Abstract
Study Objectives:
Short sleep duration and poor sleep continuity have been implicated in the susceptibility to infectious illness. However, prior research has relied on subjective measures of sleep, which are subject to recall bias. The aim of this study was to determine whether sleep, measured behaviorally using wrist actigraphy, predicted cold incidence following experimental viral exposure.
Design, Measurements, and Results:
A total of 164 healthy men and women (age range, 18 to 55 y) volunteered for this study. Wrist actigraphy and sleep diaries assessed sleep duration and sleep continuity over 7 consecutive days. Participants were then quarantined and administered nasal drops containing the rhinovirus, and monitored over 5 days for the development of a clinical cold (defined by infection in the presence of objective signs of illness). Logistic regression analysis revealed that actigraphy- assessed shorter sleep duration was associated with an increased likelihood of development of a clinical cold. Specifically, those sleeping < 5 h (odds ratio [OR] = 4.50, 95% confidence interval [CI], 1.08–18.69) or sleeping between 5 to 6 h (OR = 4.24, 95% CI, 1.08–16.71) were at greater risk of developing the cold compared to those sleeping > 7 h per night; those sleeping 6.01 to 7 h were at no greater risk (OR = 1.66; 95% CI 0.40–6.95). This association was independent of prechallenge antibody levels, demographics, season of the year, body mass index, psychological variables, and health practices. Sleep fragmentation was unrelated to cold susceptibility. Other sleep variables obtained using diary and actigraphy were not strong predictors of cold susceptibility.
Conclusions:
Shorter sleep duration, measured behaviorally using actigraphy prior to viral exposure, was associated with increased susceptibility to the common cold.
Citation:
Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. SLEEP 2015;38(9):1353–1359.
Keywords: common cold, immunity, rhinovirus, sleep continuity, sleep duration
INTRODUCTION
Growing evidence demonstrates that short sleep duration (< 6 or 7 h/night) and poor sleep continuity are associated with the onset and development of a number of chronic illnesses,1–4 susceptibility to acute infectious illness,5–7 and premature mortality.8–11 Experimental evidence in animals and humans suggests that the immune system serves as a key biological pathway.12–14 For instance, total and partial sleep deprivation in humans results in modulation of immune parameters critical to host resistance, including diminished T cell proliferation,15 shifts in T helper cell cytokine responses,16,17 decreases in natural killer (NK) cell cytotoxicity,18,19 and increased activation of proinflammatory pathways.20–23
Sleep related modulation of the immune system is also observed when sleep is measured in the natural environment, with implications for clinical outcomes.6,24 We recently reported that short sleep duration and poor sleep continuity, measured by sleep diary over 14 consecutive days, predicted the incidence of developing a biologically verified cold following viral exposure.6 One of the limitations of this prior study was a reliance on self-reported sleep, which is subject to recall bias leading to inaccurate sleep estimates. Indeed, individuals often overestimate duration and underestimate minutes awake across the night.25 Whether objectively measured sleep indices represent significant predictors of acute infectious illness following viral exposure remains unknown.
To address this gap in the literature, the current study measured sleep behavior objectively using wrist actigraphy and subjectively using sleep diaries over 7 consecutive days and investigated whether measures of sleep duration and continuity predicted susceptibility to the common cold in participants subsequently exposed to a virus (rhinovirus) that causes the common cold. Following exposure to the cold virus, participants were quarantined and monitored for cold symptoms and development of clinical illness. We hypothesized that shorter sleep duration and poorer sleep continuity would be associated with increased incidence of a biologically verified cold and that these associations would be independent of sociodemographic, psychological, and behavioral factors previously shown to predict cold incidence using this paradigm.6,7,26–28
METHODS
Participants
Data were collected between 2007 and 2011. Study participants for these analyses included 94 men and 70 women, aged between 18 and 55 y (mean age = 29.9, standard deviation [SD] = 10.9) from the Pittsburgh, Pennsylvania metropolitan area who responded to study advertisements and were judged to be in good health. Volunteers were excluded if they had a history of nasal surgery or any other chronic illness (e.g., asthma, coronary heart disease, or obstructive sleep apnea); abnormal findings based on urinalysis, complete blood count, or blood enzyme levels; were pregnant or currently lactating; were positive for the human immunodeficiency virus; or taking medications regularly, including sleep medications and oral contraceptives. They were also excluded if they had been hospitalized in the past 5 y or were currently taking medications for psychiatric conditions. In order to maximize the rate of infection by the virus, specific levels of serum antibody to the challenge virus were obtained at screening and participants were excluded with titers higher than 4. Each participant was paid $1,000 for their participation at the conclusion of the study. This study received institutional review board approval, and written, informed consent was obtained for each study participant.
