulated pathways, the majority of DNA-PKcs mediated transcriptional effects support coactivator functions. KLK3/PSA, TMPRSS2, and other well-characterized PCa-relevant AR-regulated genes (Goodwin et al., 2013; Mayeur et al., 2005) were generally reduced after DNA-PKcs depletion (Fig S3A), as expected. Analysis of genes downregulated after DNA-PKcs depletion (Fig 4A) or NU7441 (Fig S3B) revealed enrichment in pathways associated with cancer progression (Fig 4B, Fig S3C), prominently associated with cell migration and invasion. The focal adhesion gene signature was markedly suppressed by DNA-PKcs depletion (Fig 4C) or NU7441 (Fig S3D). Factors in the focal adhesion signature have previously been implicated in PCa progression and metastasis, including PREX1 (GEF for Rac1) (Qin et al., 2009), ROCK2 (effector of Rho signaling) (Kroiss et al., 2014), Integrin β4 (ITGB4, which regulates matrix organization through the Rac1 pathway) (Yoshioka et al., 2013), and VAV3 (GEF for Rho and Rac1) (Lyons and Burnstein, 2006). Expression of each was significantly reduced in hormone-therapy (HT)-sensitive cells (LNCaP), CRPC cells with limited metastatic potential (C4-2), and AR-negative CRPC cells with high metastatic potential (PC3-ML) after DNA-PKcs depletion (Fig 4D) or 24hr inhibitor treatment (Fig S3E), with the exception of ITGB4 in PC3-ML cells, which was not significantly altered, suggesting a possible role for AR in regulation. As expected, ATM levels were diminished after DNA-PKcs depletion but not after DNA-PKcs inhibition (Goodwin et al., 2013; Peng et al., 2005) (Fig 4E). ATM depletion did not significantly alter expression of the identified genes (Fig 4F), suggesting that these transcriptional events are not ATM mediated. Observations were confirmed using alternative strategies to deplete DNA-PKcs or a second highly selective DNA-PKcs inhibitor NU7026 (the lead compound in generation of NU7441) (Veuger et al., 2003) (Fig S3F). Kinetic analysis revealed a time dependent decrease in target gene expression 6 hrs after treatment (Fig S3G), suggesting direct impact of DNA-PKcs on transcriptional regulation. Consonantly, DNA-PKcs binds to the proximal promoter regions containing motifs of known DNA-PKcs associated transcription factors for PREX1 (Wong et al., 2011), ROCK2, and ITGB4 (Drake et al., 2010) (Fig 4G). Treatment with MDV3100 modestly decreased expression of ROCK2 and ITGB4 (Fig S3H), suggesting that AR is not universally required for DNA-PKcs mediated regulation of genes in this pathway. DNA-PKcs occupancy was further examined at the proximal promoter regions of PREX1, ROCK2, and ITGB4 after MDV3100 treatment or depletion of Sp1 or MAZ, the top motifs identified. MDV3100 decreased DNA-PKcs occupancy at the ROCK2 and ITGB4 promoters but not at the PREX1 promoter, consistent with the transcript data (Fig S3I, left). Sp1 depletion resulted in remarkable reduction in DNA-PKcs occupancy at the PREX1 promoter (consistent with PREX1 being regulated by Sp1 (Wong et al., 2011)) and modest but significant reduction in occupancy at both the ROCK2 and ITGB4 promoters (Fig S3I, middle), while MAZ depletion produced a significant reduction in DNA-PKcs occupancy at the ITGB4 promoter, modest reduction in occupancy at the PREX1 promoter (not statistically significant), and no change at the ROCK2 promoter (Fig S3I, right). These studies reveal that DNA-PKcs mediated expression is differentially regulated by transcription factors whose activities are modulated by DNA-PKcs. Decreased transcript expression resulted in reduced protein levels for the factors analyzed (Fig 4H), identifying DNA-PKcs as a positive regulator of metastatic signaling. PREX1, ROCK2, ITGB4, and VAV3 all interact with Rho GTPases that influence cell motility and invasion (Cook et al., 2014). DNA-PKcs depletion or inhibition decreased Rho and Rac1 activity (Fig 4I, Fig S3J). Depletion of VAV3 strongly reduced activated Rho and moderately suppressed Rac1 activity, while depletion of PREX1 diminished activated Rac1 with minimal effects on Rho (Fig S3K), demonstrating importance in DNA-PKcs mediated regulation of Rac/Rho signaling pathways, though other GEFs may be involved. Combined, these findings identify DNA-PKcs as a direct and positive regulator of Rac/Rho function and pro-metastatic pathways.
Figure 4. DNA-PKcs promotes pro-metastatic signaling.
(A) GSEA KEGG pathway analysis of genes downregulated by ≥1.5 fold compared to control after DNA-PKcs knockdown. (B,C) Heat map of transcript change of pathways in cancer (B) or focal adhesion (C) pathway genes in the DNA-PKcs knockdown groups. (D) C4-2 and PC3-ML cells in hormone proficient or LNCaP cells in hormone deficient media treated with siDNA-PKcs or siControl were subject to qPCR analysis with control data set to 1 for each cell line. (E) Immunoblot analyses of C4-2 cells depleted of DNA-PKcs or treated with 1μM NU7441. (F) C4-2 cells depleted of ATM were harvested for qPCR analysis with relative expression of indicated transcripts analyzed and normalized to GAPDH. (G) C4-2 cells harvested for ChIP-qPCR analysis and percent (input) occupancy of DNA-PKcs at the indicated regulatory regions. (H) C4-2 cells depleted of DNA-PKcs or treated with 1μM NU7441 for 48 hrs were subject to immunoblot analysis. (I) C4-2 cells depleted of DNA-PKcs or treated with 1μM NU7441 for 48 hrs were analyzed for activated (GTP-bound) Rho and Rac1 by column binding followed by immunoblot. Data are reported as mean +/− SD. *p<0.05, **p<0.01. See also Fig S3.
