Abstract
Study Objectives:
Sudden infant death syndrome (SIDS) remains an important cause of infant death, particularly among infants born preterm. Prone sleeping is the major risk factor for SIDS and this has recently been shown to alter cerebrovascular control in term infants. As preterm infants are at greater risk for SIDS than those born at term, we hypothesized that their cerebrovascular control in the prone position would be reduced compared to term infants.
Patients or Participants:
There were 35 preterm (mean gestation 31.2 ± 0.4 w) and 17 term (mean gestation 40.1 ± 0.3 w) infants.
Design:
Infants underwent daytime polysomnography at 2–4 w, 2–3 mo, and 5–6 mo postterm age. Infants slept both prone and supine and were presented with cardiovascular challenges in the form of 15° head-up tilts (HUT).
Measurements and Results:
Cerebral tissue oxygenation index (TOI) was recorded using near-infrared spectroscopy (NIRO-200 spectrophotometer, Hamamatsu Photonics KK, Japan) and mean arterial pressure (MAP) was recorded using a Finometer cuff (Finapres Medical Systems, Amsterdam, The Netherlands). In the prone position TOI increased following the HUT (P < 0.05), whereas no change was seen in the supine position. The overall pattern of response was similar in both groups, but more variable in preterm than term infants (P < 0.05).
Conclusions:
Cerebrovascular control differs between the prone and supine positions in preterm infants. Although overall the responses to head-up tilts were similar between term and preterm infants, greater variability of responses in preterm infants suggests persisting immaturity of their cerebrovascular control in the first year of life, which may contribute to their increased risk of sudden infant death syndrome.
Citation:
Fyfe KL, Odoi A, Yiallourou SR, Wong FY, Walker AM, Horne RS. Preterm infants exhibit greater variability in cerebrovascular control than term infants. SLEEP 2015;38(9):1411–1421.
Keywords: blood pressure, cerebral blood flow, cerebrovascular circulation, cerebral oxygenation, preterm birth
INTRODUCTION
Sudden infant death syndrome (SIDS) remains the leading cause of death in the postneonatal period in developed countries.1 Although the exact mechanisms are yet to be fully elucidated, SIDS is thought to occur due to immature cardiovascular control allowing an uncompensated hypotensive episode during presumed sleep, in combination with a failure to arouse from sleep.2,3 It is currently unclear in which of the two infant sleep states, active sleep (AS) or quiet sleep (QS), SIDS deaths occur. Approximately 90% of SIDS deaths occur within the first 6 mo of life, with the incidence peaking between 2–3 mo of age,4,5 a period that is associated with developmental changes in both cardiovascular6,7 and cerebrovascular control.8,9
Prone sleeping has been established as a major SIDS risk factor.4,10 Term infants sleeping prone have reduced cerebral oxygenation,8 altered cerebrovascular control9 and depressed arousal responses,11 particularly at 2–3 mo of age. Most recently we have also identified that preterm infants have lower cerebral oxygenation than infants born at term, a deficit that is most marked when they sleep prone.12 We have previously suggested that reduced cerebral oxygenation in the prone position may contribute to dysfunctional brainstem reflexes, including blunted arousal responses, that are implicated in the pathophysiology of SIDS.8
Infants born preterm are at significantly increased risk for SIDS compared with those born at term, and this risk is increased when they are slept prone.10,13 Cerebrovascular control is immature in very preterm infants in the early postnatal days, with deficits in cerebral autoregulation14–17 and cerebral blood flow-metabolism coupling.18 Furthermore, we have recently reported that preterm infants exhibited reduced cerebral oxygenation in the prone compared to the supine position and in comparison to term infants at the same postterm equivalent age.12 However, the effects of sleeping position on cerebrovascular control have not previously been assessed in preterm infants beyond term-equivalent age. To address this deficit in the literature, we assessed cerebrovascular control in preterm infants in both AS and QS in the prone and supine sleep positions across the first 6 mo postterm age. We hypothesized that cerebrovascular control would be altered in preterm infants in the prone position and in preterm compared to term infants, particularly in the prone position at 2–3 mo corrected age (CA), when SIDS risk is greatest.
PATIENTS AND METHODS
Ethical approval was obtained from the Monash Health and Monash University human research ethics committees. Written parental consent was obtained prior to the commencement of each study. No monetary incentive for participation was provided.
