Abstract
Study Objectives:
Obstructive sleep apnea syndrome (OSAS) has been associated with cardiometabolic disease in adults. In children, this association is unclear. We evaluated the effect of early adenotonsillectomy (eAT) for treatment of OSAS on blood pressure, heart rate, lipids, glucose, insulin, and C-reactive protein. We also analyzed whether these parameters at baseline and changes at follow-up correlated with polysomnographic indices.
Design:
Data collected at baseline and 7-mo follow-up were analyzed from a randomized controlled trial, the Childhood Adenotonsillectomy Trial (CHAT).
Setting:
Clinical referral setting from multiple centers.
Participants:
There were 464 children, ages 5 to 9.9 y with OSAS without severe hypoxemia.
Interventions:
Randomization to eAT or Watchful Waiting with Supportive Care (WWSC).
Measurements and Results:
There was no significant change of cardiometabolic parameters over the 7-mo interval in the eAT group compared to WWSC group. However, overnight heart rate was incrementally higher in association with baseline OSAS severity (average heart rate increase of 3 beats per minute [bpm] for apnea-hypopnea index [AHI] of 2 versus 10; [standard error = 0.60]). Each 5-unit improvement in AHI and 5 mmHg improvement in peak end-tidal CO2 were estimated to reduce heart rate by 1 and 1.5 bpm, respectively. An increase in N3 sleep also was associated with small reductions in systolic blood pressure percentile.
Conclusions:
There is little variation in standard cardiometabolic parameters in children with obstructive sleep apnea syndrome (OSAS) but without severe hypoxemia at baseline or after intervention. Of all measures, overnight heart rate emerged as the most sensitive parameter of pediatric OSAS severity.
Clinical Trial Registration:
Clinicaltrials.gov (#NCT00560859)
Citation:
Quante M, Wang R, Weng J, Rosen CL, Amin R, Garetz SL, Katz E, Paruthi S, Arens R, Muzumdar H, Marcus CL, Ellenberg S, Redline S. The effect of adenotonsillectomy for childhood sleep apnea on cardiometabolic measures. SLEEP 2015;38(9):1395–1403.
Keywords: adenotonsillectomy, metabolism, obstructive sleep apnea syndrome, pediatrics
INTRODUCTION
Childhood Obstructive Sleep Apnea Syndrome (OSAS) affects 1% to 6% of young children, and is associated with a wide range of adverse health outcomes, including behavior problems and impaired growth.1 In adult OSAS, cardiometabolic disorders, including hypertension, diabetes, coronary heart disease, heart failure, stroke, and atrial fibrillation, are widely recognized comorbidities and targets for intervention, with evidence that positive airway pressure (PAP) therapy may improve insulin resistance and blood pressure control and reduce cardiovascular events.2–7 In children, few studies have addressed the association between cardiometabolic dysfunction and OSAS8–25 or the effect of OSAS treatment on outcomes such as blood pressure, lipids, and glucose metabolism. Some studies have demonstrated improvements in lipid profiles, liver function tests, C-reactive protein (CRP), apolipoprotein B, and blood pressure control after adenotonsillectomy (AT),26–30 whereas other studies failed to show an effect of treatment.31,32 These reports have been limited by nonrandomized and uncontrolled designs, and by relatively small sample sizes, long re-study intervals, and study of heterogeneous populations and wide age ranges.
We analyzed data from the Childhood Adenotonsillectomy Trial (CHAT), a randomized controlled multicenter study of health and behavioral outcomes in children with OSAS, randomized to early AT (eAT) or to Watchful Waiting with Supportive Care (WWSC). The primary results of the trial's neurocognitive outcomes were previously reported.33 In this article, we report on the trial's secondary outcomes that addressed cardiometabolic health. We hypothesized that treatment of OSAS in children by eAT would reduce systolic, diastolic, and mean resting blood pressure and heart rate during sleep and wakefulness; improve lipid profiles and glucose homeostasis; and lower levels of inflammatory markers. Secondarily, we hypothesized that these parameters at baseline, as well as changes at follow-up, would correlate with indices of sleep disordered breathing (SDB) and sleep quality. Given the potential for subgroup differences in response to treatment, we also explored differences in groups stratified by race, OSAS severity, and body mass index (BMI).
METHODS
Study Sample
The design of the CHAT study has been previously described and the primary outcomes have been published.33–35 In brief, children ages 5.0–9.9 y with polysomnography (PSG) confirmed OSAS (obstructive apnea-hypopnea index [AHI] ≥ 2 events/h or an obstructive apnea index [OAI] ≥ 1 event/h), a history of snoring, and considered to be surgical candidates for adenotonsillectomy by an otolaryngologist were recruited from pediatric sleep centers/sleep laboratories, otolaryngology clinics, general pediatric clinics, and the general community from six clinical centers. Exclusion criteria included comorbidities, medications for psychiatric or behavioral disorders (including attention deficit hyperactivity disorder), recurrent tonsillitis, extreme obesity (defined by a body mass index > 2.99 for age group and sex, z-score) and severe OSAS (AHI ≥ 30, OAI ≥ 20 or oxyhemoglobin saturation of < 90% for > 2% of total sleep time). The study was approved by the Institutional Review Board of each institution. Informed consent was obtained from caregivers, and assent from children age 7 y or older. The study was registered at Clinicaltrials.gov (#NCT00560859). Figure 1 summarizes the flow of participants through the study.
Figure 1.
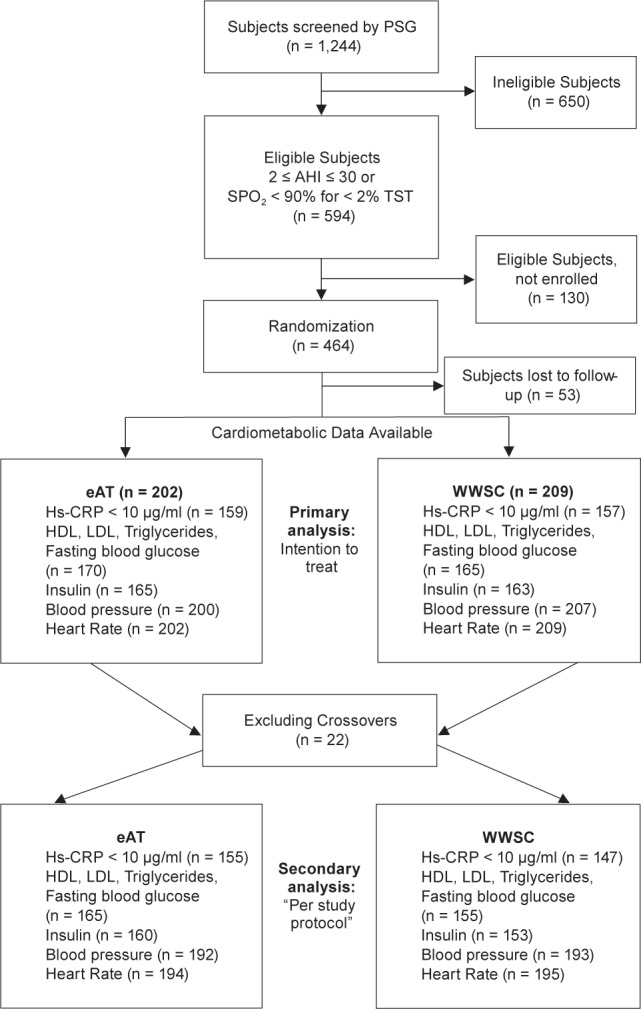
Consort diagram indicating flow of participants. eAT, early adenotonsillectomy; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; PSG, polysomonography; SpO2, saturation of peripheral oxygen; TST, total sleep time; WWSC, Watchful Waiting with Supportive Care.
