Abstract
Study Objective:
There are some data to suggest that insufficient sleep, including short sleep duration and sleep disruption, may be associated with an increased risk of cancer. We investigated the association between sleep duration and sleep disruption and risk of prostate cancer.
Design:
Prospective cohort study.
Setting:
Sweden.
Participants:
A total of 14,041 men in the Swedish National March Cohort.
Interventions:
None.
Measurements and Results:
Habitual sleep duration and sleep disruption were self-reported in 1997. Prostate cancer diagnoses, including lethal (metastases at diagnosis or death from prostate cancer) and advanced (stage T4, N1, or M1 at diagnosis or death from prostate cancer), were determined from linkage to nationwide cancer registries through 2010. We conducted Cox proportional hazards regression adjusted for potential confounding variables. During 13 years of follow-up, we identified 785 cases of incident prostate cancer, including 118 lethal and 127 advanced cases. Four percent of men reported sleeping 5 h or less a night, and 2% reported sleeping 9 h or more per night. We found no association between sleep duration and risk of prostate cancer overall or for advanced/lethal disease. We also did not find an association between prostate cancer and sleep disruption, as defined by difficulty falling asleep, difficulty maintaining sleep, sleep quality, and restorative power of sleep.
Conclusions:
In this large prospective study from Sweden, we found no association between habitual sleep duration or sleep disruption and risk of prostate cancer.
Citation:
Markt SC, Grotta A, Nyren O, Adami HO, Mucci LA, Valdimarsdottir UA, Stattin P, Bellocco R, Lagerros YT. Insufficient sleep and risk of prostate cancer in a large Swedish cohort. SLEEP 2015;38(9):1405–1410.
Keywords: prostate cancer, sleep duration, sleep disruption
INTRODUCTION
Insufficient sleep, including short sleep duration and sleep disruption, is becoming increasingly prevalent worldwide, and can lead to numerous health problems. Shift work involving circadian disruption was classified as “probably carcinogenic to humans” by the International Agency for Research on Cancer (IARC) in 2007. Much of the data leading to this conclusion were based on studies in breast cancer, with few studies conducted in prostate cancer.
Circadian disruption is hypothesized to increase cancer risk through at least three related pathways: (1) internal desynchronization of circadian rhythms, (2) suppression of the pineal hormone melatonin, and (3) inadequate sleep duration or sleep disruption. Circadian rhythm disruption can impair physiological, metabolic, and behavioral processes and may result in tumorigenesis.1–3 Experimental studies provide evidence for the role of the circadian system as a potential tumor suppressor through cell cycle arrest, inhibition of cellular proliferation, promotion of apoptosis, and anti-angiogenesis.4–7 In addition, sleep insufficiency may increase cancer risk through obstructive sleep apnea and resultant hypoxia, which has been associated with accelerated tumor progression and metastatic potential.8–10
Few epidemiological studies, often hampered by small sample size, have evaluated the association between sleep duration or sleep disruption and risk of prostate cancer, with varying results. Two studies have found an increased risk of prostate cancer for short sleep duration11,12 and another an increased risk of advanced disease for men who report sleep problems.13 Furthermore, evidence for an association between circadian disruption and prostate cancer stems from studies showing an association between shift work and prostate-specific antigen (PSA), and melatonin suppression and prostate cancer.14–16
The primary aim of this study was to prospectively investigate the association between sleep duration and sleep disruption and risk of prostate cancer in a large prospective cohort in Sweden with detailed assessment of sleep characteristics.
METHODS
Study Population
The National March Cohort (NMC), established in Sweden in September 1997, has been described in detail elsewhere.17 Briefly, the cohort includes participants in the National March, a fund-raising event for the Swedish Cancer Society. Over 43,863 individuals completed and returned a 36-page questionnaire which included demographic, lifestyle, and medical information. Participants also included their national registration number (NRN), a unique identifier given to all Swedish residents. Given the fundraising nature and almost 3,600 Swedish cities and villages participating in the event, the number of individuals offered a questionnaire is unknown.