Subjects
Thirty-five healthy preterm infants (21 males/14 females) born between 26–36 w of gestational age (GA) (mean gestation 31.2 ± 0.4, mean birth weight 1,697 ± 92 g) and 17 healthy term infants (9 males/8 females; mean gestation 40.1 ± 0.3; mean birth weight 3,666 ± 105 g) were recruited. Data relating to cerebral oxygenation from the term infants has previously been published.8,9 All infants were appropriately grown for GA, born to nonsmoking mothers, had no family history of SIDS, and routinely slept supine at home. For the preterm cohort, exclusion criteria included significant intra-cerebral pathology, congenital abnormalities, hemodynamically significant patent ductus arteriosus, and chronic lung disease requiring ongoing oxygen therapy or respiratory stimulant medication at term-equivalent age. Where twins were included only one twin was studied. All infants were discharged home prior to the first study.
Study Protocol
Infants underwent daytime polysomnography at 2–4 w, 2–3 mo, and 5–6 mo postterm corrected age (CA). Studies were conducted between 09:00 and 17:00 in the Melbourne Children's Sleep Centre, Monash Medical Centre under constant temperature (22–24°C), dim lighting and quiet conditions. Recording electrodes were applied during a morning feed, following which the infants were allowed to sleep naturally in a pram and feed on demand. Infants slept in both the prone and supine positions with the initial sleep position randomized between infants and studies. Sleep position was usually changed following a midday feed and data were collected in both QS and AS.
Data Collection
Polysomnography electrodes included two channels of electroencephalogram (EEG; C4/A1; O2/A1), electrooculo-gram (EOG), submental electromyogram (EMG), electrocardiogram (ECG), abdominal and thoracic respiratory belts (Resp-ez Piezo-electric sensor, EPM Systems, Midlothian, VA, USA), arterial oxygen saturation (Masimo Radical Oximeter, French's Forest, NSW, Australia), and abdominal skin temperature (ADInstruments, Sydney, NSW, Australia). In addition, mean arterial pressure (MAP) was recorded using a photoplethysmographic cuff (Finapres Medical Systems, Amsterdam, The Netherlands) placed around the infant's wrist as previously validated.19 Cerebral tissue oxygenation index (TOI) was also recorded using near-infrared spectroscopy (NIRS) (NIRO-200 photospectrometer, Hamamatsu Photonics KK, Tokyo, Japan).8,9 Infants were continuously observed via an infrared camera located above the pram.
Physiological data were recorded using an E series sleep recording system with Profusion 2 polysomnography software (Compumedics, Abbotsford, VIC, Australia) at a sampling rate of 512 Hz. Sleep state was determined using electroencephalographic, heart rate, and breathing patterns together with behavioral changes according to recognized guidelines.20–22
Cardiovascular Challenge
While sleeping, infants were presented with a cardiovascular challenge in the form of a 15° head-up tilt (HUT). This involved raising the head of the pram to a 15° angle over a period of 2–3 sec, which has been shown to produce a small but significant change in MAP and heart rate (HR)7 without an associated arousal from sleep. HUTs were performed following a 1-min baseline period, during which the blood pressure cuff was inflated, and infants remained in the tilted position for an additional 1 min following the HUT. We aimed to perform a total of three HUTs in each sleep state and in each sleep position. HUTs were performed when the infants were physiologically stable and free of movement and a minimum 2-min rest period was allowed between HUTs for stabilization of physiological variables.
Data Analysis
At the conclusion of each study, data were transferred via European Data Format to LabChart 7 analysis software (ADInstruments, Sydney, NSW, Australia). Beat-beat averages for cerebral TOI and MAP were calculated for the 1-min baseline period and the 1-min HUT. HUTs containing movement, arousals, sighs, or apneic pauses within the baseline or HUT periods were excluded from further analysis. The response to the HUT was determined by calculating the percentage change (Δ%) from baseline for cerebral TOI and MAP following each HUT, with the baseline being the 30 beats preceding each HUT. The HUT response period was divided into four phases: baseline (beats 1–30), early (beats 31–60), middle (beats 61–90), and late (beats 91–120) similar to methods used in our previous studies.7,9,23 Choice of these phases was based on the characteristic MAP response to HUT, which consists of an initial increase to a peak, followed by a decrease either to or below baseline, following which MAP either returns to or remains below baseline. This enabled assessment of cerebral vascular control through examination of cerebral TOI in the presence of fluctuating MAP. HUT responses were averaged for each infant in each sleep state and sleeping position.
Effect of Gestational Age on the HUT Response
In preliminary analyses we assessed the potential effect of GA at birth in preterm infants on the HUT response in two ways. First, we performed linear regression between GA at birth and the maximum and minimum change from baseline of TOI during any of the four analysis phases and found no significant correlations (data not shown). Second, we grouped infants into very preterm (< 32 w GA) and preterm (32–36 w GA) and contrasted TOI responses to a HUT; as no differences were found (data not shown) all preterm data were pooled and analysed as a single preterm group.