Interventions
Children were randomly assigned to eAT (surgery within 4 w after randomization) or a strategy of WWSC with reassessment for the need for surgery at 7 mo. All children/caregivers received information on sleep hygiene using standardized educational materials, were provided with saline nasal spray to be used as needed, and were evaluated and referred for treatment of comorbid conditions (e.g., asthma). Complete bilateral tonsillectomy and removal of obstructing adenoid tissue was performed by standard surgical techniques including cold dissection, monopolar electrocautery, coblation or microdebrider, with variation according to surgeon preference and not by patient characteristics. To ensure surgical uniformity across participating sites, intraoperative photographs were obtained on every 10th subject at each site, and were reviewed for adequacy of lymphoid tissue removal by the surgical quality control committee chair.
Protocol
Each child underwent in-laboratory baseline and follow-up PSG by study-certified technicians, using similar sensors, and following American Academy of Sleep Medicine (AASM) pediatric guidelines for both acquisition and for scoring.36 The PSGs were scored by registered sleep technicians who were specifically certified for this study after undergoing study-specific training and certification. Each PSG record was edited manually for muscle, movement, and electrical artifacts. A research clinic visit was scheduled soon after the baseline PSG, at which time certified research technicians and research nurses performed anthropometry, measured blood pressure, and obtained a fasting venous sample. Site investigators performed a physical examination. The investigators as well as CHAT study personnel involved with primary data collection were blinded to all study results. Only the study coordinator and the ear, nose, and throat surgeons were unblinded to the treatment group. Resting blood pressure was measured in triplicate after a 10-min rest period while sitting using a calibrated sphygomanometer with a cuff size chosen based on the child's arm circumference. Resting heart rate was obtained during the physical examination. Height was measured with a calibrated wall-mounted stadiometer while the child was in his/ her stocking feet; weight was measured with research quality, calibrated digital scales. All of these measurements were taken by CHAT certified site personnel in a standardized manner according to written guidelines. Caregivers were asked to complete questionnaires addressing the child's medical, social, and family histories. Sleep duration was obtained from a parent-completed 7-day sleep diary. Fasting morning blood samples were drawn by venipuncture, processed and stored frozen until assayed in batch in a centralized laboratory (Laboratory for Clinical Biochemistry, University of Vermont, Burlington, VT). The median timing between baseline PSG and research clinic visit was 28 days. Time delays largely related to scheduling requirements (family, child, and staff availability.) Measurements were repeated approximately 7 mo after randomization following a protocol similar to that of the baseline examination.
Measurements
The PSG measures for this analysis consisted of AHI, expressed as the sum of all obstructive apneas plus hypopneas associated with > 3% desaturation or arousal per hour of sleep; oxygen de-saturation index (ODI), defined as the number of oxyhemoglobin desaturations > 3% per sleep hour; peak end-tidal carbon dioxide (EtCO2); % total sleep time (TST) with EtCO2 > 50 mmHg; oxygen saturation ≤ 92% of the TST, computed as the percentage of sleep time with oxygen saturation ≤ 92%; and proportion of sleep time in stages N2, N3, and rapid eye movement (REM). The average values of triplicate determinations for systolic and diastolic blood pressure were calculated and expressed as sex-, age-, and height-specific blood pressure percentiles.37 BMI was calculated and converted to an age- and sex-adjusted BMI z-score based on the Centers for Disease Control and Prevention (CDC) growth charts (http://www.cdc.gov/growthcharts/). Weight was categorized based on percentiles for age and sex as follows: failure to thrive, < 5th percentile; normal, ≥ 5th and < 85th; overweight, ≥ 85th and < 95th; and obese, ≥ 95th.38 Mean values for heart rate were obtained from PSG software (electrocardiogram or pulse oximeter) by averaging across the sleep period. Resting heart rate during wakefulness was determined by measurement of the radial pulse during a 1-min recording made during the morning research examination. Blood was assayed for glucose and lipids (cholesterol, low-density lipoprotein [LDL] cholesterol, high-density lipoprotein [HDL] cholesterol, and triglycerides; Ortho Vitros Clinical Chemistry System 950/IRC instrument, Raritan, NJ), high sensitivity CRP [hs-CRP] (Siemens BNII analyzer, Deerfield, IL), and fasting insulin (Roche Elecsys 210 analyzer, Indianapolis, IN) levels. The interassay coefficients of variation were: glucose and lipids, < 2%; insulin, < 5%; high-sensitivity C-reactive protein (hs-CRP): 2.1–5.7%. Hs-CRP levels that were initially undetectable were repeated using a solid phase-based multiplex platform from Meso Scale Discovery (Gaithersburg, MD) (n = 156). Hs-CRP values exceeding 10 μg/mL, which likely indicate infection or acute illness, were excluded from analysis. Biochemical data were missing for approximately 20% of subjects due to specimen collection issues (child refusal, nonfasting state, inadequate blood draw). A comparison of these children to the remaining children with respect to age, sex, AHI value, and BMI-z-score indicated no differences according to missing data status.