Men with valid NRNs were included in the study population for this analysis (n = 15,673). We excluded participants with a diagnosis of cancer before baseline (n = 867); who were younger than 18 y (n = 609); invalid birthdates (n = 11); who emigrated at the time of enrollment (n = 140); and who were dead prior to enrollment (n = 5). Our final study population included 14,041 men followed prospectively for prostate cancer incidence until December 31, 2010. The study was approved by the Research Ethical Review Board at Karolinska Institutet and all study participants provided informed consent.
Assessment of Sleep Characteristics
The Karolinska sleep questionnaire was utilized to assess sleep duration and sleep disruption.18 Participants were asked to indicate “On average, how many hours do you usually sleep per day on workdays or weekdays?” and “On average, how many hours do you usually sleep per day on nonworkdays?” Response choices were: < 5, 5, 6, 7, 8, ≥ 9 h. We redefined sleep duration as ≤ 5 h, 6, 7, 8, ≥ 9 h. We created a weighted average, combining week/workdays and nonworkdays, where week/workdays received a weight of five, and nonworkdays a weight of two.
The questionnaire included 13 items related to sleep disruption. The participants were asked during the past 12 mo how often they had experienced: (1) difficulty falling asleep, (2) waking up during the night with difficulty going back to sleep, (3) waking up too early, and (4) restless sleep. Response choices included never, rarely, sometimes, mostly, or always. We combined these questions into a “sleep quality” variable, equal to “good” if participants responded rarely or never to all four questions, “poor” if they responded mostly or always to at least one of the four, and “moderate” otherwise. Additionally, we combined (1) difficulty falling asleep, (2) waking up during the night with difficulty falling back asleep, and (3) sleeping medication use into a “severe sleep problem” variable, and included waking up too early with severe problems to create a “very severe sleep problem” variable.
We created a “restorative power of sleep” variable by combining (1) difficulty waking up, (2) feeling unrested after waking up, and (3) waking up fatigued. Good, moderate and poor restorative power was defined similarly to quality. We also combined (1) feeling sleepy during daytime and (2) dozing off during daytime into a “daytime tiredness” variable. Snoring was defined as frequent if participants responded sometimes, mostly or always, and infrequent if never or rarely.
Follow-Up
Follow-up for cancer diagnoses and death information was obtained by linking each participant to nationwide and complete registries using their unique NRNs. After checking the validity of the NRNs against the population register, prostate cancer diagnoses were ascertained from the Swedish National Cancer Register, information on date and cause of death from the Swedish Death Register, and emigration from the Population register. Information on tumor stage according to the TNM classification, Gleason score, and PSA level was obtained from the National Prostate Cancer Register (NPCR).19
For this analysis, we assessed total prostate cancer incidence, and because accumulating evidence suggests that lethal and indolent prostate cancer may have distinct etiologies,20,21 we separately evaluated advanced (stage T4, N1, or M1 at diagnosis or death from prostate cancer), lethal (metastases at diagnosis or death from prostate cancer) and high-grade (Gleason score 8–10) cancers (Table S1 and Figure S1, supplemental material). We followed participants from October 1, 1997 until prostate cancer diagnosis, death, emigration or end of follow-up (December 31, 2010), whichever came first.
Statistical Analysis
We compared covariate distributions across categories of sleep duration and sleep disruption, reporting means and standard deviations for continuous covariates, and percentages for categorical variables. We compared each sleep duration category to 8 h per night. We used Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs), using age as the underlying time scale. Scaled Schoenfeld residuals were used to test the proportional hazards assumption; no deviations of model assumptions were found.
In multivariable models, we adjusted for body mass index (BMI) (continuous), employment status (employed, unemployed, retired, other), snoring (frequently, infrequently, uncertain), smoking (never, former, current), monthly alcohol consumption (none, ≤ 218 g, 219–513 g, ≥ 514 g), depressive symptoms (yes, no), physical activity (≤ 34.32 metabolic equivalent (MET-h), 34.33–46.36 MET-h, ≥ 46.37 MET-h), coffee intake (none, 1–3 cups/day, 4–6 cups/day, ≥ 7 cups/day), multivitamin use (yes, no), and diabetes (yes, no). P values for trend were determined using the median value within categories of sleep duration. We evaluated the presence of interaction of the association between sleep duration and prostate cancer by snoring, sleep disruption, coffee consumption, and daytime tiredness. We created multiplicative interaction terms and statistical significance was determined by comparing models with and without the interaction using a likelihood ratio test. We also evaluated evidence of interaction on the additive scale using Relative Excess Risk due to Interaction (RERI).22 Finally, we performed stratified analyses by the same factors.