Statistical Analysis
Statistical analysis was performed using SigmaPlot analysis software (Systat Software Inc, Chicago, IL, USA). The effects of sleep position and preterm birth on baseline data were determined using two-way analysis of variance (ANOVA) with Student-Newman-Keuls post hoc analysis at each postterm age and in each sleep state. A statistically significant change in MAP and cerebral TOI from baseline was determined within each phase (early, middle, and late) using one-way repeated-measures ANOVA. For data that did not pass normality, repeated-measures ANOVA on ranks was performed with Dunnett method post hoc analysis.
To assess the effect of preterm birth on the HUT we used two-way ANOVA within each phase, with beat number and birth (preterm or term) as factors and after nonnormal data were transformed using reverse reciprocal transformation to achieve normality.24 To assess variability of the HUT response, the mean standard deviation for each beat for MAP and TOI was determined for each infant within each sleep state and position. Preterm and term infant values were grouped and compared using two-way ANOVA within each sleep state, sleep position and HUT phase (early, middle and late).
RESULTS
Infant Characteristics
There were no differences in age or weight between term and preterm infants at the 2–4 w (preterm versus term: 3.2 ± 0.1 w versus 3.3 ± 0.2 w; weight: 3.7 ± 0.1 kg versus 3.9 ± 0.1 kg), 2–3 mo (preterm versus term: 10.7 ± 0.2 w versus 10.6 ± 0.2 w; weight: 5.2 ± 0.1 kg versus 5.2 ± 0.2 kg) or 5–6 mo studies (preterm versus term: 22.7 ± 0.3 w versus 22.4 ± 0.3 w; weight: 7.2 ± 0.2 kg versus 7.0 ± 0.2 kg).
Effects of Sleep Position and Sleep State on Baseline MAP and TOI
Sleep position did not affect MAP at any age in either group (Table 1). Sleep position had a significant effect on cerebral TOI, which in preterm infants was significantly lower in the prone compared to the supine position in both sleep states at 2–4 w and 2–3 mo CA; at 5–6 mo there was an overall effect of sleep position on TOI; however, post hoc analysis was unable to determine where the difference occurred. In term infants cerebral TOI was consistently lower in the prone compared to the supine position reaching significance in AS at 2–4 w.
Table 1.
Effects of sleep position and sleep state on baseline mean arterial pressure and cerebral tissue oxygenation index in term and preterm infants.

Sleep state had a significant effect on MAP and cerebral TOI in both groups. In preterm infants MAP was higher in AS compared to QS in both sleep positions at 2–4 w and in the prone position at 2–3 mo. In term infants MAP was higher in AS compared to QS in the prone position at 2–4 w and in both sleep positions at 2–3 mo. In preterm infants cerebral TOI was higher in QS compared to AS in both sleep positions at 2–4 w; there was no effect of sleep state at 2–3 mo and at 5–6 mo cerebral TOI was higher in AS compared to QS. In term infants cerebral TOI was higher in QS compared to AS in the prone position at 2–4 w, with no effect of sleep state seen at any other age.
Both MAP and cerebral TOI were lower in the preterm compared to the term group. Specifically, preterm infants had lower MAP compared to term infants in both sleep states in the prone position at 2–3 mo CA, with no differences seen at either 2–4 w or 5–6 mo. Cerebral TOI was also significantly lower in preterm compared to term infants in both sleep states and positions at 2–4 w CA and in both sleep states in the prone position at 2–3 mo CA. There was no effect of preterm birth on baseline cerebral TOI at 5–6 mo CA.