Procedures
Volunteers presenting for possible enrollment underwent medical screening, including a blood draw to assess specific serum neutralizing antibody titer for rhinovirus 39 (RV39). Qualifying participants were enrolled and during the approximately 2 mo that preceded viral challenge they completed questionnaire batteries, 2 w of daily interviews to assess positive emotions, and a subsequent 1 w of wrist actigraphy and concurrent sleep diary to objectively and subjectively measure sleep behavior. Another sample of blood was collected to assess antibody level just before (3–5 days) viral exposure, which provided an estimate of prechallenge antibody titers.
Participants were then isolated in a local hotel for a 6-day period. During the first 24 h of the quarantine, prior to viral exposure, participants underwent a nasal examination and nasal lavage; baseline nasal mucociliary and nasal mucus production were assessed at this time. Those showing signs or symptoms of a cold on this day were dismissed. Then, participants received nasal drops containing approximately 150 tissue culture infectious dose (TCID50)/mL of RV39. Volunteers were subsequently quarantined for 5 days. On each day, nasal lavage samples were collected to assess infection (virus culture). Additionally, daily nasal mucociliary clearance function and nasal mucus production were assessed as objective markers of illness. Approximately 28 days after viral exposure, blood was collected for serological testing.
Sleep Measures
Participants wore an Actiwatch-(64) (Philips Respironics Inc, McMurray, PA) on their nondominant wrists for 7 consecutive nights. Data were stored in 1-min epochs and validated software algorithms (Philips Respironics Inc) were used to estimate sleep parameters. The two actigraphy variables included in these analyses were total sleep time and fragmentation index. Total sleep time, which was used to estimate sleep duration, was defined as the total amount of minutes scored as sleep by the software algorithm in a given defined sleep interval. Fragmentation index is a measure of restlessness during sleep as measured by sleep epochs associated with movement (range 0 to 150, with higher values indicating poorer sleep continuity). As expected, actigraphy-assessed sleep efficiency, defined as the percentage of the sleep interval scored as sleep, was inversely correlated with fragmentation index in this sample (r = −0.59, P < 0.001). We chose fragmentation index instead of sleep efficiency given the documented poor specificity associated with actigraphy-assessed sleep efficiency.29
Self-report sleep diaries were obtained concurrently with actigraphy collection. Each morning, participants reported in their sleep diary what time they went to sleep, what time they woke up, and the min it took to fall asleep. Sleep time was calculated as the time a participant reported waking up minus the time the participant went to sleep. Self-reported sleep duration was computed by sleep time minus the minutes required to fall asleep. Sleep efficiency was calculated as sleep duration divided by sleep time multiplied by 100. Actigraphy and diary estimates for sleep (actigraphy: sleep duration and fragmentation; sleep diary: sleep duration and sleep efficiency) were obtained by averaging over the collection period for all participants with data for at least 5 of the 7 days.
Control Variables
We controlled for a number of covariates previously associated with susceptibility to the common cold, including pre-challenge viral-specific antibody levels to RV; age; sex; race; body mass index (BMI); the season in which the trial occurred; years of education; household income, health habits including current smoking status, physical activity, and alcohol consumption; and psychological variables including perceived socioeconomic status, perceived stress, extraversion, agreeableness, and positive emotional style. These covariates were assessed either during eligibility screening or in the interval between screening and the viral challenge.
Participants self-reported their age, sex, and race. They described their primary racial and ethnic group by choosing from six categories (white, Caucasian; black, African American; Native American, Eskimo, Aleut; Asian or Pacific Islander; Hispanic, Latino; Other). For the analyses, the racial or ethnic groups were dummy coded, with all but whites and blacks collapsed into a single “other” category. BMI (weight in kg/height in m2) was computed based on measurements of participants' weight and height.
Income was assessed by having participants endorse one of 13 household income categories (before taxes) that best represented them. These categories ranged from less than $5,000 to $150,000 or more; income was defined as the median income for the identified category and treated as a continuous variable. Participants' education was assessed by asking them to report on their highest educational attainment. Nine response items were provided, ranging from “didn't finish high school” to “doctoral degree.” Answers were converted into number of years of education based on their responses (e.g., high school, 12 y; PhD, 20 y). Perceived socioeconomic rank was assessed by participants placing themselves on a nine-rung of a ladder in terms of where they stand in their country based on income, education, and occupation.26
Health habits were obtained through self-report questionnaires. Participants were deemed current smokers if they answered “yes” to being asked whether they currently smoked cigarettes, cigars, or pipes on a daily basis. Physical activity was assessed by asking participants whether they engaged in regular activity at least once per week (1, yes; 0, no). Alcohol consumption was obtained by asking participants the average number of drinks they consumed per day (one drink = one glass of wine, 12 oz of beer, or one shot of hard liquor).