DNA-PKcs promotes metastatic phenotypes
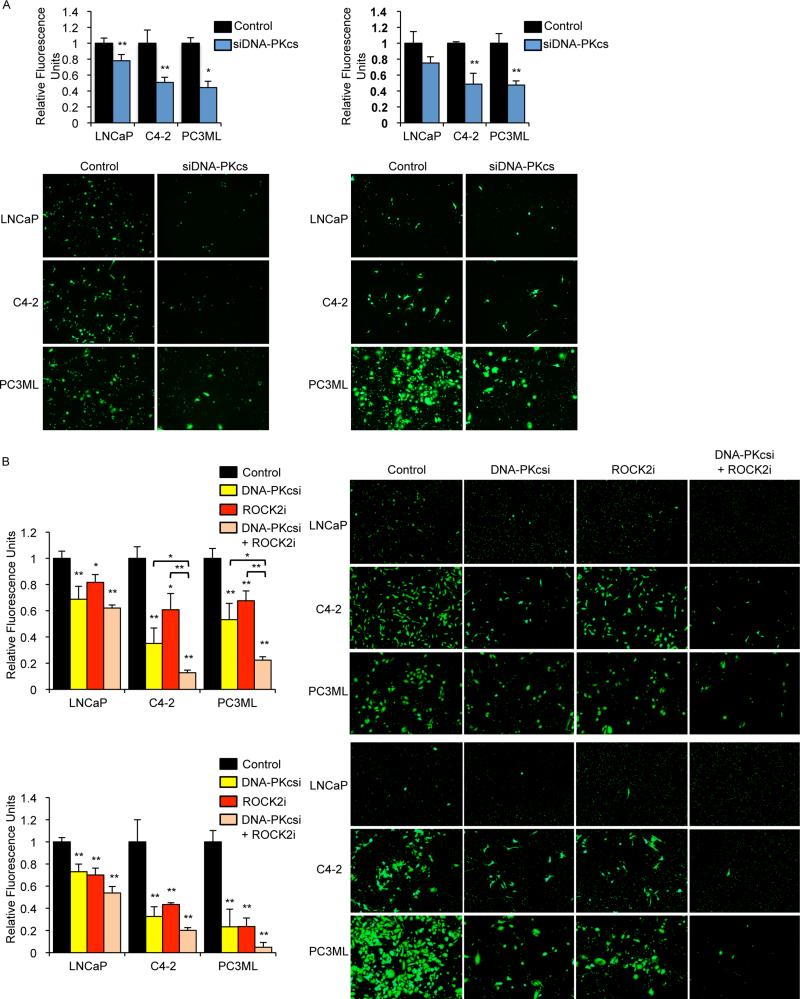
Given the impact of DNA-PKcs on pro-metastatic signaling, the consequence for metastatic potential was determined. Depletion of DNA-PKcs resulted in decreased migration in all models (Fig 5A, left) and invasion in the CRPC models (Fig 5A, right). Consonantly, DNA-PKcs inhibition suppressed migration (Fig 5B, top) and invasion (Fig 5B, bottom) in all models. Both C4-2 and PC3-ML are CRPC lines capable of proliferating in the absence of hormone, and proliferation of LNCaP cells in hormone-deficient media was not significantly altered after DNA-PKcs inhibitor treatment (Fig S4A). The ROCK2 inhibitor reduced migration and invasion similar to that observed with NU7441 (Fig 5B). Combination of the DNA-PKcs and ROCK2 inhibitors resulted in modest but significant decreases in migration in C4-2 and PC3-ML cells, and further suppressed invasion in all models compared to either inhibitor alone, suggesting that DNA-PKcs regulates migration and invasion through pathways in addition to Rho signaling. Further, cells depleted of UGT2B15 or 2B17 failed to demonstrate significant changes in migratory or invasive potential (Fig S4B), suggesting that DNA-PKcs impact on metastatic phenotypes are independent from effects on metabolism. In sum, these findings establish DNA-PKcs as a positive regulator of gene expression events that induce migration and invasion.
Figure 5. DNA-PKcs induces metastatic phenotypes.
(A) Cells depleted of DNA-PKcs were seeded into hormone deficient media and allowed to migrate (left) or invade through matrigel (right) towards hormone proficient media. (B) Cells pretreated with 1μM NU7441, SLx-2119 or combination of both for 24 hrs were seeded into hormone deficient media and allowed to migrate for 24 hrs (top) or invade through matrigel for 72 hrs (bottom) towards hormone proficient media. Data are reported as mean +/− SD. *p<0.05, **p<0.01 compared to control unless otherwise indicated. See also Fig S4.
DNA-PKcs inhibition delays formation of metastases in vivo
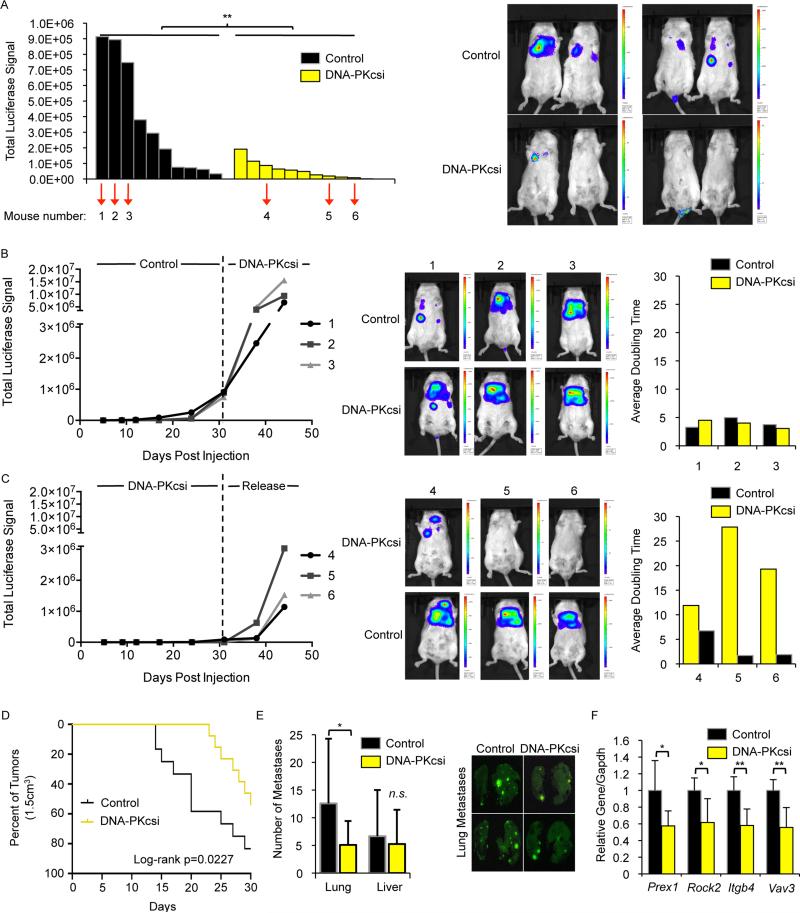
To determine the impact of DNA-PKcs on metastatic development in vivo, PC3-ML cells expressing luciferase were pre-treated for 48 hrs with NU7441 or vehicle and injected into the tail veins of SCID mice. Mice were treated every 24 hrs (5 days/week) with 25mg/kg NU7441 or vehicle, and tumor formation monitored by live imaging (Fig S5A). Parallel studies wherein cells were maintained in culture revealed no significant differences in cell number or viability between the cohorts (Fig S5A). Whereas robust metastases were observed in the control arm, total tumor burden observed in the DNA-PKcs inhibitor treated cohort was significantly reduced, demonstrating that DNA-PKcs inhibition delays formation of productive metastases in vivo (Fig 6A). These findings provide evidence linking DNA-PKcs enzymatic activity to development of metastases.
Figure 6. DNA-PKcs inhibitors delay formation of metastases in vivo.
(A) Mice were injected with luciferin 31 days post tail vein injection of PC3-ML cells and imaged using the IVIS imaging system with total luciferase signal reported (left) and representative images shown (right). Indicated mice were selected for crossover studies. (B,C) Mice were injected with luciferin and imaged for 2 weeks after initiation of crossover studies with total luciferase signal reported (left), representative images shown (middle), and average doubling times pre and post crossover calculated. (D) CASP-NPK-YFP tumors were measured twice weekly for 30 days after initiation of treatment (end point for survival was the predefined tumor volume of 1.5cm3) with volumes calculated using the formula volume=(width)2xlength/2. (E) At time of sacrifice, metastases were documented ex vivo in the lungs and livers by visualizing fluorescence with the total number of metastatic nodules for the lungs and livers assessed. (F) CASPNPK-YFP tumors were harvested for qPCR analysis with the indicated transcripts set relative to Gapdh mRNA. Data are reported as mean +/− SD. **p<0.01. See also Fig S5.