To evaluate the association between sleep disruption and prostate cancer, within each sleep disruption domain, we compared “sometimes” and “mostly/always” to “never/rarely”, respectively. For sleep quality and restorative power of sleep, we compared moderate and poor to good, respectively. We also cross-classified sleep duration and feeling unrested, sleep quality, and restorative sleep, respectively, to evaluate the joint effect of sleep duration and disruption on prostate cancer risk.
We performed several secondary analyses to compare with previous studies, including categorizing sleep duration as ≤ 6 h and ≥ 9 h compared to 7–8 h,11 and evaluated risk of fatal prostate cancer (death from prostate cancer) after restricting follow-up to 8 y.12
All analyses were performed using SAS version 9.4 (SAS Institute, Inc; Cary, NC, USA). All P values were two-sided, with a value < 0.05 considered to be statistically significant.
RESULTS
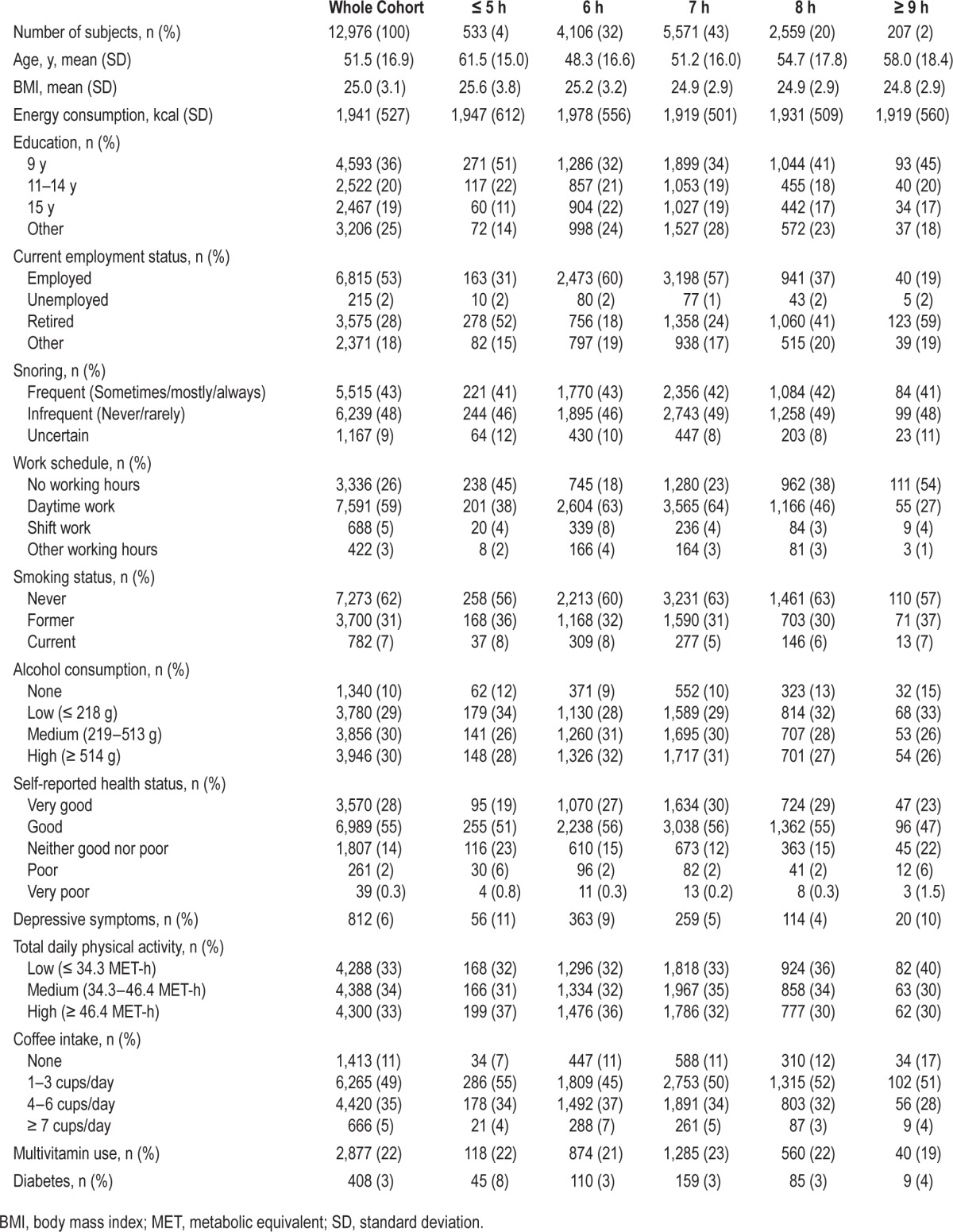
During 13 years of follow-up, 785 of the 12,976 men with information on sleep duration received a diagnosis of prostate cancer. Of these cancers, 118 were defined as lethal, 127 advanced, and 112 were high grade. The baseline characteristics of the cohort by sleep duration are shown in Table 1. Overall, 20% of men reported sleeping 8 h per night, 4% reported sleeping 5 h or fewer, and 2% reported 9 h or more of sleep per night. Men at the extremes of sleep duration (≤ 5 h and ≥ 9 h) were more likely to be retired and therefore have no working hours, and were more likely to report depressive symptoms. We did not find meaningful differences by BMI, snoring, or smoking. As