Psychological variables that were assessed by questionnaire included a 10-item perception of stress over the past month30; extraversion and agreeableness were assessed using the 10-item versions from the International Personality Item Pool (IPIP) Big Five Factor Markers.31 Finally, positive emotional style was measured as part of an evening interview assessment that was conducted over 14 consecutive days. During each of the 14 daily interviews participants reported the extent to which they felt happy, calm, lively, full of pep, and cheerful throughout the preceding day; ratings for each item were averaged to create a daily total positive affect score across the interview period.27
Virus Culture and Antibody Response
Virus-specific neutralizing antibody titers were measured in serum samples obtained before and approximately 28 days after viral exposure. The results were expressed as reciprocals of the final dilution of serum.32 Daily nasal lavage samples were frozen at −80°C and later cultured for RV using standard techniques.32
Signs of Illness
Daily mucus production was obtained by collecting used tissues in sealed plastic bags.33 The bags were weighed and the weights of the tissues and bags were subtracted. Nasal mucociliary clearance function was measured by administering a dye into the anterior area of the nose and calculating the time taken for the dye to reach the nasopharynx.33
Clinical Cold Criteria
Study participants were considered to have a clinical cold if they were both infected and met illness criteria. Infection was defined as the recovery of the challenge virus on any of the postchallenge days or a fourfold or greater increase in the virus-specific serum neutralizing antibody titer measured pre-exposure to 28 days post-exposure.33 Illness criterion for an objective cold required a total adjusted mucus weight of ≥ 10 g or a total adjusted nasal clearance time of ≥ 35 min.7
Statistical Analysis
All analyses were carried out using SPSS version 22 (SPSS Inc., Chicago, IL). Data were drawn from 212 volunteers who participated in this study. Of those, actigraphy measures were collected from 165 participants. One participant was identified as a clear outlier (> 9 standard deviations above the mean on sleep duration) and excluded, yielding 164 participants for these analyses. Self-report sleep measures obtained by sleep diary were available on 159 participants. Income, BMI, and alcohol consumption were log (base-10) transformed to better approximate a normal distribution. Logistic regression was used to predict colds (1, yes; 0, no). Sleep measures were treated as continuous variables with the exception of self-reported sleep efficiency, which was negatively skewed, and was modeled as a categorical (quartile) predictor. We reported regression coefficients with standard errors and probability values.
Age and prechallenge viral-specific antibody titers were included as covariates in all analyses. Next, we conducted a series of regressions entering one of the 14 separate covariates, along with age and prechallenge antibody titers. The approach reduces the risks of “overfitting” the regression models34,35; however, we also computed single models that included all study covariates simultaneously. In addition, to better elucidate the independent and interactive contributions of duration and continuity measures on cold susceptibility, we fit models that included both actigraphy assessed sleep duration and fragmentation simultaneously as predictors as well as tested the interaction between them (sleep duration × fragmentation).
Finally, to better clarify associations between actigraphy assessed sleep duration and cold incidence and to provide an estimate effect size, sleep duration was categorized based on hours of sleep (< 5 h, n = 36; 5 to 6 h, n = 54; 6.01 to 7 h, n = 52; > 7 h, n = 22). We fitted a logistic regression using this categorical sleep variable and reported odds ratios (OR) with 95% confidence intervals (CIs).