To further investigate the impact of DNA-PKcs, crossover studies were performed wherein animals in the control arm with the greatest tumor burden (denoted 1, 2, and 3) were switched to the NU7441 arm; conversely, 3 mice randomly selected from the NU7441 arm (denoted 4, 5, and 6) were removed from treatment. After 2 weeks, animals moved from control to NU7441 failed to show reductions in tumor burden at established sites of metastases, consistent with the concept that DNA-PKcs inhibitors block development of productive metastases rather than suppressing tumor growth (Fig 6B). Conversely, animals released from NU7441 incurred dramatic induction of metastatic burden, with the tumor-doubling time reduced by ~50-90% (Fig 6C), suggesting that resurgent DNA-PKcs activity drives metastatic development. Mice not selected for crossover were continued on study, and total tumor burden remained suppressed in the NU7441 cohort but not the control arm (Fig S5B). Proliferation rates of the metastatic lesions in the lungs were similar in both crossover cohorts (Fig S5C) again suggesting that tumor changes in animals released from inhibitor illustrate the impact of DNA-PKcs on metastases and not proliferation, though it is possible that NU7441 treatment of large tumors in the crossover may be less effective due to tumor size. Experiments utilizing AR-positive 22Rv1 cells also demonstrated a significant decrease in overall metastatic tumor burden (Fig S5D), though this model is less aggressive in developing metastatic lesions. Combined, these findings clearly reveal that DNA-PKcs induces tumor metastases in vivo, confirming the importance of DNA-PKcs regulated pathways in metastatic development.
To further characterize the impact of DNA-PKcs on metastatic development in AR-positive but aggressive models of spontaneous metastasis, CASP-NPK-YFP tumor cells (Aytes et al., 2013) were engrafted into nude mice. Post-engraftment (5 days), mice were randomized for treatment with 25mg/kg NU7441 or vehicle (5 days/week for 30 days) (Fig S5E). DNA-PKcs suppression decreased overall tumor burden (Fig 6D), though primary tumor weight was not significantly altered between the treatment groups (Fig S5F); by contrast, significant reduction of metastatic lung lesions was observed in the inhibitor treated cohort, with a less pronounced but similar trend in liver metastases (Fig 6E). Finally, analysis of tumors harvested at sacrifice revealed significant decrease in transcript expression of Prex1, Rock2, Itgb4, and Vav3 (Fig 6F), demonstrating that DNA-PKcs modulates expression of these four metastatic genes and promotes development of metastatic lesions in vivo. Thus, DNA-PK promotes metastatic signaling and tumor metastases in both AR-positive and AR-negative cancers.
DNA-PKcs inhibition modulates expression of pro-metastatic factors in primary human tumors
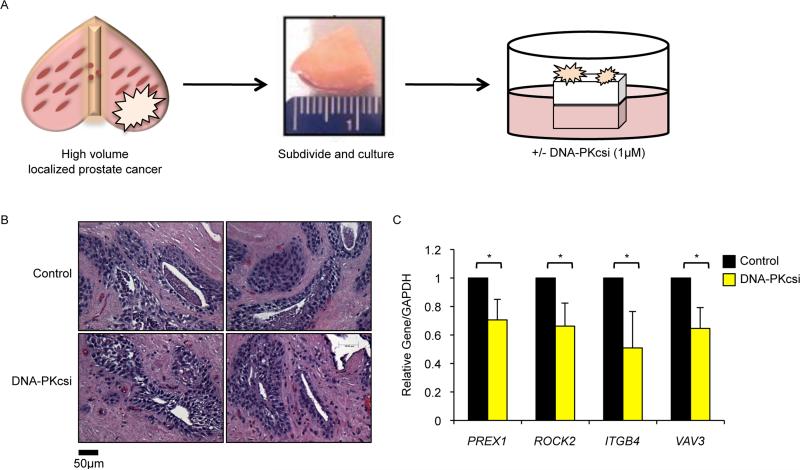
Transcriptional regulatory functions of DNA-PKcs on pro-metastatic factors were further assessed using an ex vivo culture system of primary human PCa, in which tissue obtained immediately upon surgical resection can be subdivided, cultured, and subjected to targeted therapy as previously described (Centenera et al., 2013) (Fig 7A). Explant specimens retain the complex 3D structure and microenvironment of the original tumor, and can be used for clinical assessment of targeted agents (Centenera et al., 2013; Schiewer et al., 2012). While major alterations in histoarchitecture were not observed after exposure to NU7441 (Fig 7B), DNA-PKcs inhibition effectively suppressed expression of PREX1, ROCK2, ITGB4, and VAV3 (Fig 7C). In sum, these findings confirm that DNA-PKcs inhibition regulates expression of pro-metastatic factors in primary human tumors.
Figure 7. DNA-PKcs inhibition modulates expression of pro-metastatic factors in primary human disease.
(A) Schematic of explant assay, adapted from (Schiewer et al., 2012). (B) Representative images of explant tissues treated with control or 1μM NU7441 and stained with hematoxylin & eosin. (C) Explant tissues were harvested on day 6 for qPCR analysis with indicated transcripts set relative to GAPDH. Data are reported as mean +/− SD. *p<0.05.