Effects of Sleep Position and Sleep State on Preterm Infant MAP and TOI Responses to HUT: Supine Position
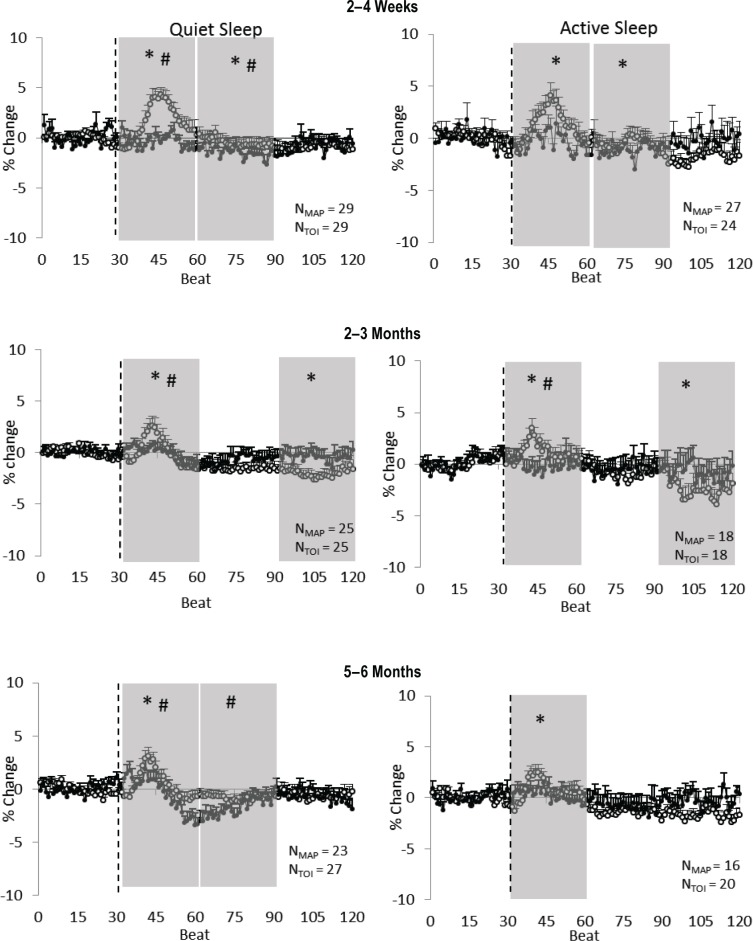
In preterm infants in the supine position, in both sleep states and at all three ages, MAP showed a characteristic surge in the early phase of the HUT response (Figure 1). Following the initial surge, MAP fell below baseline in both sleep states at 2–4 w (middle phase P < 0.05) and 2–3 mo CA (late phase P < 0.05). At 5–6 mo CA, following the initial surge, MAP remained at baseline for the remainder of the HUT response in both sleep states.
Figure 1.
Preterm infants: supine position. Mean arterial pressure (MAP) (open circles) and cerebral tissue oxygenation index (TOI) (closed circles) responses to a head-up tilt (HUT) (indicated by the dashed line) in quiet sleep (left) and active sleep (right) at 2–4 w (top), 2–3 mo (middle) and 5–6 mo (bottom) corrected age (CA). Results are mean ± standard error of the mean; N values represent numbers of infants. Shaded areas represent phases in which significant changes from baseline occurred; *P < 0.05 MAP versus baseline; #P < 0.05 TOI versus baseline.
In QS, there was a small, but statistically significant fall in TOI below baseline at 2–4 w CA (early phase P < 0.05; middle phase P < 0.05), 2–3 mo (early phase P < 0.05) and 5–6 mo CA (early phase P < 0.05 and middle phase P < 0.05). In AS, TOI remained at baseline for the duration of the HUT response at 2–4 w CA and 5–6 mo CA but fell below baseline at 2–3 mo CA (early phase P < 0.05).
Effects of Sleep Position and Sleep State on Preterm Infant MAP and TOI Responses to HUT: Prone Position
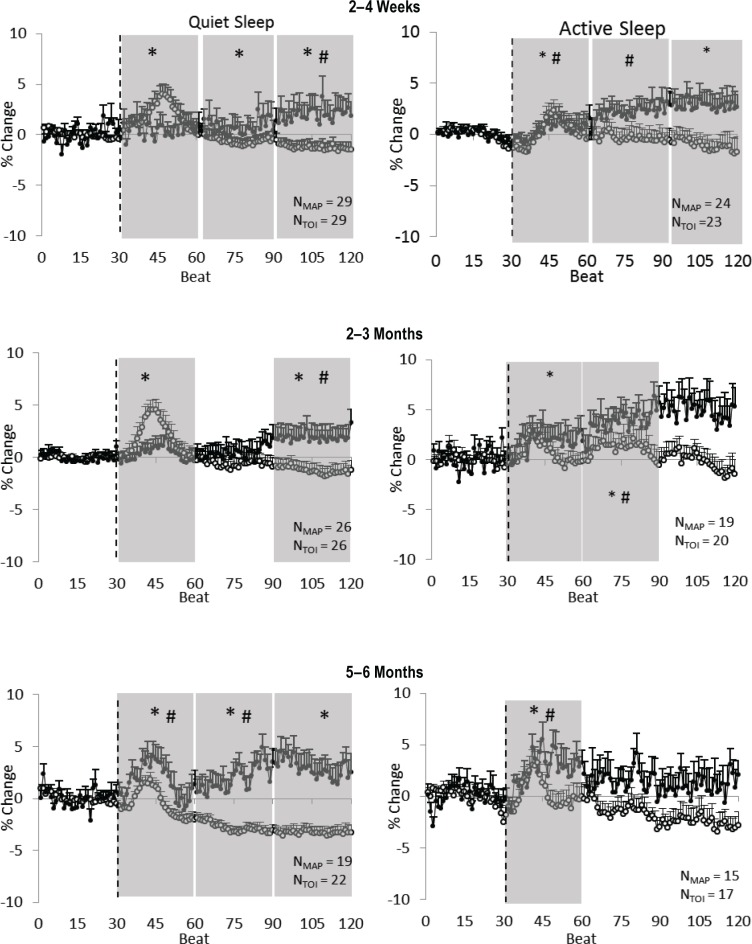