RESULTS
Sample Characteristics and Sleep Scores
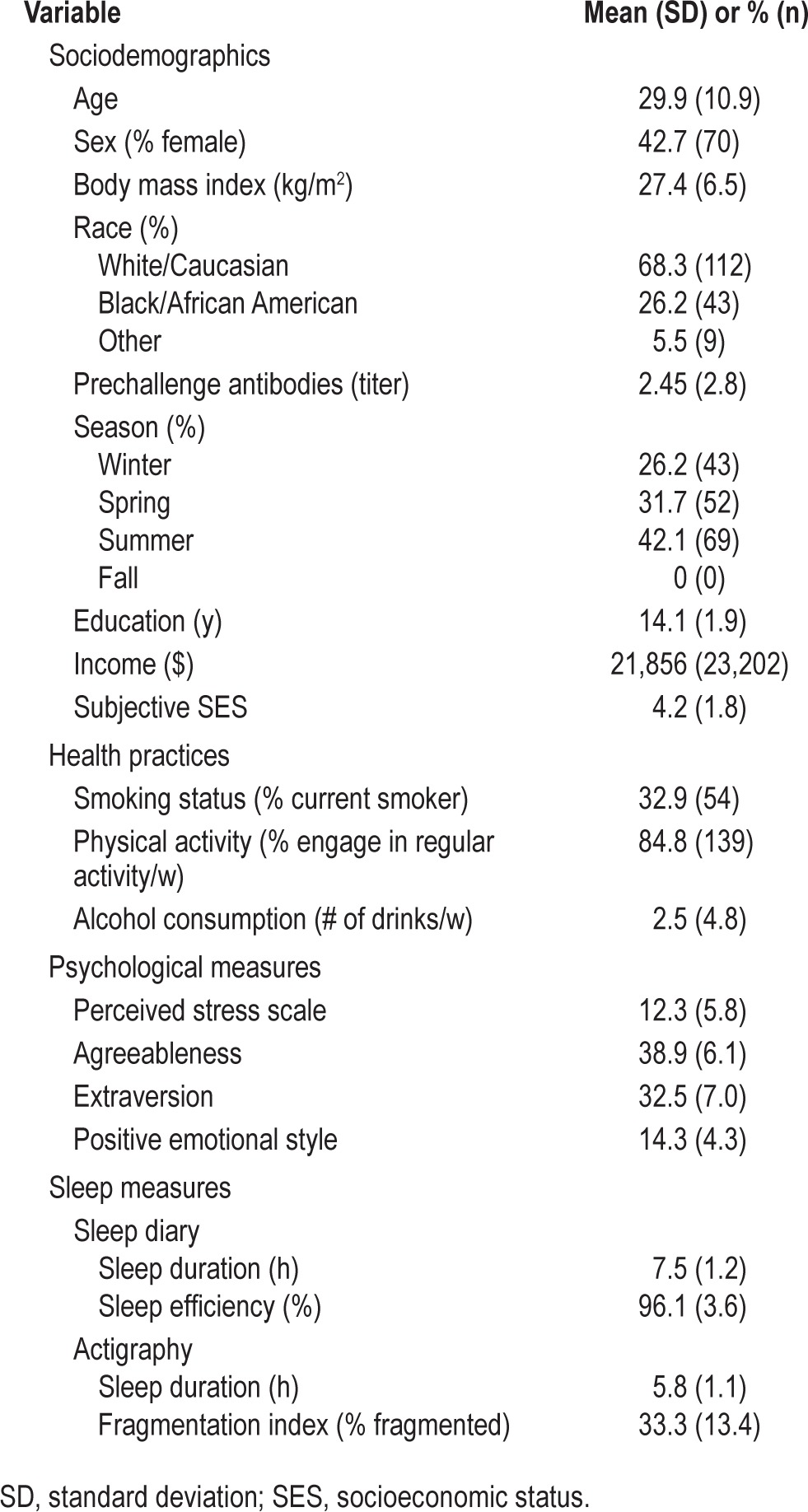
Table 1 presents descriptive data for all variables involved in the analyses. Of the 164 participants, 124 (75.6%) were infected and 48 (29.3%) developed a biologically verified cold, which was defined as infection and objective cold criterion. As expected, sleep measures were intercorrelated (actigraphy sleep duration and fragmentation, r = −0.37, P < 0.001; actigraphy sleep duration and self-reported sleep duration, r = 0.49, P < 0.001; actigraphy sleep duration and self-reported sleep efficiency, r = 0.27, P < 0.001; actigraphy fragmentation and self-reported sleep efficiency, r = −0.12, P = 0.14).
Table 1.
Sample characteristics (n = 164).
Sleep and Susceptibility to the Common Cold
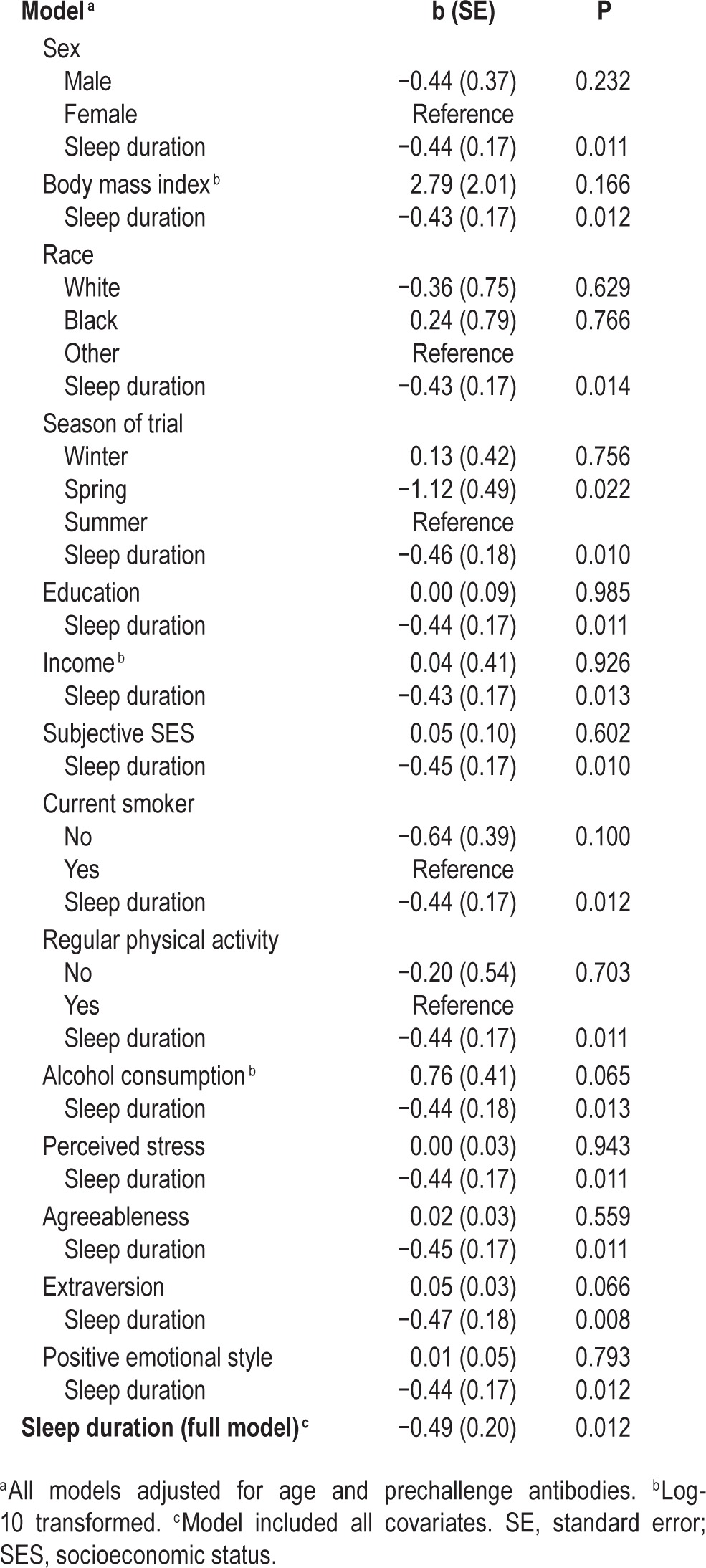
Adjusting for age and prechallenge antibody titers, shorter sleep duration, assessed using actigraphy, was associated with increased risk for the development of the cold (b = −0.44, standard error [SE] = 0.17, P = 0.011). In contrast, sleep fragmentation and self-reported sleep duration were not significant predictors of cold susceptibility (fragmentation: b = −0.01, SE = 0.01, P = 0.715; self-reported sleep duration: b = −0.15, SE = 0.16, P = 0.325). Similarly, participants reporting sleep efficiency in the bottom quartile were no more likely to develop the cold than individuals in the top quartile (b = 0.57, SE = 0.51, P = 0.258).