DNA-PKcs expression and activity predicts clinical disease recurrence and metastatic development
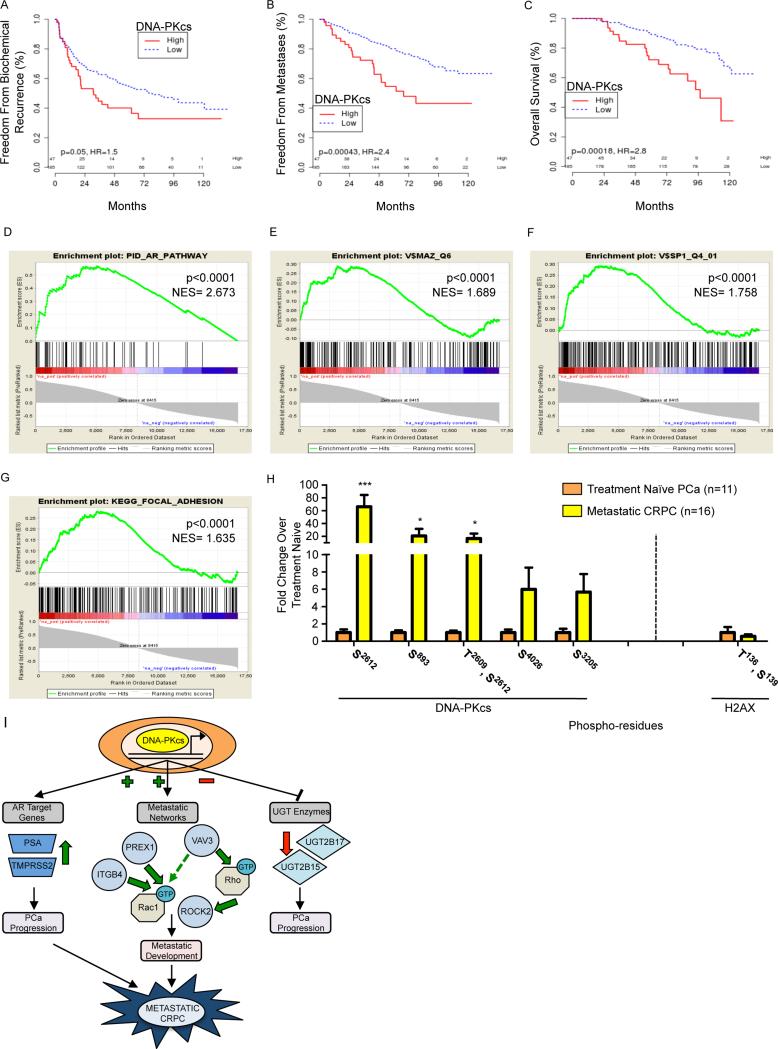
Finally, the prognostic impact of DNA-PKcs in the clinical setting was investigated. A cohort of 232 patients with high-risk localized PCa was examined to assess the relevance of DNA-PKcs expression on outcomes following prostatectomy. As shown, elevated DNA-PKcs conferred reduced freedom from biochemical recurrence (Fig 8A, p=0.050, HR=1.5), and dramatically worse freedom from metastatic progression (Fig 8B, p=0.0004, HR=2.4), PCa-specific survival (Fig S6A, p=0.001, HR=2.8), and overall survival (Fig 8C, p<0.0002, HR=3.1). These results were comparable to the hazard ratios of high Gleason score for these same outcomes (BCR: HR=1.3, p=0.1; metastasis: HR=2.2, p=0.0007; PCSS: HR=4.4, p<0.0001; OS: HR=2.2, p=0.003). As Gleason score is one of the strongest known predictors for aggressive disease (Van der Kwast, 2014), these data illustrate the potent role of DNA-PKcs in promoting lethal PCa. Further, analysis of DNA-PKcs correlated genes showed significant enrichment in the AR pathway (Fig 8D, p<0.0001, normalized enrichment score (NES)= 2.673), the AR transcription factor pathway (Fig S6B, p<0.0001, NES= 2.474), MAZ targets (Fig 8E, p<0.0001, NES= 1.689), Sp1 targets (Fig 8F, p<0.0001, NES= 1.758), and the focal adhesion pathway (Fig 8G, p<0.0001, NES= 1.635), thus validating the preclinical findings. As expected, multiple pathways associated with DDR were also enriched (Table S1). DNA-PKcs was significantly positively correlated with AR, Sp1, and MAZ expression in the clinical samples (Fig S6C, correlation coefficients of 0.68, 0.77, and 0.70, respectively, all p<0.0001), further supporting the functional connectivity. Finally, elevated UGT2B15, but not 2B17, was associated with decreased freedom from metastases (Fig S6D). These findings, compared with previous reports (Mitsiades et al., 2012; Paquet et al., 2012), provide the basis for future studies directed at discerning the potentially divergent roles of UGT2B15 and 2B17 in CRPC progression. These observations identify DNA-PKcs as markedly upregulated in advanced disease, confirm the link between DNA-PKcs and metastatic signaling, and strongly support the contention that DNA-PKcs mediated transcriptional regulation is a major effector of lethal tumor phenotypes.
Figure 8. DNA-PKcs is associated clinically with disease recurrence and metastases.
(A-C) Tumor samples were profiled for DNA-PKcs mRNA, which was split into high vs. low by the 80th percentile for Kaplan Meier analysis. (D-G) GSEA analyses showed enrichment of the AR pathway (D), MAZ (E) and SP1 (F) targets, and the focal adhesion pathway (G) in genes correlated to DNA-PKcs. (H) DNA-PKcs and histone H2AX phosphorylation were measured by mass spectrometry in organ confined, treatment naïve PCa and metastatic CRPC tissues. (I) DNA-PKcs modulates cancer-associated transcriptional networks, inducing expression of AR targets and genes that regulate pro-metastatic Rho/Rac signaling pathways and suppressing expression of UGT enzymes known to impact DHT metabolism, identifying DNA-PKcs as a clinically actionable driver of metastatic CRPC. ***p<0.001, *p<0.05. See also Fig S6, Table S1, S2.
To further interrogate the link between DNA-PKcs and metastasis, an independent cohort was analyzed wherein DNA-PKcs phosphorylation was quantified by phospho-proteomic analyses of fresh clinical specimens from organ confined, treatment naïve PCa vs. metastatic CRPC. Multiple DNA-PKcs residues were hyper-phosphorylated in metastatic CRPC, including Thr2609, an autophosphorylation residue also reported to be phosphorylated by ATM (Chen et al., 2007) and indicative of enzymatic activation (Chan et al., 2002) (Fig 8H, Table S2). These findings reveal that DNA-PKcs is not only present, but highly active in late stage, metastatic CRPC. By contrast, analysis of γH2AX, a marker of DNA DSBs, in metastatic tissues demonstrated no detectable change in phosphorylation levels compared to treatment naïve tissues (Fig 8H), suggesting that the heightened DNA-PKcs activation is not the result (or readout) of elevated DNA damage in metastatic tissues. Combined, these clinical analyses reveal that DNA-PKcs expression predicts for disease recurrence and DNA-PKcs phosphorylation suggests significant activation in metastatic tissues independent of heightened damage response, validating the preclinical evidence that DNA-PKcs is a master regulator of transcriptional events driving disease progression and development of metastatic lesions (Fig 8I).
DISCUSSION
Understanding mechanisms contributing to tumor progression and metastatic development is crucial for development of effective therapeutic strategies targeting advanced cancers. This study identifies DNA-PKcs as a key contributor to metastatic progression, mediated through transcriptional regulation. Key findings reveal that i. DNA-PKcs interacts with AR and is recruited to regulatory loci of AR target genes upon DHT stimulation, facilitating transcriptional activation; ii. DNA-PKcs selectively modifies transcriptional networks associated with tumor progression, and is recruited to loci regulated by DNA-PKcs-associated transcription factors; iii. UGT enzymes are negatively regulated by DNA-PKcs, implicating DNA-PKcs in pathways associated with therapeutic relapse; iv. DNA-PKcs positively regulates a transcriptional network that promotes pro-metastatic signaling, resulting in DNA-PKcs-induced tumor cell migration and invasion; v. pharmacological DNA-PKcs inhibition prevents formation of metastases in vivo; vi. analyses of clinical specimens reveal that DNA-PKcs is elevated and highly active in advanced disease, distinct from marks of DNA damage; and vii. DNA-PKcs dysregulation is strongly associated with development of distant metastases and reduced survival. In sum, these findings strongly support a model wherein the transcriptional regulatory functions of DNA-PKcs induce a pro-metastatic signaling program that drives tumor metastases and lethal disease. These studies not only define DNA-PKcs as a metastatic driver and a putative biomarker of disease progression, but nominate DNA-PKcs as a therapeutic target.