Statistical Analysis
Demographic, anthropometric, and cardiometabolic characteristics were summarized and compared between the groups utilizing analysis of variance (ANOVA) or chi-square and Fisher exact tests. Cardiometabolic characteristics were also compared with published normative data in pediatric populations.39–41 Unadjusted associations were assessed using Spearman correlation coefficients. Outcome measurements were hs-CRP, LDL cholesterol, HDL cholesterol, triglycerides, fasting blood glucose, fasting insulin, average heart rate during total sleep, REM sleep and non-REM sleep and during wake, and systolic and diastolic blood pressure percentile. Independent variables were treatment assignment (eAT, WWSC), AHI, ODI, peak EtCO2, EtCO2 > 50 mmHg, oxygen saturation ≤ 92%, stage N1, N2, N3, and REM sleep. To address each of the study hypotheses, we conducted three series of analyses: The initial analysis assessed the association between sleep variables and cardiometabolic variables at baseline using multiple linear regression analysis. To evaluate the effect of intervention on cardiometabolic outcomes, we conducted an analysis of covariance (ANCOVA) for change in outcomes by treatment group. Finally, we used linear regression analysis to model the associations between changes in cardiometabolic outcomes in relationship to changes in indices of OSAS and sleep quality. Stratified analyses further explored groups defined by race, median baseline AHI, and median BMI z-score change. Analyses did not include total cholesterol or glucose homeostasis model assessment, because of their strong correlation with LDL cholesterol and insulin, respectively. All statistical models were adjusted for the trial's stratification variables: age (5 to 7 y of age vs. 8 to 9 y of age), race (African American vs. other), overweight/obesity status, and study site. Average heart rate during REM and non-REM sleep was only reported if results differed from average heart rate during total sleep. The primary analysis was an intention-to-treat analysis. Per-protocol analyses (i.e., excluding the 22 children who did not receive the assigned therapy) were performed, but not presented as they showed findings similar to the primary analysis. No corrections were made for multiple comparisons. Results are presented as mean ± standard deviation. All analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
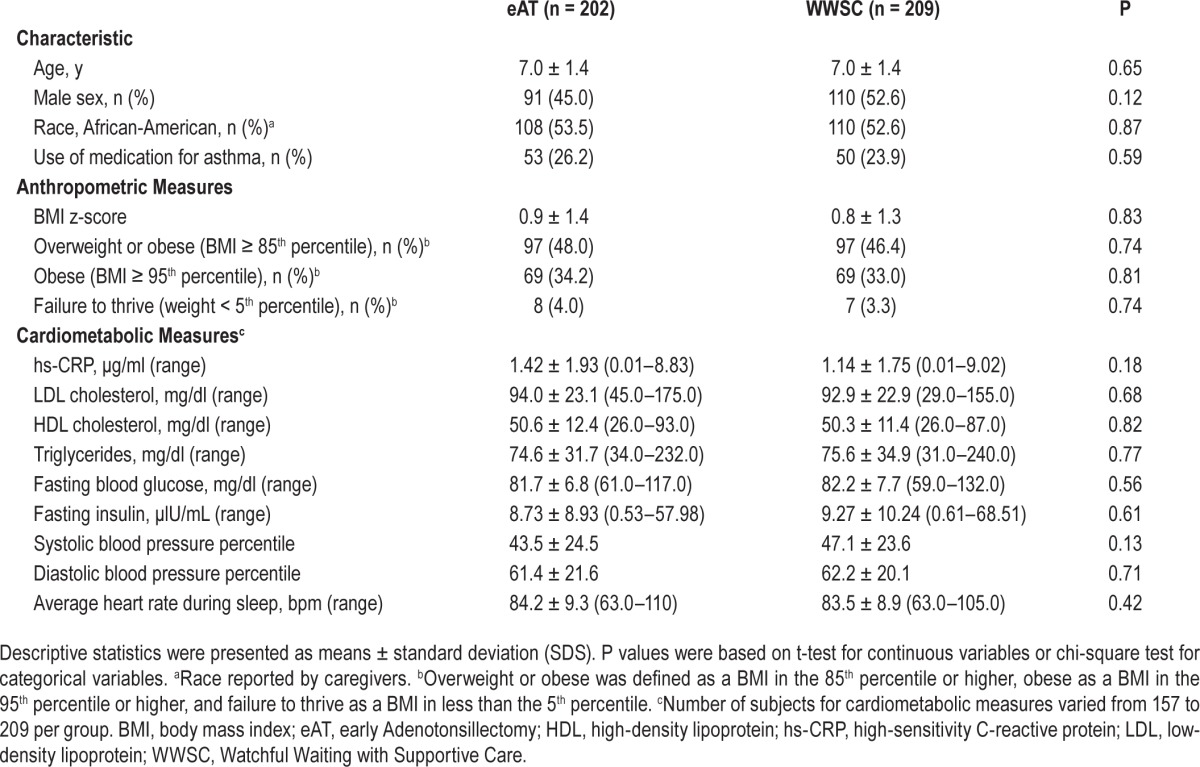
Characteristics for the analytic sample at baseline and by intervention group are shown in Table 1. Approximately half of the subjects were male and half were overweight or obese. Baseline anthropometric, sleep, and cardiometabolic characteristics were comparable across intervention groups. On average, compared with published normative data in pediatric populations, 7% of overall lipid levels, 8% of markers of glycemic control, and 3% of blood pressure values exceeded the normal range for age and sex.39–41 Thus, although there was a high proportion of overweight or obese participants, relatively few participants had indices of cardiometabolic health that exceeded normative values. As reported before, over the intervention period, key measures of OSAS improved more while BMI z-score increased more in the eAT compared to the WWSC group.33,35 (Table S1, supplemental material)
Table 1.
Baseline characteristics of subjects.
Associations between Baseline PSG Indices and Study Outcomes
Adjusted linear regression analysis of the baseline data with cardiac parameters as the outcomes showed that AHI, ODI, EtCO2 > 50 mmHg, and stage N1 sleep were positively associated with average heart rate during total sleep, REM sleep, and non-REM sleep, but not with wake heart rate (Table 2). Specifically, an AHI of 2 versus 10 was associated with an average sleep heart rate increase of 3 bpm (standard error [SE] = 0.60). Further adjustment for percentage of REM sleep had no effect on these associations (data not shown). Lower proportions of time in stages N2 and REM sleep and higher proportions of N1 and N3 were associated with higher average heart rate during sleep. There were no significant associations between sleep indices (stage N1, stage N2, stage N3, REM sleep, AHI, ODI, EtCO2 > 50 mmHg, and oxygen saturation ≤ 92%) and systolic and diastolic blood pressure percentile.
Table 2.
Associations of baseline cardiac parameters with sleep measures.