shown in Table S2 (supplemental material), men who reported sleeping 5 h or less were more likely to take sleeping medications, and report problems falling asleep, staying asleep, early morning awakening, and feeling unrested; they were also more likely to have poor restorative sleep.
Table 1.
Baseline characteristics of the Swedish National March Cohort by sleep duration, 1997.
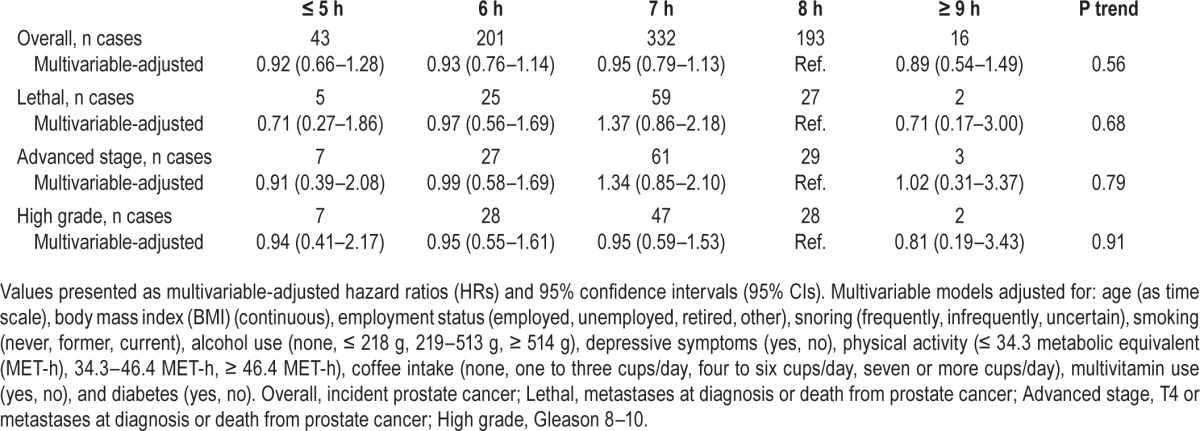
In multivariable-adjusted analyses, sleep duration was not associated with risk of any of our prostate cancer outcomes (Table 2). However, sample size was low in extreme categories of sleep duration, particularly when prostate cancer was broken down into subcategories. Adjustment for potential confounders did not change our estimates from age-adjusted models. Results were similar when we used 7 h per night as the reference group and when we restricted the analysis to men at least 40 y old at baseline (data not shown). For comparison with other studies, we combined sleep duration as ≤ 6 h and ≥ 9 h, compared to 7–8 h per night.11 We found no association with overall prostate cancer (HR: 0.93, 95% CI: 0.76–1.14 for ≤ 6 h; HR: 0.89, 95% CI: 0.54–1.49 for ≥ 9 h), or with any of our other prostate cancer outcomes (data not shown). Although restricted by small numbers, we were unable to confirm an association with fatal prostate cancer limited to the first 8 y of follow-up (compared to 7 h: ≤ 5 h HR: 0.60, 95% CI: 0.17–2.07; ≥ 9 h HR: 0.43, 95% CI: 0.06–3.27). Finally, we found similar results when we examined the association between sleep duration and risk of prostate cancer with a 4-y lag period (data not shown).