To follow up on the significant association between actigraphy-assessed sleep duration and the likelihood of developing a biologically verified cold, additional models were computed adjusting for study covariates. Here, we carried out a set of regressions that entered each covariate one by one in separate models (14 separate models). As displayed in Table 2, shorter sleep duration continued to be associated with increased rates of developing a cold (all Ps < 0.015). Furthermore, shorter sleep duration predicted increased odds of developing a cold when all covariates were included in a single model (b = −0.49, SE = 0.20, P = 0.012). Sleep fragmentation was not significantly related to cold incidence when all covariates were included in a single model (b = −0.01, SE = 0.02, P = 0.755). This was similarly the case for self-reported sleep duration and efficiency (data not shown).
Table 2.
Logistic regression models with actigraphy-based sleep duration predicting incidence of the cold, adjusting for each study covariate separately.
To better characterize the effect of sleep duration on odds of developing a cold, sleep categories were created. As illustrated in Figure 1, the predictive influence of sleep duration on cold susceptibility indicates a threshold effect at 6 or fewer hours of sleep (< 5 h, OR = 4.50; 95% CI 1.08–18.69; 5–6 h, OR = 4.24; 95% CI 1.08–16.71; 6.01–7 h, OR = 1.66; 95% CI 0.40–6.95; > 7 h, 1 [reference]).
Figure 1.