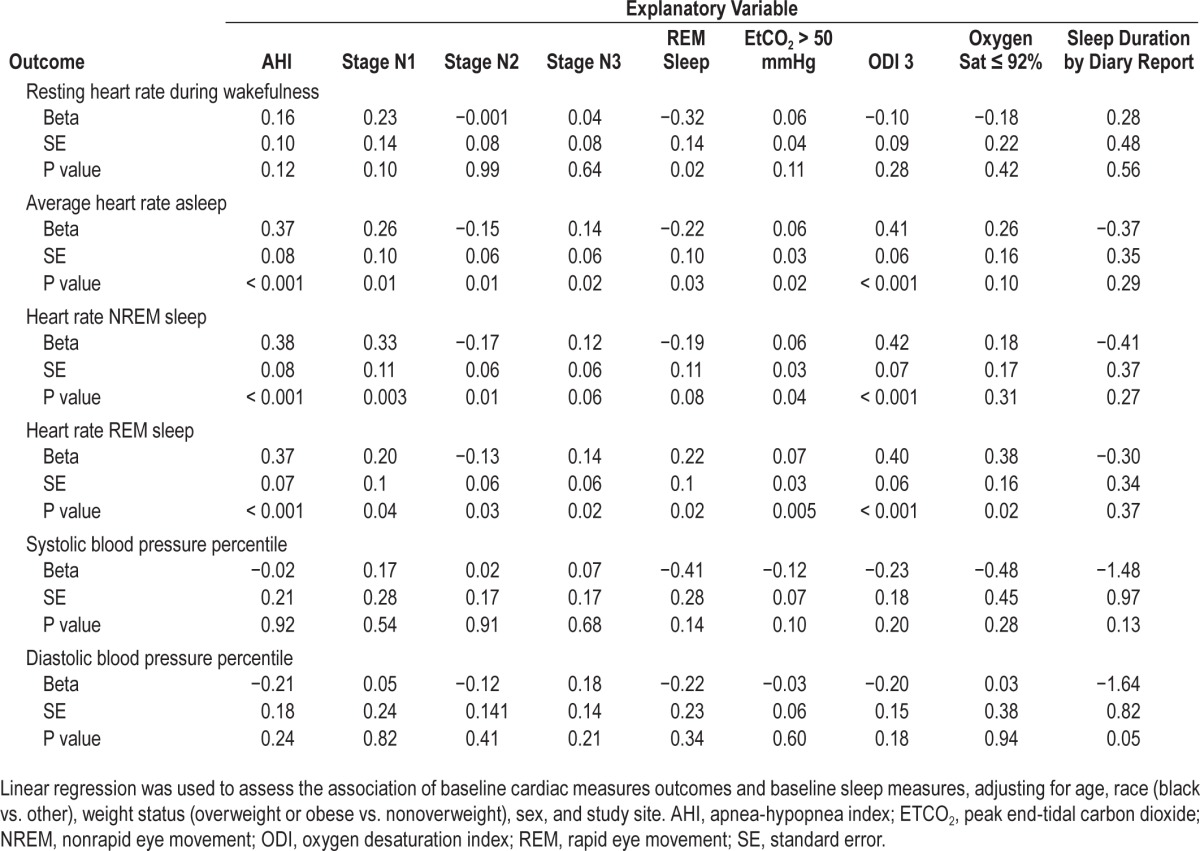
Regarding metabolic outcomes, no associations were observed between various baseline sleep measures with LDL cholesterol, HDL cholesterol, hs-CRP, and fasting blood glucose other than for triglyceride level. Triglycerides level was positively associated with stage N3 (β = 0.735, SE = 0.23, P = 0.002,) and negatively with stage N2 sleep (β = −0.618, SE = 0.23, P = 0.009).
Treatment Effect on Study Outcomes
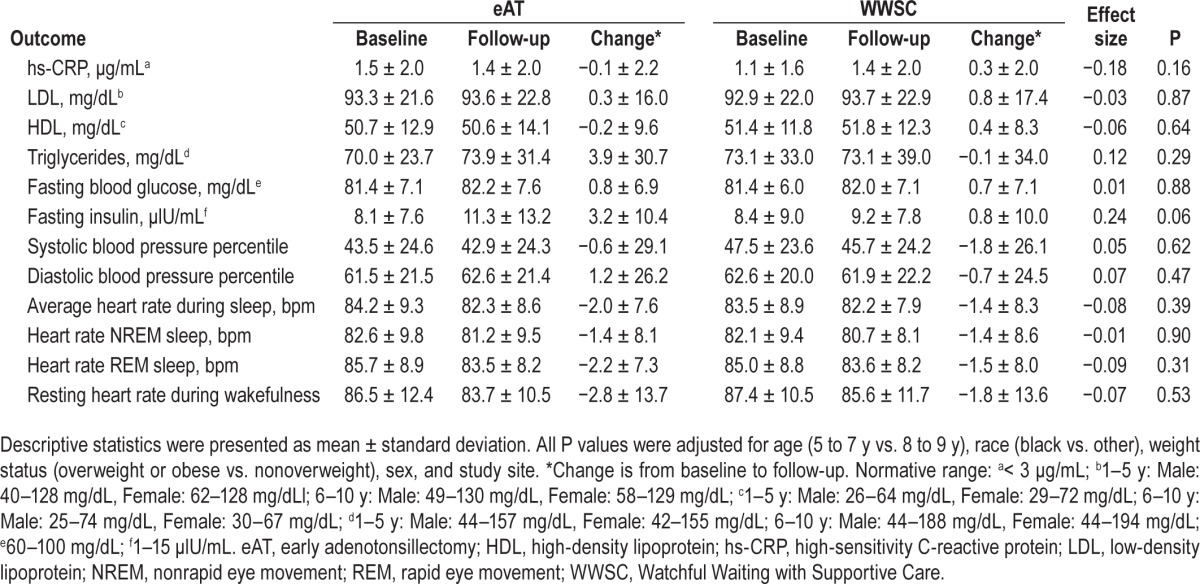
Fasting LDL cholesterol, blood glucose, and insulin tended to increase during the study interval in both eAT and WWSC groups, whereas heart rate during sleep and systolic blood pressure percentile decreased. After adjusting for the trial's stratification factors, no significant intervention effect was observed for these cardiometabolic measures (Table 3). Additional exploratory analyses were conducted to assess if there was subgroup differences in treatment response by race (black vs. other), baseline median AHI (4.7) and median BMI z-score change. These results also showed no treatment effect on cardiometabolic outcomes in any of these subgroups (data not shown).
Table 3.
Change of cardiometabolic measures from baseline to follow-up in the early Adenotonsillectomy compared to the Watchful Waiting with Supportive Care group.
Follow-up Analysis of Associations between Change in Polysomnographic Indices and Change in Study Outcomes
Comparable associations between change in sleep measures and change in cardiac measures were observed in analysis of each intervention group separately. We found no interaction between treatment and any of the predictors (data not shown). Therefore, data from both groups were combined in subsequent multiple regression analysis modeling with the change in cardiac measures as the outcome, adjusting for age, race, baseline overweight/ obesity status, sex, and study site. Positive associations between change in AHI, peak EtCO2 and EtCO2 > 50 mmHg with change in average heart rate were observed (Table 4). Similar findings were observed for ODI and oxygen saturation ≤ 92%. Specifically, each 5-unit improvement in AHI and 5 mmHg improvement in peak EtCO2 were estimated to reduce heart rate during sleep by 1 and 1.5 bpm, respectively. We also observed similar associations for resting heart rate during wakefulness with AHI and ODI but not with peak EtCO2 and EtCO2 > 50 mmHg (β = 0.317, SE = 0.08, P = < 0.001 and β = 0.287, SE = 0.08, P = < 0.001, for AHI and ODI, respectively).
Table 4.