Data here are consistent with literature identifying DNA-PKcs as associated with sequence-specific transcription factors. Recent studies identified DNA-PKcs in ER/coregulator complexes (Foulds et al., 2013) and as an AR coactivator (Goodwin et al., 2013; Mayeur et al., 2005). This study provides direct insight into the mechanism of coordinated transcriptional regulation between AR and DNA-PKcs, wherein DNA-PKcs is recruited with delayed kinetics to sites of AR function, and is required for maximum AR activity. Amongst the AR target genes sensitive to DNA-PKcs regulation, TMPRSS2 was recently shown to promote metastasis (Lucas et al., 2014), providing another mechanism by which DNA-PKcs may modulate metastatic development. Ongoing investigation is directed at discerning the impact of DNA-PKcs on the chromatin microenvironment surrounding AR and DNA-PKcs binding. The studies herein identify DNA-PKcs as an AR coregulator, supporting a role for DNA-PKcs in cancer-relevant transcriptional events.
Consistent with these findings, emerging evidence links DNA repair factors to transcriptional regulation. Initial studies reported that recruitment of DDR machinery was primarily the result of transient, site-specific DSBs required for transcriptional activation (Ju et al., 2006). Further, the gene rearrangements observed in PCa can result from fusion events in transcriptional hubs bringing together distant chromosomal regions (Tomlins et al., 2005), suggesting that DNA repair capacity is needed at sites of active transcription. However, recent findings suggest that repair factors hold transcriptional regulatory functions independent of damage response, as exemplified by PARP1, a DNA repair factor with roles in transcriptional regulation whose functions can be segregated (Steffen et al., 2014). While the effects of DNA-PKcs on transcriptional activation reported here occurred in the absence of exogenous damage, it is possible that transcription-associated DNA breaks may contribute to observed DNA-PKcs activation. Irrespective of the means of activation, the findings herein demonstrate that DNA-PKcs interacts with known transcriptional modulators, binds to sites of transcriptional activation, and selectively engages a transcriptional network of strong cancer relevance.
The concept that DNA-PKcs suppresses UGT2B15 and 2B17 enzyme expression at least partially through NCoR and SMRT provides insight into how this PCa-relevant pathway is governed (Chouinard et al., 2006). Deregulation of androgen metabolism contributes to PCa progression (Chang et al., 2013) and may contribute to metastatic development (Mitsiades et al., 2012). Gene suppressive roles for DNA-PKcs have previously been reported (Hill et al., 2011; Jeyakumar et al., 2007; Yu et al., 2006), suggesting that DNA-PKcs-mediated transcriptional repression is not unique. While AR is required for basal expression of both UGT2B15 and 2B17, stimulation with androgen results in gene downregulation (Bao et al., 2008), suggesting that resurgent AR signaling in CRPC may have a role in DNA-PKcs-mediated transcriptional repression of UGT enzyme expression. Factors influencing UGT expression in non-prostatic tissues include NRF and Sp1 (Mackenzie et al., 2010), and influence on these factors may contribute to the impact of DNA-PKcs. Since UGT2B15 and 2B17 are being evaluated as pharmacologic targets for PCa management (Grosse et al., 2013), the identified link to DNA-PKcs may prove important in designing therapeutic regimens.
Identification of DNA-PKcs as a master regulator of pro-metastatic signaling complements previous studies linking the kinase to cancer-associated transcription factors (Brenner et al., 2011). The top scoring pathway for positively regulated DNA-PKcs genes is focal adhesion, hallmarked by factors that contribute to progression of multiple malignancies. Though the mechanisms regulating ITGB4, PREX1, ROCK2, and VAV3 expression are not well defined, previous reports identified binding sites for DNA-PKcs interacting transcription factors within regulatory regions. Moreover, promoter motif analysis of genes sensitive to DNA-PKcs depletion revealed enrichment for binding sites of DNA-PKcs interacting transcription factors (e.g. Sp1, LEF1, and MYC). The AR binding sequence was not among the top motifs identified, likely influenced by the fact that androgen response elements (AREs) are present at only ~40% of known AR-binding sites, and AR primarily regulates transcription from enhancers (Yu et al., 2010). However, one of the top motifs identified was ELK1, an ETS domain factor required for expression of a major subset of AR target genes (Patki et al., 2013), supporting the finding that DNA-PKcs modulates AR-dependent transcription. Characterization of genome-wide DNA-PKcs occupancy combined with identification of the DNA-PKcs-associated proteome is a focus of current studies, and will help to completely define partners of DNA-PKcs used to selectively modulate transcription.
A major consequence of DNA-PKcs mediated transcriptional regulation is tumor metastasis, and the Rho/Rac pathway was identified as a critical effector of DNA-PKcs activity. Previous studies established a role for Rho/Rac signaling in metastases (Matsuoka and Yashiro, 2014). The finding that ROCK2 and DNA-PKcs inhibitors functioned cooperatively to suppress migration and invasion suggests that pathways in addition to Rho/Rac signaling may contribute to DNA-PKcs induced metastasis (eg Wnt-β-catenin, TGFβ), and it is intriguing to speculate that DNA-PKcs forms a central signaling point modulating metastatic networks. The importance of DNA-PKcs in metastatic formation was confirmed in multiple in vivo models, as inhibition of DNA-PKcs activity strongly delayed formation of metastases, and crossover studies suggest that DNA-PKcs functions early in establishment of metastatic lesions. Combined, these findings provide comprehensive analysis of cancer-associated factors regulated by DNA-PKcs, and identify DNA-PKcs mediated transcriptional regulation as a driver of metastasis.
Finally, findings herein provide robust clinical evidence of DNA-PKcs as promoting metastasis in human disease and as a candidate biomarker to predict poor outcome. Despite recent advances (Mitsiades et al., 2012), clinical biomarkers predicting progression or therapeutic response in PCa are lacking. Analyses of clinical samples demonstrated that high DNA-PKcs expression strongly correlates with decreased freedom from recurrence, freedom from metastases, and survival, implicating DNA-PKcs as a major driver of lethal cancer development. Strikingly, DNA-PKcs held similar prognostic value to Gleason score, underscoring its importance in disease progression. Additionally, a second independent analysis revealed that DNA-PKcs phosphorylation on residues associated with activation (Thr2609) and chromatin binding (Thr2609, Ser2612) is highly enriched in metastatic vs. treatment naïve tissues, indicating that DNA-PKcs is highly active in metastatic PCa, independent of DNA damage markers. While it was previously thought that DNA-PKcs activation occurs only through Ku-mediated binding to broken DNA, recent studies identified additional mechanisms that contribute to DNA-PKcs activation, such as interaction with factors including AKT, EGFR, CK2, and multiple protein phosphatases (Douglas et al., 2001; Goodwin and Knudsen, 2014). While future studies are required to determine which (if any) of these mechanisms contribute to DNA-PKcs activation in the context of transcription, the kinase activity of DNA-PKcs is targetable, and DNA-PKcs inhibitors are currently in clinical trials for advanced solid tumors, hematologic malignancies, and metastases (clinicaltrials.gov, NCT01353625). As development of metastases is nearly universally lethal in solid tumors, the clinical value in targeting DNA-PKcs for prevention of metastatic development in multiple malignancies should be evaluated.