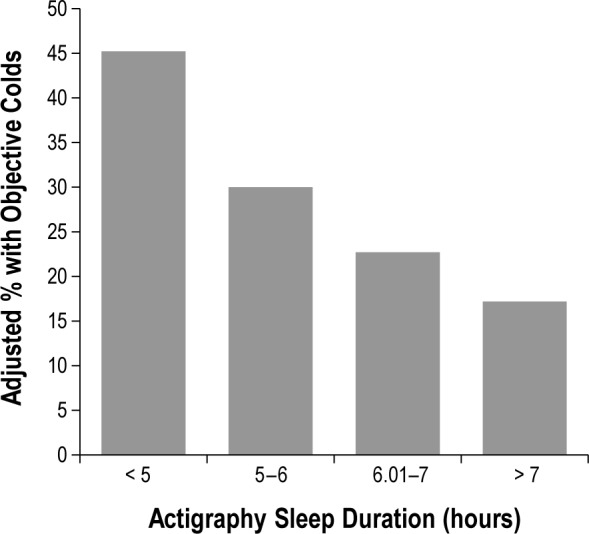
Sleep duration (measured by wrist actigraphy) averaged over a 7-day period before virus exposure is associated with percentage of participants who subsequently developed a cold. The percentage of colds is based on predicted values (adjusted for age and prechallenge viral-specific antibody levels).
The observed elevated risk of developing the cold in participants experiencing shorter sleep duration may have been due to increased susceptibility to infection and/or increased illness expression among those infected. In this regard, in adjusted analyses, actigraphy assessed sleep duration was unrelated to rates of infection (b = −0.11, SE = 0.17, P = 0.543). Similarly, among those who were infected (n = 124), shorter sleep duration was not significantly related to increased odds of meeting illness criteria for mucus production or nasal clearance time (b = −0.32, SE = 0.19, P = 0.090). Although there were no significant relationships of actigraphy-assessed sleep duration with either infection or expression of illness, the association with cold incidence appears to be primarily driven by illness expression.
Because measures of actigraphy assessed sleep duration and fragmentation capture different aspects of an individual's sleep, we tested whether the effects of duration operated independent of fragmentation in predicting risk for a biologically verified cold. To this end, we fit a regression model with both measures entered together. Analyses revealed that sleep duration continued to predict cold incidence adjusting for age and prechallenge antibody levels (b = −0.53, SE = 0.19, P = 0.005) as well as in the fully adjusted (16 covariates and fragmentation) model (b = −0.56, SE = 0.21, P = 0.006). There was no evidence that sleep duration and fragmentation interacted to predict cold incidence (P = 0.92).
DISCUSSION
Shorter sleep duration, measured by wrist actigraphy over a 7-day period, was prospectively associated with increased incidence of the common cold following experimental viral challenge. This association was independent of a cadre of covariates, including age, prechallenge antibody levels, sex, body mass index, race, season of trial, income, education, perceived socioeconomic status, smoking, physical activity, alcohol consumption, perceived stress, agreeableness, extraversion, and positive emotional style. This study provides the first prospective evidence that behaviorally assessed sleep duration serves as a predictor of cold susceptibility.
Analyses revealed a linear association between sleep duration and cold susceptibility; however, when categorized based on hours of sleep, a threshold effect was observed such that individuals sleeping fewer than 6 h of sleep per night were at elevated risk whereas those sleeping more than 6 h were not. This is consistent with some epidemiologic evidence that find strong effects on morbidity and mortality in short sleepers compared to normal sleepers.1,11,36 For instance, Patel and colleagues found that in a sample of nearly 57,000 women, those who reported sleeping ≤ 6 h per night were at significantly greater risk of developing pneumonia compared to those sleeping 8 h per night.5 Those sleeping 7 h were at no greater risk than 8-h sleepers. Emerging evidence also suggests that long sleepers (≥ 9 h per night) are at increased risk of disease.10,11,37 The underlying mechanisms linking negative health and long sleep are poorly understood38–40; however, depression and medical comorbidities have been implicated.38 Very few participants in this study reported sleeping more than 9 h per night (11.3% by sleep diary, 0.6% by actigraphy), making it difficult to determine whether long sleep was a risk factor of cold incidence. The small sample of long sleepers in this study may be due to the fact that the study sample was carefully screened to meet good health standards, including being free from psychiatric illness.
Self-reported diary measures of duration and sleep efficiency were unrelated to cold incidence. This is in contrast to our prior work that found that poorer sleep efficiency and shorter sleep duration, measured via a 14-day daily interview, predicted cold susceptibility.6 There are several possible explanations for differences across studies. First, fewer participants became infected in this sample, which may have limited our power to detect effects using self-report measures. Second, this study relied on a shorter 7-day sleep diary rather than a 14-day daily interview, which may have produced less stable averages as well as less accurate estimates of sleep. In regard to sleep estimates, employment of a daily interview in the prior study helped ensure timely assessments of self-reported sleep, which potentially decreased recall bias. Third, given that actigraphy has been well correlated with polysomnography,41 the gold standard of measurement in sleep research, it is also possible that had our prior study included actigraphy assessment concurrently with the daily interview sleep data, those findings would have been even more robust. Future studies incorporating both actigraphy and sleep diaries are needed to understand when and why certain sleep measures significantly predict immune function.