In preterm infants in the prone position, MAP also showed a characteristic surge in the early phase of the HUT response in both sleep states at all three ages (Figure 2). In QS, following the initial surge, MAP fell below baseline at 2–4 w (late phase P < 0.05), 2–3 mo (late phase P < 0.05) and at 5–6 mo (middle phase P < 0.05 and late phase P < 0.05). In AS, following the initial surge MAP fell below baseline at 2–4 w CA (late phase P < 0.05), rose above baseline at 2–3 mo (middle phase P < 0.05) and remained at baseline at 5–6 mo CA.
Figure 2.
Preterm infants: prone position. Mean arterial pressure (MAP) (open circles) and cerebral tissue oxygenation index (TOI) (closed circles) responses to a head-up tilt (HUT) (indicated by the dashed line) in quiet sleep (left) and active sleep (right) at 2–4 w (top), 2–3 mo (middle), and 5–6 mo (bottom) corrected age (CA). Results are mean ± standard error of the mean. Shaded areas represent phases in which significant changes from baseline occurred; *P < 0.05 MAP versus baseline; #P < 0.05 TOI versus baseline.
In QS, TOI rose above baseline at 2–4 w (late phase, P < 0.05), 2–3 mo CA (late phase, P < 0.05) and 5–6 mo (early phase, P < 0.05 and middle phase, P < 0.05). In AS, TOI also rose above baseline at 2–4 w CA (early phase P < 0.05 and middle phase P < 0.05), 2–3 mo CA (early phase P < 0.05 and middle phase P < 0.05) and at 5–6 mo CA (early phase P < 0.05).
Comparison of the TOI Response to a HUT between Preterm and Term Infants
The overall responses of cerebral TOI to a HUT were generally similar between term and preterm infants with only small (∼ 1–2%) differences observed.
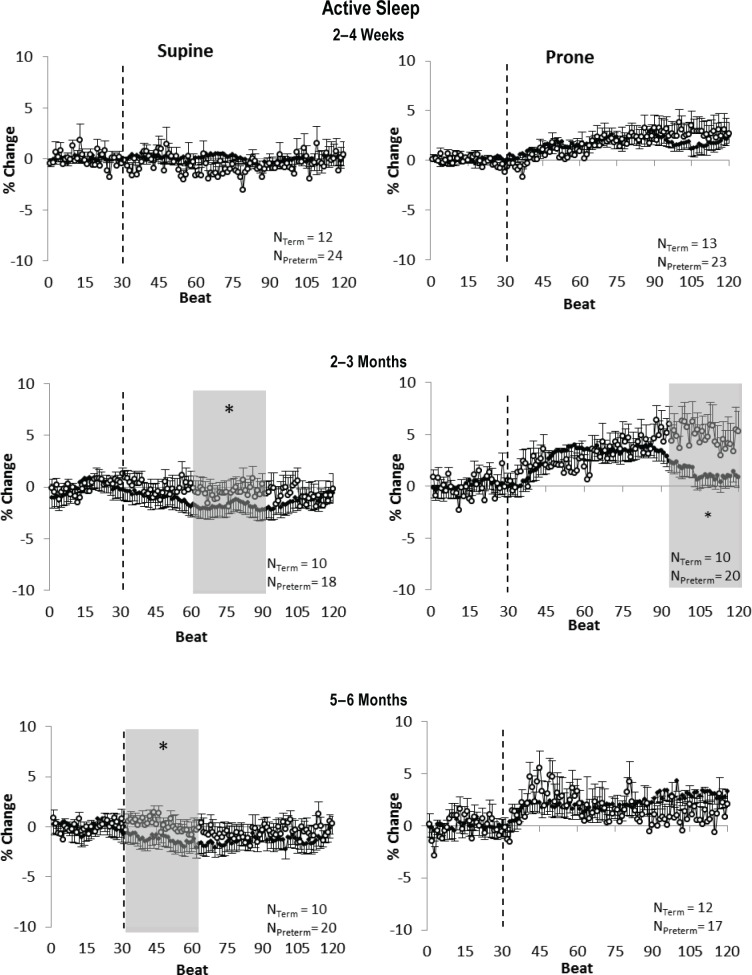
In QS (Figure 3) at 2–4 w, there were no differences in the TOI response to a HUT between term and preterm infants in either sleep position. At 2–3 mo in the prone position, there was a greater increase in TOI in preterm infants than term infants (late phase P < 0.05). At 5–6 mo in the supine position, TOI fell lower in preterm compared to term infants (middle phase P < 0.05 and late phase P < 0.05), but there were no differences between the term and preterm infant groups in the prone position.