In sum, the studies herein reveal paradigms for DNA-PKcs activity, unveil definitive transcriptional regulatory functions that promote the development of lethal tumor phenotypes, and nominate DNA-PKcs as a therapeutic target.
EXPERIMENTAL PROCEDURES
Tail Vein Assays
Mouse studies were performed with Thomas Jefferson University IACUC approval. PC3-ML or 22Rv1 cells expressing luciferase were pre-treated for 48 hrs with 1 μM NU7441 or DMSO. After 48 hrs, 1×105 cells were seeded in hormone proficient media for viability studies and 5×105 cells in 100uL PBS were injected into the tail vein of 6 week old SCID mice. Cell number and viability were determined via trypan blue exclusion. Mice were treated every 24 hrs 5 days/week with 25mg/kg NU7441 or control through IP injection. Tumor volume was monitored by IP injection of 150uL RediJect D-Luciferin followed by IVIS imaging, with tumor volume quantified by Living Image Software. At day 31 of the PC3-ML study, 3 mice per cohort were selected for crossover studies. Mice not selected continued original treatment for an additional week. Crossover mice received new treatment for 2 weeks prior to sacrifice. Average doubling time pre- and post-crossover was determined using Td= (t2-t1)*((ln2)/ (ln(q2/q1))).
Clinical Analyses
DNA-PKcs expression
Tumor samples were obtained from Mayo Clinic utilizing a case-cohort study design to randomly sample 20% of patients for analysis, in addition to all who developed metastases, from a cohort of 1,010 high-risk men who underwent radical prostatectomy between 2000-2006, for a total cohort of 232 patients as described (Karnes et al., 2013). Studies were approved by the Mayo Clinic IRB and informed consent obtained from all subjects. DNA-PKcs expression was profiled using Affymetrix Human Exon 1.0 ST arrays. Expression data was normalized and summarized using the SCAN algorithm (Karnes et al., 2013). Expression was split into high versus low by the 80th percentile of DNA-PKcs expression. Gleason was split into high (8-10) versus intermediate/low (≤7). Kaplan Meier curves and p-values were generated using the log-rank test. Expression of other genes was correlated with DNA-PKcs using Spearman's correlation. Pre-ranked GSEA analyses were run using spearman's rho and indicated pathways analyzed.
DNA-PKcs phosphorylation
DNA-PKcs and H2AX phosphorylation were measured in organ confined, treatment naïve PCa and metastatic CRPC tissues (Drake et al., 2013). Studies were approved by the UCLA IRB and informed consent obtained from all subjects. Phosphopeptide enrichment was performed as previously described (Zimman et al., 2010) with minor modifications. LC-MS/MS was performed using a Q-Exactive mass spectrometer. MS/MS fragmentation spectra were searched using Andromeda (Cox et al., 2011) against the Uniprot human reference proteome database with canonical and isoform sequences (downloaded January 2012 from uniprot.org) and a reversed decoy database with an FDR <0.01. Search parameters included N-terminal acetylation and oxidized methionine as variable modifications and carbamidomethyl cysteine as a fixed modification. Variable modifications included phosphorylated serine, threonine, or tyrosine [phospho (STY)]. In addition, group-specific parameters included max missed cleavages of 2. Search scores are reported in Table S2. Quantitation was performed using Skyline 2.6.0.6851 (Schilling et al., 2012). Prior to analysis, redundant spectral libraries were generated from Proteome Discoverer search results of the raw data files using the same Uniprot human reference proteome database. Retention time filtering was used so that only scans within 2 minutes of an MS/MS id were included. The precursor isotopic import filter was set to include only the first isotopomer (M0) at a Skyline resolution setting of 70,000. Reintegration of the peaks was performed with mProphet to improve peak picking, with a scoring model based on precursor mass error, identification and co-elution count. Results were reported as areas under the curve (AUC) for each peptide. AUC values were compared across the treatment naïve PCa and metastatic CRPC for DNA-PKcs and H2AX phosphopeptides. Relative fold changes for each phosphoresidue as determined by the average of treatment naïve and metastatic CRPC tissues were plotted. To calculate significance, two-tailed t-tests or Mann-Whitney U tests were used for normally and non-normally distributed phosphopeptide data.
Supplementary Material
HIGHLIGHTS.
Identification of DNA-PKcs-modulated transcriptional networks and consequence
DNA-PKcs-mediated gene regulation promotes migration, invasion, and metastases
DNA-PKcs is upregulated and highly activated in aggressive human tumors
DNA-PKcs independently predicts for metastases, recurrence and poor survival
SIGNIFICANCE.
Mechanisms underlying metastatic development remain incompletely defined, and few therapeutic regimens effectively target the metastatic process. Studies here identify DNA-PKcs as a master driver of pro-metastatic signaling and tumor metastasis through transcriptional regulation, thus shifting paradigms with regard to DNA-PKcs activity and illuminating critical functions in human malignancy. Preclinical findings are strongly supported by clinical observations which demonstrate that DNA-PKcs is significantly upregulated in advanced disease, and predicts for tumor metastases, recurrence, and poor survival. Moreover, DNA-PKcs was shown to be highly activated in metastatic tumors, independent of DNA damage indicators. These collective findings transform understanding of DNA-PKcs function, establish clinical relevance, and nominate DNA-PKcs as a therapeutic target to suppress metastases.
ACKNOWLEDGMENTS
The authors thank Dr. A Fatatis and TJ Stanek for reagents, and N Erho (GenomeDx) and members of the Knudsen laboratory for input. This work was supported by grants to: MJS (PCF), FYF, SAT and KEK (PCF/Movember and Evans Foundation), KEK (PA CURE and NCI, CA159945, CA176401), JMD (DOD PCa Research program W81XWH-14-1-0148), NAG (UCLA SOMI and NIH R25T CA098010), JAW (NIH GM089778), TGG (NCI, CA168585 and ACS RSG-12-257-01-TBE), TGG and ONW (NCATS UCLA UL1TR000124), ONW (PCF), and CA-S (NCI CA173481, CA183929). ONW is an Investigator of the Howard Hughes Medical Institute and partially supported by a Stand Up to Cancer-PCF-Prostate Dream Team Translational Cancer Research Grant (co-PI), a grant made possible through the Movember Foundation. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by AACR. KEK receives research support from Celgene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number- GSE63480.
For details on other experiments, please refer to Sup Experimental Procedures.