Associations between changes from baseline to follow-up in cardiac measures and changes in corresponding sleep measures.
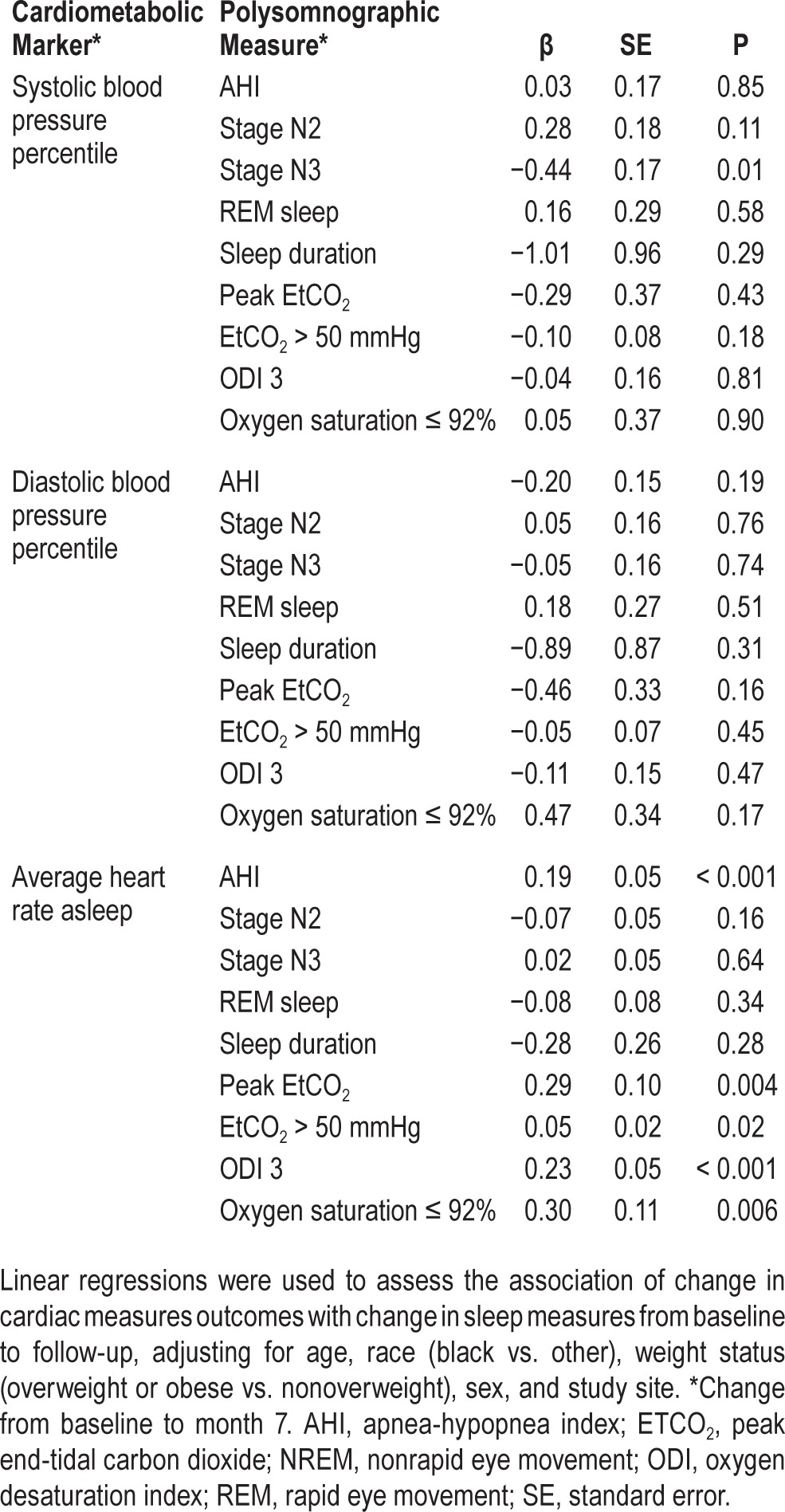
An increased proportion of time in stage 3 sleep from baseline to follow-up was associated with reduction in systolic blood pressure percentile. Further adjustment for percentage of REM sleep had no effect on these associations (data not shown).
No associations were observed between change in AHI, stage N2, stage N3, and REM sleep with any of the metabolic measures.
In an exploratory analysis assessing change in cardiometabolic parameters from baseline to follow-up within the 253 children who had “resolution of OSAS” (reduction in AHI to < 2 and apnea index to < 1), we found significantly increased levels of insulin and decreased heart rate (heart rate during sleep and resting heart rate during wakefulness) following intervention (P = 0.004 and P = < 0.001).
DISCUSSION
In this large, randomized controlled trial of eAT for PSG-confirmed childhood OSAS, we found that eAT compared to the WWSC group did not result in a significant change in fasting glucose, insulin, lipids, hs-CRP, blood pressure or heart rate over a 7-mo intervention period in children ages 5 to 9 y, the majority of whom had normal cardiometabolic parameters at baseline examination. We also did not observe significant correlations between polysomnographic indices and cardiometabolic variables other than an association between measures of OSAS severity (AHI, ODI, EtCO2) and heart rate in sleep (particularly REM sleep). We also observed that over the 7-mo intervention period, improvements in measures of oxygen saturation and end tidal CO2 levels were associated with decreases in heart rate during sleep. Wake heart rate also improved in association with improved AHI and ODI. These results indicate that of all the measured cardiometabolic parameters, relatively simple indices of average heart rate during wakefulness and sleep were sensitive to OSAS severity. We also observed several interesting associations between N3 sleep and cardiometabolic parameters. In particular, increases in stage N3 sleep over the intervention period were associated with decreases in systolic blood pressure. However, the positive correlation between stage N3 sleep and triglyceride level was unexpected and of unclear significance.