Table 2.
Association between sleep duration and prostate cancer, Swedish National March Cohort, Sweden, 1997–2010.
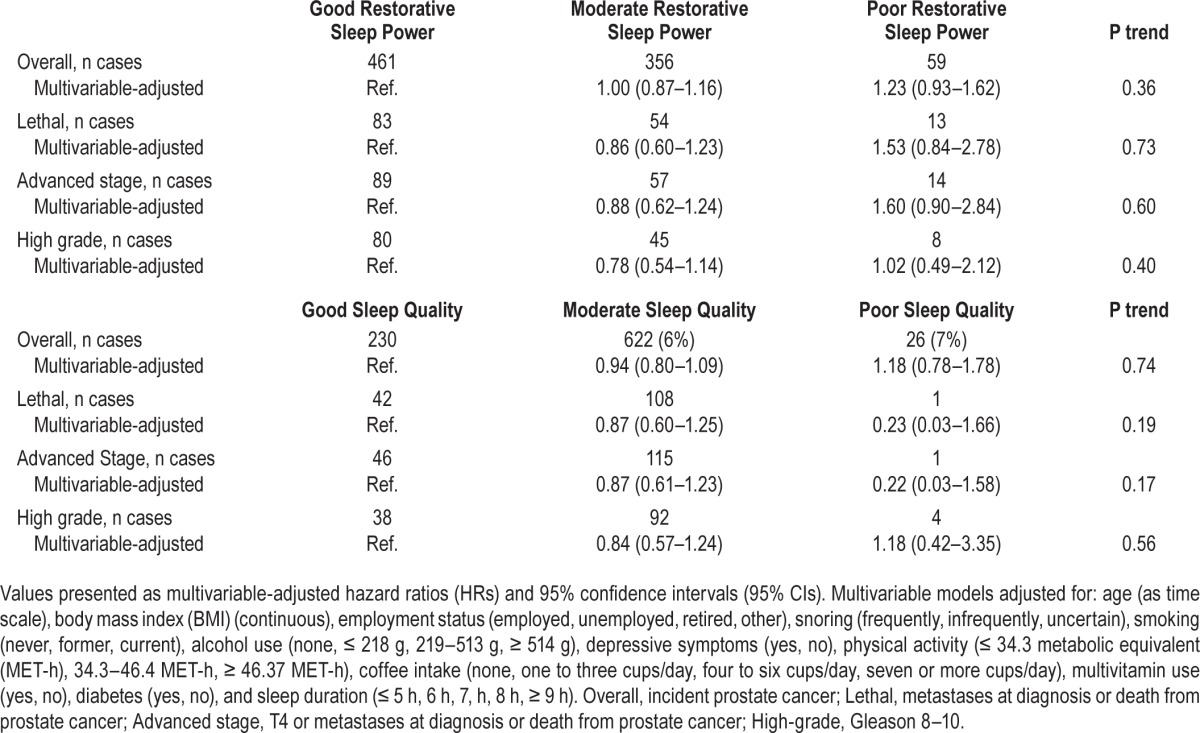
Overall, 5,660 (41%), 6,990 (51%), and 1,170 (8%) men reported good, moderate, or poor restorative power of sleep, respectively. Men with poor restorative sleep had no statistically significant increased risk of overall (HR: 1.23, 95% CI: 0.93–1.62), lethal (HR: 1.53, 95% CI: 0.84–2.78) or advanced disease (HR: 1.60, 95% CI: 0.90–2.84), compared to men with good restorative power (Table 3). The majority of men (70%, n = 9,748) reported moderate quality of sleep, whereas 27% (n = 3,712) and 3% (n = 384) reported good or poor quality of sleep, respectively. We found no evidence for an association between quality of sleep and prostate cancer risk (Table 3). We similarly did not find any significant associations with any of the prostate cancer outcomes when we combined moderate and poor quality and compared to good quality (data not shown). We also did not find evidence for an association with restorative power or quality of sleep when we combined moderate and poor categories together and compared to good. We did not find an association between prostate cancer risk and sleep disruption as defined by difficulty falling asleep, difficulty staying asleep, early morning awakening, and feeling unrested (Table S3, supplemental material). Finally, severe and very severe sleep problems, as defined in a previous study,13 were not associated with any of our prostate cancer outcomes (data not shown).
Table 3.
Association between restorative power and quality of sleep and prostate cancer, Swedish National March Cohort, Sweden, 1997–2010.