Figure 3.
Comparison between term (closed circles) and preterm (open circles) infants of the response of cerebral tissue oxygenation index (TOI) to a head-up tilt (HUT) in quiet sleep in the supine position (left) and prone position (right) at 2–4 w (top), 2–3 mo (middle), and 5–6 mo (bottom). Shaded areas represent the early, middle, and late phases of the tilt response, shown only when there was an overall significant difference between term and preterm infants detected on analysis of variance. *P < 0.05 term versus preterm.
In AS (Figure 4) at 2–4 w, there were no differences in the TOI response to a HUT between term and preterm infants in either sleep position. At 2–3 mo in the supine position, there was a smaller fall in TOI in preterm infants compared to term infants (middle phase P < 0.05) and a greater increase in TOI in the prone position (late phase P < 0.05). At 5–6 months in the supine position, TOI fall was larger in term infants compared to preterm infants (early phase P < 0.05), and there were no differences in the TOI response between the infants groups in the prone position.
Figure 4.
Comparison between term (closed circles) and preterm (open circles) infants of the response of cerebral tissue oxygenation index (TOI) to a head-up tilt (HUT) in active sleep in the supine position (left) and prone position (right) at 2–4 w (top), 2–3 mo (middle), and 5–6 mo (bottom). Shaded areas represent the early, middle, and late phases of the tilt response, shown only when there was an overall significant difference between term and preterm infants detected on analysis of variance. *P < 0.05 term versus preterm.
It is important to note that the absolute tilt-related change in TOI in preterm and term infants was small and did not overcome the baseline differences in cerebral TOI between term and preterm infants as highlighted in (Figure 5) for QS and AS in the prone position at 2–3 mo.
Figure 5.
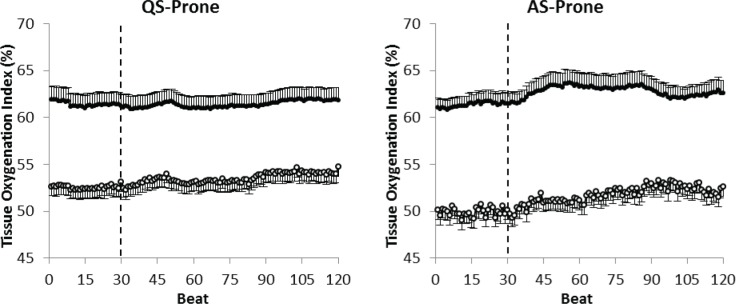
Comparison of the absolute change in cerebral tissue oxygenation index (TOI) in response to a head-up tilt (HUT) quiet sleep (left) and active sleep (right) in the prone position at 2–3 mo postterm age between term (closed circles) and preterm (open circles) infants. Note that the baseline TOI level in the preterm infants is lower than in the term infants, and remains significantly lower despite the greater increase during HUT.
Comparison of Variability of Responses to HUT between Preterm and Term Infants
In both sleeping positions and sleep states, the response of cerebral TOI to a HUT was significantly more variable in pre-term compared to term infants in all three phases at all three ages (Table 2).
Table 2.
Effect of preterm birth on variability in the response of tissue oxygenation index to a head-up tilt in the prone and supine positions.