Animal, adult, and pediatric studies implicate intermittent hypoxemia and sleep fragmentation in the pathogenesis of OSAS-related dysregulation of blood pressure, autonomic function, inflammation, and glucose and lipid metabolism.9,17–23,42–45 Because children with severe hypoxemia were excluded from our study, it is possible that the lack of a significant effect of eAT on cardiometabolic outcomes reflects the modest level of hypoxemia in the CHAT sample. Our findings are not inconsistent with the limited and observational literature, which has shown variable cardiometabolic effects of treatment in children with OSAS. A study of 62 obese and nonobese children with OSAS reported significant improvements in serum lipid profiles and CRP after surgical removal of hypertrophic tonsils and adenoids in the entire cohort. However, significant improvements of insulin were only present in obese children.26 Another small study found that children with resolution of OSAS (either by AT, positive pressure ventilation, or spontaneously) experienced a small but significant decrease in total cholesterol levels. However, no changes in insulin, glucose, triglycerides, or HDL cholesterol could be shown, whether or not treatment occurred.27 Li et al.46 demonstrated a significant reduction of CRP following treatment (AT, nasal corticosteroids, or positive pressure ventilation) in 16 children with OSAS, whereas lipid profiles remained unchanged. Similar to the findings of two other studies, we could not find a significant association between severity of OSAS with levels of fasting insulin and glucose.47,48 Shamsuzzaman et al.24 found that ODI rather than AHI was associated with increased insulin resistance in children with OSAS, an interesting result that could not be replicated in our study. One study on long-term outcomes of children with OSAS of whom 29% were treated with either AT, nasal steroids or a combination of treatments, including a nonsnoring control group, revealed that improvement of SDB (spontaneously or as a result of treatment) was associated with improved baroreflex control of blood pressure. However, there was no difference in either wake or overnight systolic blood pressure in the control, resolved, and unresolved groups at follow-up, which is in line with our study results.29 In contrast to our study, most prior research reported on patients with more severe OSAS, were not randomization trials, and did not robustly consider potential confounders.
In contrast to a null effect of eAT on cardiometabolic outcomes, we observed that improvement in AHI (or ODI or EtCO2) and increased stage N3 sleep in the intervention period was associated with reduction in heart rate and systolic blood pressure, respectively. The more significant associations observed for changes in PSG parameters than an effect of intervention per se (eAT vs. WWSC) may reflect the fact that there was within-group heterogeneity in changes in OSAS severity over the intervention period. In particular, 46% of children in the WWSC group had resolution of their OSAS and 21% of the eAT group had persistence of OSAS at follow-up, potentially reducing the between-group differences for the surgical and control arms.33 Furthermore, the eAT group gained more weight than the WWSC group,35 and weight gain may have confounded other treatment effects. Consistent with this notion, insulin sensitivity as measured by fasting insulin and glucose showed a trend toward worsening in the eAT group compared to the WWSC group at follow-up, and in fact was significantly increased in the subgroup in whom OSAS was resolved.
The sensitivity of change in heart rate to improvement in OSAS indices across the study period was consistent with the results of the baseline analysis showing that higher AHI, ODI, and EtCO2 were each associated with higher heart rate. Heart rate has been described to be elevated in association with respiratory disturbances.2,30,49 Recently, Walter et al.25 comprehensively investigated nocturnal autonomic function in school children with SDB. In all sleep stages, children with moderate to severe OSAS had significantly higher heart rates compared with the control group. Moreover, children with SDB showed a significant lower heart rate variability during sleep than control children without SDB.25 In another study, Muzumdar et al.30 showed a significant reduction in heart rate during sleep in association with a reduction in AHI after adenotonsillectomy with a decrease in sympathetic balance as demonstrated by heart rate variability. It has been proposed that an accelerated heart rate induced by sympathetic nervous system over activity is responsible for the reported risk of long-term cardiovascular events and all-cause mortality.50,51 Our results extend those reports by showing that OSAS is associated not only with heart rate measures during sleep as well as during wakefulness, albeit changes are small.
Increase in stage N3 sleep (also known as slow wave sleep; SWS) from baseline to post intervention was associated with a decrease in systolic blood pressure percentile. Stage N3 sleep is when parasympathetic tone is highest and sympathetic tone the lowest, corresponding to the stage when heart rate and blood pressure decline. In adults, decreased SWS has been linked to incident hypertension in older men.52 In children, support for a relationship between blood pressure and SWS can be found in a recent study from Hannon et al.53 that reported an association between decreased amounts of REM and N3 sleep with greater morning blood pressure in obese adolescents.
In baseline analyses, stages N1 and N3 sleep were positively associated with heart rate while stages N2 and REM sleep were negatively associated with heart rate. Although speculative, it is possible that these associations reflect differences in homeo-static drive (i.e., resulting in more N3 sleep when studied in the laboratory) in some of the children who may be sleep deprived and have elevated sympathetic tone. Because sleep architecture changes and cardiometabolic function changes with puberty, it is also possible that pubertal influences may have confounded this association.54,55 Although our analysis controls for age and sex and analyzed children younger than 9 y, there is a trend for earlier puberty, especially among African Americans who constituted a majority of our sample.56
This study has a number of significant strengths, including its large sample, inclusion of a racially diverse group, randomized design, and rigorous collection of measurements. However in the interpretation of our findings, several limitations must be considered. First, cardiometabolic parameters were assessed on only two time points, 6 to 7 mo apart. Measurement variability could bias the findings toward the null. It is possible that associations between cardiometabolic parameters and sleep indices may be stronger or weaker at later time points. Second, findings are applicable to children with mild to moderate levels of OSAS and should not be generalized to children who are markedly hypoxemic. Furthermore, results should not be generalized to primary snorers or those with upper airway resistance syndrome. Finally, analyses were not subject to correction for multiple comparisons, so that the likelihood of false positive findings is increased. The analyses in this paper were secondary to the primary results of the CHAT study, and thus require future independent replication.
In summary, this study evaluated the effect of eAT for OSAS on cardiometabolic parameters using a randomized controlled design. In contrast to our expectations, changes in levels of glucose, lipids, insulin, CRP, blood pressure, and heart rate were not different between eAT and WWSC groups. However, we identified the potential sensitivity of heart rate as an index of OSAS severity and a measure of responsiveness to OSAS improvement. We also identified a negative association between N3 sleep and lower blood pressure, a finding previously reported in adults. Because elevated heart rate and blood pressure are associated with increased cardiovascular risk, our results support further research aimed at clarifying the role of OSAS and its treatment of cardiovascular health of children.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr Redline has received support from ResMed Foundation and equipment donation from ResMed Inc. and Philips Respironics. Dr Marcus had the use of research equipment loaned to her by Philips Respironics and Ventus for research unrelated to this study. Dr Rosen has consulted for Jazz Pharmaceuticals, Natus Medical, and Advance-Medical. Dr Ellenberg has received grant support from Abbvie Pharmaceuticals; has lectured for Janssen Pharmaceuticals and Merck; has consulted for Intermune, GSK, Salix Pharmaceuticals, Merck, Philips Lytle, and Chelsea Pharmaceuticals; and has provided data monitoring committee service for Bristol-Myers Squibb. Her spouse has consulted for Entero-medics. Dr Paruthi has received royalties from UpToDate. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Participating sites: Children's Hospital of Philadelphia, Philadelphia, PA; Cincinnati Children's Hospital Medical Center, Cincinnati, OH; Rainbow Babies and Children's Hospital, Cleveland, OH; Boston Children's Hospital, Boston, MA; Cardinal Glennon Children's Hospital, St. Louis, MO; and Montefiore Medical Center, Bronx, NY
The CHAT gratefully acknowledges the superb support of the CHAT research staff: Jean Arnold, Mary Ellen Carroll, Mary Anne Cornaglia, Beth Ann Compton, Casey Critchlow, Judith Emancipator, Melissa Fernando, Theresa Friederich, Amanda Goodman, Xiaoling Hou, Elise Hodges, Laurie Karamessinis, Kim Lacy, Megan McDougall, Daniel Mobley, Michelle Nicholson, Angela Orlando, Deborah L. Ruzicka, Gauri Sathe, Nancy Scott, Susan Surovec, Omarya Vega, Xingmei Wang, and Catherine Williams. We also appreciate the generous participation of the families enrolled in the study. We are grateful for the helpful guidance during the study of the CHAT Data and Safety Monitoring Board: Lynn Taussig, MD (Chair); Thomas Anders, MD; Julie Buring, ScD; Karina Davidson, PhD; Estelle Gauda, MD; Steven Piantadosi, MD, PhD; Bennett Shaywitz, MD; Benjamin Wilfond, MD; Tucker Woodson, MD; Robert Zeiger, MD.