What are the mechanisms that might link sleep and susceptibility to acute infectious illness? Sleep, along with circadian rhythms, exerts substantial regulatory effects on the immune system.42,43 Circulating immune cells, including T and B cells, peak early in the night and then decline throughout the nocturnal hours moving out of circulation into lymphoid organs where exposure to virally infected cells occur.43–45 Studies employing experimental sleep loss also support functional changes relevant to host resistance. Sleep deprivation results in down regulation in T cell production of interleukin-219,44 and a shift away from T-helper 1 responses, marked by a reduction in the ratio of interferon-γ/IL-4 production.16 Sleep loss is associated with diminished proliferative capacity of T cells in vitro15 as well as modulation of the function of antigen presenting cells critical to virus uptake.46
Illness expression in colds is generally attributed to blunted downregulation of local inflammatory responses.47,48 Emerging evidence demonstrates bidirectional links between sleep and inflammation.14,42,49 Proinflammatory activity has a role in the homeostatic regulation of sleep.50,51 Likewise, some but not all studies that employ partial and total sleep restriction find substantial increases in systemic levels of proinflammatory cytokines52 as well as enhanced inflammatory gene expression and transcriptional pathways that support inflammatory processes.20,21 In addition, recent evidence suggests that elevated systemic inflammation mediates prospective associations between short sleep duration and premature mortality.53 Future studies characterizing the immunologic mediators of cold incidence in the context of sleep duration and our viral challenge paradigm are needed to clarify when in the infection process sleep has the most potent effects.
Like prior work, we find that infectious risk is strongest in the shortest of sleepers, suggesting that “normal” sleepers (e.g., 7 to 9 h per night for adults) would be protected in this context. Whether sleep interventions aimed at increasing sleep duration would protect individuals from cold incidence remains an open question. In this regard, recent findings that cognitive behavioral therapy for older adults with insomnia resulted in decreased levels of systemic inflammation54 raises the possibility that a similar enhancement in cell-mediated immunity could also be observed. Given that infectious illness (i.e., influenza and pneumonia) remains one of the top 10 leading causes of death in the United States,55 the current data suggest that a greater focus on sleep duration, as well as sleep health more broadly,56 is indicated.
In summary, these novel findings provide the first evidence that sleep duration assessed behaviorally through actigraphy predicts incidence of infectious illness using an experimental viral challenge. Although this study does not provide direct evidence of causality, the prospective nature of the viral challenge design does eliminate concerns of reverse causation. It is recognized that actigraphy is a behavioral measure of rest/ activity patterns and is not an objective measure of sleep per se. Although actigraphy has been shown to correlate well with polysomnography in healthy samples,41 actigraphy-assessed indices of sleep duration cannot identify specific dimensions of sleep (e.g., decreased slow wave sleep) that may be contributing to infectious risk. In addition, future studies investigating the immunologic mechanisms underlying these effects as well as generalizability of these findings to other samples (i.e., older adults; sleep disordered patients) are warranted.
DISCLOSURE STATEMENT
This was not an industry supported study. Preparation of this paper was supported by the National Center for Complementary and Alternative Medicine (AT006694), and data collection by the National Institute of Allergy and Infectious Diseases (AI066367). Clinical and regulatory assistance for the study was provided by National Institute of Health grants (UL1 RR024153 and UL1 TR000005) to the University of Pittsburgh Clinical and Translational Science Institute. Dr. Prather's participation was supported by a grant from the National Heart, Lung, & Blood Institute (K08HL112961). The authors have indicated no financial conflicts of interest.
Footnotes
A commentary on this article appears in this issue on page 1341.