We did not find any statistically significant interactions between sleep duration and prostate cancer related to snoring, sleep disruption, coffee consumption, and daytime tiredness on either the multiplicative or additive scales (data not shown). To further explore the potential impact of sleep on prostate cancer risk, we cross-classified sleep duration and disruption (as defined by restorative power of sleep). We did not find an association with overall prostate cancer for men with moderate/poor restorative power of sleep who slept ≤ 5 h per night (HR: 1.22, 95% CI: 0.79–1.88) or men who slept 9 h or more (HR: 1.16, 95% CI: 0.57–2.34), compared to those who slept 6–8 h per night with good restorative power of sleep (Figure 1). We also did not find evidence of an association for the combination of sleep quality and sleep duration with risk of prostate cancer (data not shown).
Figure 1.
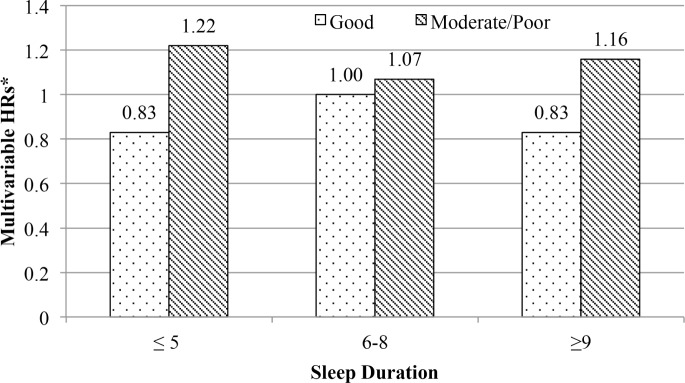
Risk of prostate cancer associated with combinations of sleep duration and restorative power of sleep among participants in the Swedish National March Cohort, Sweden, 1997–2010. *Hazard ratios (HRs) are compared to 6–8 h of sleep duration and good restorative power of sleep. HRs are adjusted for: age (as time scale, body mass index (BMI) (continuous), employment status (employed, unemployed, retired, other), snoring (frequently, infrequently, uncertain), smoking (never, former, current), alcohol use (none, ≤ 218 g, 219–513 g, ≥ 514 g), depressive symptoms (yes, no), physical activity (≤ 34.3 metabolic equivalent (MET-h), 34.3–46.4 MET-h, ≥ 46.4 MET-h), coffee intake (none, one to three cups/day, four to six cups/day, seven or more cups/day), multivitamin use (yes, no), and diabetes (yes, no).
DISCUSSION
In this large prospective study, we found no association between sleep duration and risk of prostate cancer, either overall or more advanced disease. In addition, we did not find a consistent association with sleep disruption and risk of prostate cancer.