Grant support: NIH grants: HL083075, HL083129, UL1 RR024134, UL1 RR024989; Max Kade Postdoctoral Research Exchange Grant, Max Kade Foundation, NY; Year 4 Within-Center Developmental Award, National Cancer Institute Centers for Transdisciplinary Research on Energetics and Cancer (TREC).
For the Childhood Adenotonsillectomy Trial (CHAT): Boston Children's Hospital, Harvard University, Boston, MA (Eliot Katz, MD; Janice Ware, PhD; Dwight Jones, MD); Brigham and Women's Hospital and Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA (Susan Redline, MD MPH; Rui Wang PhD); Cardinal Glennon Children's Hospital, Saint Louis University, St. Louis, MO (Ron Mitchell, MD; Shalini Paruthi, MD; Karen Snyder, MS); University of Pennsylvania/Children's Hospital of Philadelphia, (Carole Marcus, MBBCh; Nina H. Thomas, PhD; Lisa Elden, MD); Cincinnati Children's Medical Center, University of Cincinnati, Cincinnati, OH (Raouf Amin, MD; Dean Beebe, PhD; Paul Willging, MD); Montefiore Children's Hospital, Albert Einstein College of Medicine, Yeshiva University, Bronx, NY (Raanan Arens, MD; Hiren Muzumdar, MD; Shelby Harris, PsyD CBSM); Rainbow Babies and Children's Hospital, University Hospitals Case Medical Center, Case Western Reserve University School of Medicine, Cleveland, OH (Carol Rosen, MD; H. Gerry Taylor, PhD; Robert Sprecher, MD; James Arnold, MD); University of Kentucky, Louisville, Kentucky (David Gozal, MD); University of Michigan, Ann Arbor, MI (Ronald Chervin, MD; Susan Garetz, MD, Bruno Giordani, PhD; Tim Hoban, M.D.); University of Pennsylvania, Philadelphia, PA (Susan Ellenberg PhD; Reneé H. Moore, PhD; Kim Lacy, RN, BSN; Melissa Fernando).
Footnotes
A commentary on this article appears in this issue on page 1343.
SUPPLEMENTAL MATERIAL
Change of sleep measures and BMI z-score from baseline to follow-up in the early Adenotonsillectomy compared to the Watchful Waiting with Supportive Care group.
REFERENCES
- 1.Marcus CL, Brooks LJ, Draper KA, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130:e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 2.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26:541–7. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iftikhar IH, Khan MF, Das A, Magalang UJ. Meta-analysis: continuous positive airway pressure improves insulin resistance in patients with sleep apnea without diabetes. Ann Am Thorac Soc. 2013;10:115–20. doi: 10.1513/AnnalsATS.201209-081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63:203–9. doi: 10.1161/HYPERTENSIONAHA.113.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbe F, Duran-Cantolla J, Sanchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161–8. doi: 10.1001/jama.2012.4366. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234:446–53. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock TG, Wang X, Rueschman M, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–25B. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larkin EK, Rosen CL, Kirchner HL, et al. Variation of C-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–84. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 9.Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhattacharjee R, Kheirandish-Gozal L, Pillar G, Gozal D. Cardiovascular complications of obstructive sleep apnea syndrome: evidence from children. Prog Cardiovasc Dis. 2009;51:416–33. doi: 10.1016/j.pcad.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 11.de la Eva RC, Baur LA, Donaghue KC, Waters KA. Metabolic correlates with obstructive sleep apnea in obese subjects. J Pediatr. 2002;140:654–9. doi: 10.1067/mpd.2002.123765. [DOI] [PubMed] [Google Scholar]
- 12.Zintzaras E, Kaditis AG. Sleep-disordered breathing and blood pressure in children: a meta-analysis. Arch Pediatr Adolesc Med. 2007;161:172–8. doi: 10.1001/archpedi.161.2.172. [DOI] [PubMed] [Google Scholar]
- 13.Li AM, Au CT, Sung RY, et al. Ambulatory blood pressure in children with obstructive sleep apnoea: a community based study. Thorax. 2008;63:803–9. doi: 10.1136/thx.2007.091132. [DOI] [PubMed] [Google Scholar]
- 14.Li AM, Au CT, Ho C, Fok TF, Wing YK. Blood pressure is elevated in children with primary snoring. J Pediatr. 2009;155:362–8. e1. doi: 10.1016/j.jpeds.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 15.Bixler EO, Vgontzas AN, Lin HM, et al. Blood pressure associated with sleep-disordered breathing in a population sample of children. Hypertension. 2008;52:841–6. doi: 10.1161/HYPERTENSIONAHA.108.116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nisbet LC, Yiallourou SR, Walter LM, Horne RS. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Med Rev. 2014;18:179–89. doi: 10.1016/j.smrv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines, adiponectin, and insulin resistance as measured by HOMA in children with obstructive sleep apnea. Sleep. 2010;33:1185–91. doi: 10.1093/sleep/33.9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Hoorenbeeck K, Franckx H, Debode P, et al. Metabolic disregulation in obese adolescents with sleep-disordered breathing before and after weight loss. Obesity (Silver Spring) 2013;21:1446–50. doi: 10.1002/oby.20337. [DOI] [PubMed] [Google Scholar]
- 19.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–6. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 20.Nisbet LC, Yiallourou SR, Nixon GM, et al. Nocturnal autonomic function in preschool children with sleep-disordered breathing. Sleep Med. 2013;14:1310–6. doi: 10.1016/j.sleep.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Nisbet LC, Yiallourou SR, Biggs SN, et al. Preschool children with obstructive sleep apnea: the beginnings of elevated blood pressure? Sleep. 2013;36:1219–26. doi: 10.5665/sleep.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horne RS, Yang JS, Walter LM, et al. Elevated blood pressure during sleep and wake in children with sleep-disordered breathing. Pediatrics. 2011;128:e85–92. doi: 10.1542/peds.2010-3431. [DOI] [PubMed] [Google Scholar]
- 23.Liao D, Li X, Vgontzas AN, et al. Sleep-disordered breathing in children is associated with impairment of sleep stage-specific shift of cardiac autonomic modulation. J Sleep Res. 2010;19:358–65. doi: 10.1111/j.1365-2869.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shamsuzzaman A, Szczesniak RD, Fenchel MC, Amin RS. Glucose, insulin, and insulin resistance in normal-weight, overweight and obese children with obstructive sleep apnea. Obesity Res Clin Pract. 2014;8:e584–91. doi: 10.1016/j.orcp.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter LM, Nixon GM, Davey MJ, Anderson V, Walker AM, Horne RS. Autonomic dysfunction in children with sleep disordered breathing. Sleep Breath. 2013;17:605–13. doi: 10.1007/s11325-012-0727-x. [DOI] [PubMed] [Google Scholar]