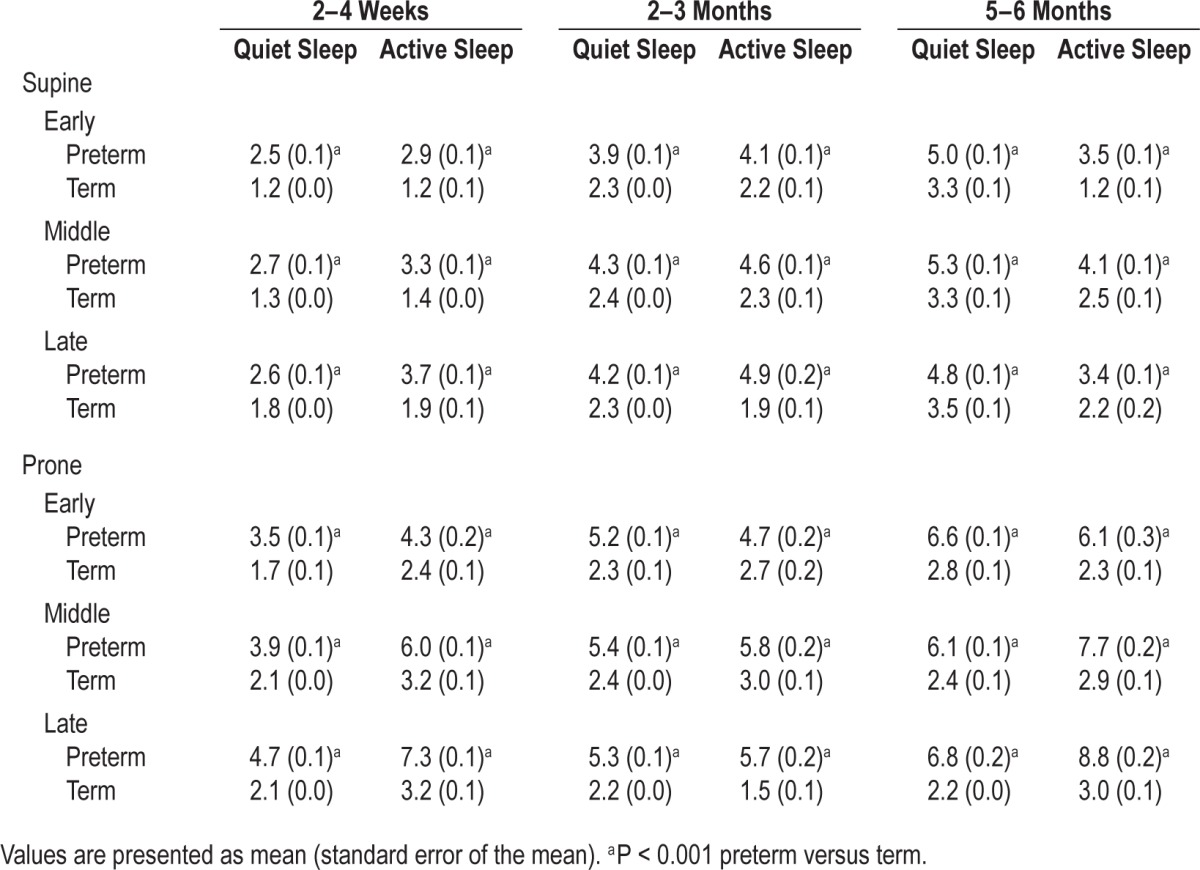
At 2–4 w the response of MAP to a HUT was more variable in preterm compared to term infants, reaching significance in all three phases in both QS-prone and AS-supine, and in the middle and late phases in QS-supine and AS-prone. The response of MAP was more variable in term infants in the early phase in QS-supine. At 2–3 mo the response of MAP to a HUT was also more variable in preterm compared to term infants, reaching significance in the middle and late phases in QS-supine, in all three phases in QS-prone and AS-supine, and in the middle phase in AS-prone. In the early phase in AS-prone, the response of MAP was more variable in term compared to preterm infants. At 5–6 mo the response of MAP to a HUT was more variable in term compared to preterm infants in the middle and late phases in QS-supine and in the early and middle phases in AS-prone. The response was more variable in preterm infants in all three phases in QS-prone and in the early and middle phases in AS-supine.
DISCUSSION
Our study provides novel data on cerebrovascular control in preterm infants beyond term-equivalent age. Consistent with findings in term infants, in the supine position cerebral oxygenation is tightly controlled even in the presence of a surge in MAP. In contrast, in the prone position, an orthostatic challenge results in an increase in cerebral oxygenation possibly due to cerebral vasodilation. Although the response to a HUT was similar between term and preterm infants, it is important to note that baseline cerebral oxygenation was reduced in pre-term compared to term infants, particularly in the prone position, potentially representing greater vulnerability to cerebral hypoxia in infants born preterm. Furthermore, preterm infants showed significantly greater variability in their responses compared to term infants, suggesting cerebrovascular control is less mature in preterm infants at matched postterm ages.