Two previous studies have found significant associations between sleep duration and risk of prostate cancer. The Ohsaki National Health Insurance Cohort study found a 38% non-statistically significant increased risk of prostate cancer for men who slept ≤ 6 h per night and a 64% statistically significant decreased risk of prostate cancer for men who slept ≥ 9 h per night, compared to those who slept 7–8 h per night; however, there was no association between short or long sleep duration and risk of advanced or metastatic disease.11 The Cancer Prevention Study-II found during the first 8 y of follow-up that short sleep duration (3–5 h) was associated with a 64% increased risk of fatal prostate cancer compared to 7 h/night.12 We were unable to replicate these results in this cohort; however, we had few fatal cases in the extremes of sleep duration.
Sleep disruption and risk of prostate cancer has been evaluated in few studies. In a cohort of Icelandic men, those with problems falling asleep and staying asleep had an increased risk of advanced prostate cancer, compared to men without sleep disruption.13 Other measures of circadian disruption, such as shift work, have been associated with elevated PSA levels and an increased risk of prostate cancer.14,23–26 We were unable to find an association between any of our sleep disruption domains and risk of prostate cancer.
Strengths of this study include the prospective design, detailed assessment of sleeping characteristics, capturing of rich covariate data to adjust for potential confounding, complete follow-up for prostate cancer incidence and mortality through linkage to nationwide registries, as well as information on subtypes of prostate cancer by stage and Gleason score. A limitation of this study is that sleep duration and disruption may be subject to misclassification, since it was not objectively measured, but assessed from patient responses; however, it is not feasible to validate in this study population. We also lacked information on and were unable to evaluate sleep disordered breathing and risk of prostate cancer, which has been shown to be associated with increased cancer mortality.27 We did not have repeated measurements of sleep duration or disruption to assess the potential impact of change in sleeping habits during follow-up with risk of prostate cancer. We also lacked information on history of PSA testing, which might be correlated with sleep duration and disruption. However, possible confounding by PSA testing should affect primarily early stage disease. Finally, we were limited by small sample size and precision may have been insufficient to rule out relevant associations. Further research is needed including men in extreme sleep categories, also allowing for sufficient latency between exposure and outcome to rule out reverse causation.
In conclusion, we found no statistically significant association between insufficient sleep, either short sleep duration or sleep disruption, and risk of overall, advanced, or lethal prostate cancer in this prospective cohort study in Sweden. Given the mixed results of studies reported to date, further research investigating the interplay between sleep duration, sleep disruption, and risk of prostate cancer in large, prospective studies should be conducted. In addition, future studies should investigate the role of sleep disordered breathing in prostate cancer initiation and progression.
DISCLOSURE STATEMENT
This work was supported by funding from Ericsson, ICA Sweden, and The Swedish Cancer Society (CAN 2012/591); the National Cancer Institute at the National Institutes of Health Training Grant (NIH T32 CA09001 to Sarah Markt), the Prostate Cancer Foundation (to Lorelei Mucci) and the regional agreement on medical training and clinical research between Stockholm County Council and Karolinska Institutet (to Ylva Trolle Lagerros). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Cancerfonden and volunteers who worked with the National March. We also would like to thank ICA AB and Ericsson for financial support and Statistics Sweden for scanning the questionnaires.
SUPPLEMENTAL MATERIAL
Definitions of prostate cancer outcomes.
Baseline sleep disruption characteristics of the Swedish National March Cohort by sleep duration, 1997.
Multivariable-adjusted association (hazard ratios and 95% confidence intervals) between sleep disruption parameters and overall, lethal, and advanced stage prostate cancer, Swedish National March Cohort, Sweden, 1997–2010.
Diagram of number of cases within each category of prostate cancer outcome.