REFERENCES
- 1.Al-Ubaidi FL, Schultz N, Loseva O, Egevad L, Granfors T, Helleday T. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res. 2013;19:1547–56. doi: 10.1158/1078-0432.CCR-12-2795. [DOI] [PubMed] [Google Scholar]
- 2.An J, Huang YC, Xu QZ, Zhou LJ, Shang ZF, Huang B, Wang Y, Liu XD, Wu DC, Zhou PK. DNA-PKcs plays a dominant role in the regulation of H2AX phosphorylation in response to DNA damage and cell cycle progression. BMC Mol Biol. 2010;11:18. doi: 10.1186/1471-2199-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, LeKaye HC, Koutcher JA, Cardiff RD, Califano A, et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proc Natl Acad Sci U S A. 2013;110:E3506–15. doi: 10.1073/pnas.1303558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao BY, Chuang BF, Wang Q, Sartor O, Balk SP, Brown M, Kantoff PW, Lee GS. Androgen receptor mediates the expression of UDP-glucuronosyltransferase 2 B15 and B17 genes. Prostate. 2008;68:839–48. doi: 10.1002/pros.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beskow C, Skikuniene J, Holgersson A, Nilsson B, Lewensohn R, Kanter L, Viktorsson K. Radioresistant cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs, Ku70 and Ku86. Br J Cancer. 2009;101:816–21. doi: 10.1038/sj.bjc.6605201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchaert P, Guerif S, Debiais C, Irani J, Fromont G. DNA-PKcs expression predicts response to radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1179–85. doi: 10.1016/j.ijrobp.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Brenner JC, Ateeq B, Li Y, Yocum AK, Cao Q, Asangani IA, Patel S, Wang X, Liang H, Yu J, et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19:664–78. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centenera MM, Raj GV, Knudsen KE, Tilley WD, Butler LM. Ex vivo culture of human prostate tissue and drug development. Nat Rev Urol. 2013;10:483–7. doi: 10.1038/nrurol.2013.126. [DOI] [PubMed] [Google Scholar]
- 9.Chan DW, Chen BP, Prithivirajsingh S, Kurimasa A, Story MD, Qin J, Chen DJ. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes Dev. 2002;16:2333–8. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen BP, Chan DW, Kobayashi J, Burma S, Asaithamby A, Morotomi-Yano K, Botvinick E, Qin J, Chen DJ. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J Biol Chem. 2005;280:14709–15. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 12.Chen BP, Uematsu N, Kobayashi J, Lerenthal Y, Krempler A, Yajima H, Lobrich M, Shiloh Y, Chen DJ. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J Biol Chem. 2007;282:6582–7. doi: 10.1074/jbc.M611605200. [DOI] [PubMed] [Google Scholar]
- 13.Chouinard S, Tessier M, Vernouillet G, Gauthier S, Labrie F, Barbier O, Belanger A. Inactivation of the pure antiestrogen fulvestrant and other synthetic estrogen molecules by UDP-glucuronosyltransferase 1A enzymes expressed in breast tissue. Mol Pharmacol. 2006;69:908–20. doi: 10.1124/mol.105.015891. [DOI] [PubMed] [Google Scholar]
- 14.Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33:4021–35. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M. Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res. 2011;10:1794–805. doi: 10.1021/pr101065j. [DOI] [PubMed] [Google Scholar]
- 16.Douglas P, Moorhead GB, Ye R, Lees-Miller SP. Protein phosphatases regulate DNA-dependent protein kinase activity. J Biol Chem. 2001;276:18992–8. doi: 10.1074/jbc.M011703200. [DOI] [PubMed] [Google Scholar]
- 17.Drake JM, Barnes JM, Madsen JM, Domann FE, Stipp CS, Henry MD. ZEB1 coordinately regulates laminin-332 and {beta}4 integrin expression altering the invasive phenotype of prostate cancer cells. J Biol Chem. 2010;285:33940–8. doi: 10.1074/jbc.M110.136044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drake JM, Graham NA, Lee JK, Stoyanova T, Faltermeier CM, Sud S, Titz B, Huang J, Pienta KJ, Graeber TG, et al. Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proc Natl Acad Sci U S A. 2013;110:E4762–9. doi: 10.1073/pnas.1319948110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dvir A, Peterson SR, Knuth MW, Lu H, Dynan WS. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992;89:11920–4. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evert M, Frau M, Tomasi ML, Latte G, Simile MM, Seddaiu MA, Zimmermann A, Ladu S, Staniscia T, Brozzetti S, et al. Deregulation of DNA-dependent protein kinase catalytic subunit contributes to human hepatocarcinogenesis development and has a putative prognostic value. Br J Cancer. 2013;109:2654–64. doi: 10.1038/bjc.2013.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foulds CE, Feng Q, Ding C, Bailey S, Hunsaker TL, Malovannaya A, Hamilton RA, Gates LA, Zhang Z, Li C, et al. Proteomic analysis of coregulators bound to ERalpha on DNA and nucleosomes reveals coregulator dynamics. Mol Cell. 2013;51:185–99. doi: 10.1016/j.molcel.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodwin JF, Knudsen KE. Beyond DNA repair: DNA-PK function in cancer. Cancer Discov. 2014;4:1126–39. doi: 10.1158/2159-8290.CD-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodwin JF, Knudsen KE. Beyond DNA Repair: DNA-PK Function in Cancer. Cancer Discov. 2014;4:1126–1139. doi: 10.1158/2159-8290.CD-14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, Ma T, Den RB, Dicker AP, Feng FY, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3:1254–71. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosse L, Paquet S, Caron P, Fazli L, Rennie PS, Belanger A, Barbier O. Androgen glucuronidation: an unexpected target for androgen deprivation therapy, with prognosis and diagnostic implications. Cancer Res. 2013;73:6963–71. doi: 10.1158/0008-5472.CAN-13-1462. [DOI] [PubMed] [Google Scholar]
- 26.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill R, Madureira PA, Waisman DM, Lee PW. DNA-PKCS binding to p53 on the p21WAF1/CIP1 promoter blocks transcription resulting in cell death. Oncotarget. 2011;2:1094–108. doi: 10.18632/oncotarget.378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Jackson SP, MacDonald JJ, Lees-Miller S, Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990;63:155–65. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- 29.Jeyakumar M, Liu XF, Erdjument-Bromage H, Tempst P, Bagchi MK. Phosphorylation of thyroid hormone receptor-associated nuclear receptor corepressor holocomplex by the DNA-dependent protein kinase enhances its histone deacetylase activity. J Biol Chem. 2007;282:9312–22. doi: 10.1074/jbc.M609009200. [DOI] [PubMed] [Google Scholar]
- 30.Ju BG, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, Rosenfeld MG. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312:1798–802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 31.Karnes RJ, Bergstralh EJ, Davicioni E, Ghadessi M, Buerki C, Mitra AP, Crisan A, Erho N, Vergara IA, Lam LL, et al. Validation of a genomic classifier that predicts metastasis following radical prostatectomy in an at risk patient population. J Urol. 2013;190:2047–53. doi: 10.1016/j.juro.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15:4792–8. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroiss A, Vincent S, Decaussin-Petrucci M, Meugnier E, Viallet J, Ruffion A, Chalmel F, Samarut J, Allioli N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene Epub. 2014:1–10. doi: 10.1038/onc.2014.222. [DOI] [PubMed] [Google Scholar]