- 26.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters KA, Sitha S, O'Brien LM, et al. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174:455–60. doi: 10.1164/rccm.200401-110OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kheirandish-Gozal L, Sans Capdevila O, Kheirandish E, Gozal D. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest. 2008;133:92–9. doi: 10.1378/chest.07-0773. [DOI] [PubMed] [Google Scholar]
- 29.Vlahandonis A, Yiallourou SR, Sands SA, et al. Long-term changes in blood pressure control in elementary school-aged children with sleep-disordered breathing. Sleep Med. 2014;15:83–90. doi: 10.1016/j.sleep.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Muzumdar HV, Sin S, Nikova M, Gates G, Kim D, Arens R. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 2011;139:1050–9. doi: 10.1378/chest.10-1555. [DOI] [PubMed] [Google Scholar]
- 31.Chu L, Yao H, Wang B. Impact of adenotonsillectomy on high-sensitivity C-reactive protein levels in obese children with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2012;147:538–43. doi: 10.1177/0194599812444419. [DOI] [PubMed] [Google Scholar]
- 32.Apostolidou MT, Alexopoulos EI, Damani E, et al. Absence of blood pressure, metabolic, and inflammatory marker changes after adenotonsillectomy for sleep apnea in Greek children. Pediatr Pulmonol. 2008;43:550–60. doi: 10.1002/ppul.20808. [DOI] [PubMed] [Google Scholar]
- 33.Marcus CL, Moore RH, Rosen CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368:2366–76. doi: 10.1056/NEJMoa1215881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redline S, Amin R, Beebe D, et al. The Childhood Adenotonsillectomy Trial (CHAT): rationale, design, and challenges of a randomized controlled trial evaluating a standard surgical procedure in a pediatric population. Sleep. 2011;34:1509–17. doi: 10.5665/sleep.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katz ES, Moore RH, Rosen CL, et al. Growth after adenotonsillectomy for obstructive sleep apnea: an RCT. Pediatrics. 2014;134:282–9. doi: 10.1542/peds.2014-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 38.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip PM, Chan MK, Nelken J, Lepage N, Brotea G, Adeli K. Pediatric reference intervals for lipids and apolipoproteins on the VITROS 5,1 FS Chemistry System. Clin Biochem. 2006;39:978–83. doi: 10.1016/j.clinbiochem.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 41.Ten S, Maclaren N. Insulin resistance syndrome in children. J Clin Endocrinol Metab. 2004;89:2526–39. doi: 10.1210/jc.2004-0276. [DOI] [PubMed] [Google Scholar]
- 42.Montesano M, Miano S, Paolino MC, et al. Autonomic cardiovascular tests in children with obstructive sleep apnea syndrome. Sleep. 2010;33:1349–55. doi: 10.1093/sleep/33.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polak J, Shimoda LA, Drager LF, et al. Intermittent hypoxia impairs glucose homeostasis in C57BL6/J mice: partial improvement with cessation of the exposure. Sleep. 2013;36:1483–90. doi: 10.5665/sleep.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanno S, Tanigawa T, Saito I, et al. Sleep-related intermittent hypoxemia and glucose intolerance: a community-based study. Sleep Med. 2014;15:1212–8. doi: 10.1016/j.sleep.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 45.Drager LF, Yao Q, Hernandez KL, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–8. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li AM, Chan MH, Yin J, et al. C-reactive protein in children with obstructive sleep apnea and the effects of treatment. Pediatr Pulmonol. 2008;43:34–40. doi: 10.1002/ppul.20732. [DOI] [PubMed] [Google Scholar]
- 47.Tauman R, O'Brien LM, Ivanenko A, Gozal D. Obesity rather than severity of sleep-disordered breathing as the major determinant of insulin resistance and altered lipidemia in snoring children. Pediatrics. 2005;116:e66–73. doi: 10.1542/peds.2004-2527. [DOI] [PubMed] [Google Scholar]
- 48.Kaditis AG, Alexopoulos EI, Damani E, et al. Obstructive sleep-disordered breathing and fasting insulin levels in nonobese children. Pediatr Pulmonol. 2005;40:515–23. doi: 10.1002/ppul.20306. [DOI] [PubMed] [Google Scholar]
- 49.Rosen CL, Debaun MR, Strunk RC, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134:273–81. doi: 10.1542/peds.2013-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ó Hartaigh B, Gill TM, Shah I, et al. Association between resting heart rate across the life course and all-cause mortality: longitudinal findings from the Medical Research Council (MRC) National Survey of Health and Development (NSHD) J Epidemiol Community Health. 2014;68:883–9. doi: 10.1136/jech-2014-203940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho JE, Larson MG, Ghorbani A, et al. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham Heart Study. J Am Heart Assoc. 2014;3:e000668. doi: 10.1161/JAHA.113.000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javaheri S, Redline S. Sleep, slow-wave sleep, and blood pressure. Curr Hypertens Rep. 2012;14:442–8. doi: 10.1007/s11906-012-0289-0. [DOI] [PubMed] [Google Scholar]
- 53.Hannon TS, Tu W, Watson SE, Jalou H, Chakravorty S, Arslanian SA. Morning blood pressure is associated with sleep quality in obese adolescents. J Pediatr. 2014;164:313–7. doi: 10.1016/j.jpeds.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hagenauer MH, Lee TM. Adolescent sleep patterns in humans and laboratory animals. Horm Behav. 2013;64:270–9. doi: 10.1016/j.yhbeh.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janz KF, Dawson JD, Mahoney LT. Predicting heart growth during puberty: the Muscatine Study. Pediatrics. 2000;105:E63. doi: 10.1542/peds.105.5.e63. [DOI] [PubMed] [Google Scholar]
- 56.Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics. 2010;126:e583–90. doi: 10.1542/peds.2009-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change of sleep measures and BMI z-score from baseline to follow-up in the early Adenotonsillectomy compared to the Watchful Waiting with Supportive Care group.