REFERENCES
- 1.Davis S, Mirick DK. Circadian disruption, shift work and the risk of cancer: a summary of the evidence and studies in Seattle. Cancer Causes Control. 2006;17:539–45. doi: 10.1007/s10552-005-9010-9. [DOI] [PubMed] [Google Scholar]
- 2.Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med Rev. 2009;13:257–64. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Costa G, Haus E, Stevens R. Shift work and cancer - considerations on rationale, mechanisms, and epidemiology. Scand J Work Environ Health. 2010;36:163–79. doi: 10.5271/sjweh.2899. [DOI] [PubMed] [Google Scholar]
- 4.Reiter RJ. Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab. 2003;17:273–85. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- 5.Sainz RM, Mayo JC, Tan DX, León J, Manchester L, Reiter RJ. Melatonin reduces prostate cancer cell growth leading to neuroendocrine differentiation via a receptor and PKA independent mechanism. Prostate. 2005;63:29–43. doi: 10.1002/pros.20155. [DOI] [PubMed] [Google Scholar]
- 6.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–8. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo SS, Yoo Y-M. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 8.Almendros I, Montserrat JM, Ramirez J, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. European Respir J. 2012;39:215–7. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 9.Ahn GO, Brown M. Targeting tumors with hypoxia-activated cytotoxins. Front Biosci. 2007;12:3483–501. doi: 10.2741/2329. [DOI] [PubMed] [Google Scholar]
- 10.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol. 2003;76:S11–22. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 11.Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99:176–8. doi: 10.1038/sj.bjc.6604425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gapstur SM, Diver WR, Stevens VL, Carter BD, Teras LR, Jacobs EJ. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am J Prev Med. 2014;46:S26–33. doi: 10.1016/j.amepre.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 13.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:872–9. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurdardottir LG, Valdimarsdottir UA, Fall K, et al. Circadian disruption, sleep loss, and prostate cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2012;21:1002–11. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigurdardottir LG, Markt SC, Rider JR, et al. Urinary melatonin levels, sleep disruption, and risk of prostate cancer in elderly men. Eur Urol. 2015;67:191–4. doi: 10.1016/j.eururo.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J Natl Cancer Inst. 2013;105:1292–7. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagerros YT, Bellocco R, Adami HO, Nyren O. Measures of physical activity and their correlates: the Swedish National March Cohort. Eur J Epidemiol. 2009;24:161–9. doi: 10.1007/s10654-009-9327-x. [DOI] [PubMed] [Google Scholar]
- 18.Akerstedt T, Ingre M, Broman JE, Kecklund G. Disturbed sleep in shift workers, day workers, and insomniacs. Chronobiol Int. 2008;25:333–48. doi: 10.1080/07420520802113922. [DOI] [PubMed] [Google Scholar]
- 19.Van Hemelrijck M, Wigertz A, Sandin F, et al. Cohort profile: the National Prostate Cancer Register of Sweden and Prostate Cancer Data Base Sweden 2.0. Int J Epidemiol. 2013;42:956–67. doi: 10.1093/ije/dys068. [DOI] [PubMed] [Google Scholar]
- 20.Wilson KM, Giovannucci EL, Mucci LA. Lifestyle and dietary factors in the prevention of lethal prostate cancer. Asian J Androl. 2012;14:365–74. doi: 10.1038/aja.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol. 2007;36:1111–8. doi: 10.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- 23.Conlon M, Lightfoot N, Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–3. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 24.Kubo T, Oyama I, Nakamura T, et al. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int J Urol. 2011;18:206–11. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 25.Pukkala E, Martinsen JI, Weiderpass E, et al. Cancer incidence among firefighters: 45 years of follow-up in five Nordic countries. Occup Environ Med. 2014;71:398–404. doi: 10.1136/oemed-2013-101803. [DOI] [PubMed] [Google Scholar]
- 26.Parent ME, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–9. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 27.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered Breathing and Cancer Mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Definitions of prostate cancer outcomes.
Baseline sleep disruption characteristics of the Swedish National March Cohort by sleep duration, 1997.
Multivariable-adjusted association (hazard ratios and 95% confidence intervals) between sleep disruption parameters and overall, lethal, and advanced stage prostate cancer, Swedish National March Cohort, Sweden, 1997–2010.
Diagram of number of cases within each category of prostate cancer outcome.