- 34.Kurimasa A, Kumano S, Boubnov NV, Story MD, Tung CS, Peterson SR, Chen DJ. Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining. Mol Cell Biol. 1999;19:3877–84. doi: 10.1128/mcb.19.5.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas JM, Heinlein C, Kim T, Hernandez SA, Malik MS, True LD, Morrissey C, Corey E, Montgomery B, Mostaghel E, et al. The Androgen-Regulated Protease TMPRSS2 Activates a Proteolytic Cascade Involving Components of the Tumor Microenvironment and Promotes Prostate Cancer Metastasis. Cancer Discov. 2014;4:1310–25. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons LS, Burnstein KL. Vav3, a Rho GTPase guanine nucleotide exchange factor, increases during progression to androgen independence in prostate cancer cells and potentiates androgen receptor transcriptional activity. Mol Endocrinol. 2006;20:1061–72. doi: 10.1210/me.2005-0346. [DOI] [PubMed] [Google Scholar]
- 37.Mackenzie PI, Hu DG, Gardner-Stephen DA. The regulation of UDP-glucuronosyltransferase genes by tissue-specific and ligand-activated transcription factors. Drug Metab Rev. 2010;42:99–109. doi: 10.3109/03602530903209544. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado E, Shiekhattar R, Sheldon M, Cho H, Drapkin R, Rickert P, Lees E, Anderson CW, Linn S, Reinberg D. A human RNA polymerase II complex associated with SRB and DNA-repair proteins. Nature. 1996;381:86–9. doi: 10.1038/381086a0. [DOI] [PubMed] [Google Scholar]
- 39.Matsuoka T, Yashiro M. Rho/ROCK signaling in motility and metastasis of gastric cancer. World J Gastroenterol. 2014;20:13756–13766. doi: 10.3748/wjg.v20.i38.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayeur GL, Kung WJ, Martinez A, Izumiya C, Chen DJ, Kung HJ. Ku is a novel transcriptional recycling coactivator of the androgen receptor in prostate cancer cells. J Biol Chem. 2005;280:10827–33. doi: 10.1074/jbc.M413336200. [DOI] [PubMed] [Google Scholar]
- 41.Mitsiades N, Sung CC, Schultz N, Danila DC, He B, Eedunuri VK, Fleisher M, Sander C, Sawyers CL, Scher HI. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 2012;72:6142–52. doi: 10.1158/0008-5472.CAN-12-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paquet S, Fazli L, Grosse L, Verreault M, Tetu B, Rennie PS, Belanger A, Barbier O. Differential expression of the androgen-conjugating UGT2B15 and UGT2B17 enzymes in prostate tumor cells during cancer progression. J Clin Endocrinol Metab. 2012;97:E428–32. doi: 10.1210/jc.2011-2064. [DOI] [PubMed] [Google Scholar]
- 43.Patki M, Chari V, Sivakumaran S, Gonit M, Trumbly R, Ratnam M. The ETS domain transcription factor ELK1 directs a critical component of growth signaling by the androgen receptor in prostate cancer cells. J Biol Chem. 2013;288:11047–65. doi: 10.1074/jbc.M112.438473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Y, Woods RG, Beamish H, Ye R, Lees-Miller SP, Lavin MF, Bedford JS. Deficiency in the catalytic subunit of DNA-dependent protein kinase causes down-regulation of ATM. Cancer Res. 2005;65:1670–7. doi: 10.1158/0008-5472.CAN-04-3451. [DOI] [PubMed] [Google Scholar]
- 45.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, Arora VK, Yen WF, Cai L, Zheng D, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3:1245–53. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qin J, Xie Y, Wang B, Hoshino M, Wolff DW, Zhao J, Scofield MA, Dowd FJ, Lin MF, Tu Y. Upregulation of PIP3-dependent Rac exchanger 1 (P-Rex1) promotes prostate cancer metastasis. Oncogene. 2009;28:1853–63. doi: 10.1038/onc.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rowland A, Miners JO, Mackenzie PI. The UDP-glucuronosyltransferases: their role in drug metabolism and detoxification. Int J Biochem Cell Biol. 2013;45:1121–32. doi: 10.1016/j.biocel.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 48.Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, Dean JL, Liu F, Planck JL, Ravindranathan P, Chinnaiyan AM, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012;2:1134–49. doi: 10.1158/2159-8290.CD-12-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schilling B, Rardin MJ, MacLean BX, Zawadzka AM, Frewen BE, Cusack MP, Sorensen DJ, Bereman MS, Jing E, Wu CC, et al. Platform-independent and label-free quantitation of proteomic data using MS1 extracted ion chromatograms in skyline: application to protein acetylation and phosphorylation. Mol Cell Proteomics. 2012;11:202–14. doi: 10.1074/mcp.M112.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steffen JD, Tholey RM, Langelier MF, Planck JL, Schiewer MJ, Lal S, Bildzukewicz NA, Yeo CJ, Knudsen KE, Brody JR, et al. Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer. Cancer Res. 2014;74:31–7. doi: 10.1158/0008-5472.CAN-13-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 52.Van der Kwast TH. Prognostic prostate tissue biomarkers of potential clinical use. Virchows Arch. 2014;464:293–300. doi: 10.1007/s00428-014-1540-7. [DOI] [PubMed] [Google Scholar]
- 53.Veuger SJ, Curtin NJ, Richardson CJ, Smith GC, Durkacz BW. Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res. 2003;63:6008–15. [PubMed] [Google Scholar]
- 54.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, Gospodarowicz M, Sanders K, Kostashuk E, Swanson G, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378:2104–11. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willmore E, Elliott SL, Mainou-Fowler T, Summerfield GP, Jackson GH, O'Neill F, Lowe C, Carter A, Harris R, Pettitt AR, et al. DNA-dependent protein kinase is a therapeutic target and an indicator of poor prognosis in B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:3984–92. doi: 10.1158/1078-0432.CCR-07-5158. [DOI] [PubMed] [Google Scholar]
- 56.Wong CY, Wuriyanghan H, Xie Y, Lin MF, Abel PW, Tu Y. Epigenetic regulation of phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 gene expression in prostate cancer cells. J Biol Chem. 2011;286:25813–22. doi: 10.1074/jbc.M110.211292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res. 1999;27:4679–86. doi: 10.1093/nar/27.24.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoshioka T, Otero J, Chen Y, Kim YM, Koutcher JA, Satagopan J, Reuter V, Carver B, de Stanchina E, Enomoto K, et al. beta4 Integrin signaling induces expansion of prostate tumor progenitors. J Clin Invest. 2013;123:682–99. doi: 10.1172/JCI60720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu J, Palmer C, Alenghat T, Li Y, Kao G, Lazar MA. The corepressor silencing mediator for retinoid and thyroid hormone receptor facilitates cellular recovery from DNA double-strand breaks. Cancer Res. 2006;66:9316–22. doi: 10.1158/0008-5472.CAN-06-1902. [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–54. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao Y, Thomas HD, Batey MA, Cowell IG, Richardson CJ, Griffin RJ, Calvert AH, Newell DR, Smith GC, Curtin NJ. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66:5354–62. doi: 10.1158/0008-5472.CAN-05-4275. [DOI] [PubMed] [Google Scholar]
- 62.Zimman A, Chen SS, Komisopoulou E, Titz B, Martinez-Pinna R, Kafi A, Berliner JA, Graeber TG. Activation of aortic endothelial cells by oxidized phospholipids: a phosphoproteomic analysis. J Proteome Res. 2010;9:2812–24. doi: 10.1021/pr901194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.