REFERENCES
- 1.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207–16. doi: 10.1046/j.1365-2796.2002.00941.x. [DOI] [PubMed] [Google Scholar]
- 3.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28:2762–7. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 4.King CR, Knutson KL, Rathouz PJ, Sidney S, Liu K, Lauderdale DS. Short sleep duration and incident coronary artery calcification. JAMA. 2008;300:2859–66. doi: 10.1001/jama.2008.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel SR, Malhotra A, Gao X, Hu FB, Neuman MI, Fawzi WW. A prospective study of sleep duration and pneumonia risk in women. Sleep. 2012;35:97–101. doi: 10.5665/sleep.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–7. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–4. [PubMed] [Google Scholar]
- 8.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 9.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin MR. Why sleep Is important for health: a psychoneuroimmunology perspective. Annu Rev Psychology. 2015;66:143–72. doi: 10.1146/annurev-psych-010213-115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265:R1148–54. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 14.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nature reviews. Immunology. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 15.Bollinger T, Bollinger A, Skrum L, Dimitrov S, Lange T, Solbach W. Sleep-dependent activity of T cells and regulatory T cells. Clin Exp Immunol. 2009;155:231–8. doi: 10.1111/j.1365-2249.2008.03822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimitrov S, Lange T, Tieken S, Fehm HL, Born J. Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain Behav Immunity. 2004;18:341–8. doi: 10.1016/j.bbi.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sakami S, Ishikawa T, Kawakami N, et al. Coemergence of insomnia and a shift in the Th1/Th2 balance toward Th2 dominance. Neuroimmunomodulation. 2002;10:337–43. doi: 10.1159/000071474. [DOI] [PubMed] [Google Scholar]
- 18.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–8. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–53. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 20.Irwin M, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–62. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 21.Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–40. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 23.Moldofsky H, Lue FA, Davidson JR, Gorczynski R. Effects of sleep deprivation on human immune functions. FASEB J. 1989;3:1972–7. doi: 10.1096/fasebj.3.8.2785942. [DOI] [PubMed] [Google Scholar]
- 24.Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35:1063–9. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychol. 2008;27:268–74. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 27.Cohen S, Alper CM, Doyle WJ, Treanor JJ, Turner RB. Positive emotional style predicts resistance to illness after experimental exposure to rhinovirus or influenza a virus. Psychosom Med. 2006;68:809–15. doi: 10.1097/01.psy.0000245867.92364.3c. [DOI] [PubMed] [Google Scholar]
- 28.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–7. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 29.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–55. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 31.Goldberg LR. The intenational personality item pool and the future of public-domain personality measures. J Research Personality. 2006;40:84–96. [Google Scholar]
- 32.Gwaltney JM, Jr., Colonno RI, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NI, Ernmons RW, editors. Diagnostic procedures for viral, rickettsial, and chlaymdial infections. 6th ed. Washington, DC: American Public Healh Association; 1989. pp. 579–614. [Google Scholar]
- 33.Doyle WJ, McBride TP, Skoner DP, Maddern BR, Gwaltney JM, Jr, Uhrin M. A double-blind, placebo-controlled clinical trial of the effect of chlorpheniramine on the response of the nasal airway, middle ear and eustachian tube to provocative rhinovirus challenge. Pediatr Infect Dis J. 1988;7:229–38. doi: 10.1097/00006454-198803000-00033. [DOI] [PubMed] [Google Scholar]
- 34.Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979–85. doi: 10.1016/s0895-4356(01)00372-9. [DOI] [PubMed] [Google Scholar]
- 35.Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–21. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- 36.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–9. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 37.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26:380–4. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 38.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandner MA, Kripke DF. Self-reported sleep complaints with long and short sleep: a nationally representative sample. Psychosom Med. 2004;66:239–41. doi: 10.1097/01.psy.0000107881.53228.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep. 2006;29:881–9. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 42.Opp MR, Born J, Irwin MR. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. 4th ed. New York, NY: Academic Press; 2007. pp. 570–618. [Google Scholar]
- 43.Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- 44.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158:4454–64. [PubMed] [Google Scholar]
- 45.Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch. 2012;463:121–37. doi: 10.1007/s00424-011-1044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimitrov S, Lange T, Nohroudi K, Born J. Number and function of circulating human antigen presenting cells regulated by sleep. Sleep. 2007;30:401–11. doi: 10.1093/sleep/30.4.401. [DOI] [PubMed] [Google Scholar]
- 47.Proud D, Gwaltney JM, Jr, Hendley JO, Dinarello CA, Gillis S, Schleimer RP. Increased levels of interleukin-1 are detected in nasal secretions of volunteers during experimental rhinovirus colds. J Infect Dis. 1994;169:1007–13. doi: 10.1093/infdis/169.5.1007. [DOI] [PubMed] [Google Scholar]
- 48.Zhu Z, Tang W, Ray A, et al. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J Clin Invest. 1996;97:421–30. doi: 10.1172/JCI118431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–64. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci. 2001;933:211–21. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- 51.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nature reviews. Immunology. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solarz DE, Mullington JM, Meier-Ewert HK. Sleep, inflammation and cardiovascular disease. Front Bioscience. 2012;4:2490–501. doi: 10.2741/e560. [DOI] [PubMed] [Google Scholar]
- 53.Hall MH, Smagula SF, Boudreau RM, et al. Association between sleep duration and mortality is mediated by markers of inflammation and health in older adults: the health, aging and body composition study. Sleep. 2015;38:189–95. doi: 10.5665/sleep.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin MR, Olmstead R, Carrillo C, et al. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. 2014;37:1543–52. doi: 10.5665/sleep.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61:1–117. [PubMed] [Google Scholar]
- 56.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]