There is evidence to suggest that cerebral autoregulation is immature in preterm infants prior to term-equivalent age resulting in pressure passivity between systemic blood pressure and cerebral blood flow.14–16 This is particularly true among unwell preterm infants, but there is evidence to suggest that cerebral autoregulation is also impaired in clinically stable preterm infants.25 However, to our knowledge cerebral auto-regulation in preterm infants has not previously been assessed beyond term-equivalent age. Our data suggest that cerebral autoregulation is functioning effectively in preterm infants at 2–4 w CA. In the supine position and in the prone position in QS, cerebral TOI was maintained despite a surge in MAP, suggesting an autoregulatory increase in cerebrovascular resistance prevents a concomitant increase in cerebral blood flow. In QS in the supine position, cerebral TOI tended to fall below baseline approximately 15–20 beats after the HUT (20 beats at 2–4 w and 2–3 mo CA, 15 beats at 5–6 mo CA), coinciding with a fall in MAP. This is a pattern similar to that seen in term infants,9,25 and is likely to be due to the response time required for autoregulation-mediated cerebral vasodilation resulting in a temporary and minor decrease in cerebral blood flow in the presence of falling MAP.26,27 In the prone position in QS at both 2–4 w and 2–3 mo CA, cerebral TOI increased in the late phase of the HUT response when MAP decreased. This suggests increased cerebral blood flow due to cerebral vasodilation in response to reduced MAP. This increase in cerebral blood flow appears to slightly overshoot cerebral metabolic requirements leading to increased cerebral TOI.
In contrast, in the prone position in QS at 5–6 mo CA and at all three ages in AS, cerebral TOI increased immediately in response to the HUT. The increase in cerebral TOI preceded the surge in MAP induced by the HUT, consistent with findings in term infants.9,28 This suggests that changes in cerebral blood flow occur due to a vestibular-mediated response to a change in position and not in response to changing MAP. A previous study in preterm infants assessed at 36 w GA, sleeping in the supine position, found immature vestibular responses to side-ways shifts compared to healthy term infants assessed at 3 mo of age. The authors reported diminished heart rate and blood pressure responses to sideways motion tests in preterm infants and concluded that vestibular-mediated cardiovascular control is immature in preterm infants between 34 to 39 w postconceptional age.29 Our data suggest improvement in vestibular responses to a positional change by 42–44 w postconceptional age in healthy preterm infants, particularly in AS. However, failure of this immediate increase in cerebral oxygenation in response to a HUT in the prone position at 2–4 w and 2–3 mo in QS may represent immaturity of the vestibular response during the period of peak risk for SIDS.
Contrary to our hypothesis, we found that the response of cerebral oxygenation to a HUT was generally similar between term and preterm infants and there was no effect of gestational age at birth. However, despite the similarity, we believe that preterm infants may be at relatively greater risk of cerebral hypoxia than age-matched term infants. We have previously suggested in term infants9 that the observed increase in cerebral oxygenation in response to a HUT in the prone position may be a protective response to safeguard against critically reduced cerebral oxygenation in a position where baseline cerebral oxygenation is already reduced. Consistent with our previously reported results in the same cohorts of infants,12 in this study baseline cerebral oxygenation was significantly lower in preterm compared to term infants, with the difference approaching 10 percentage points at both 2–4 w and 2–3 mo CA in the prone position. Thus, baseline cerebral oxygenation in preterm infants is positioned significantly lower than in age-matched term infants, so that a comparatively greater increase in cerebral oxygenation following a change in position would be required to ensure similar protection against cerebral hypoxia. Studies in both humans and animals suggest that cerebral hypoxic damage occurs with cerebral oxygenation levels below 40%.30,31 Preterm infants with a baseline cerebral TOI of approximately 50% are dangerously close to pathologically impaired cerebral oxygenation in the prone position at 2–3 mo CA. Thus, although the percentage increase in cerebral TOI in response to a HUT may be greater in preterm infants at 2–3 mo in QS in the prone position, this increase remains insufficient to overcome the baseline deficit in cerebral TOI in preterm compared to term infants as highlighted in Figure 5; thus, absolute cerebral TOI remains lower in the preterm cohort during a HUT. Therefore, despite generally similar HUT responses between term and preterm infants, preterm infants may still be at greater comparative risk due to their lower baseline cerebral oxygenation levels in the prone position.
Despite generally similar responses between preterm and term infants of cerebral TOI to a HUT, we found significantly and consistently greater variability in the responses of the pre-term cohort even when variability in the MAP response was greater in the term cohort. Greater variability in the cerebrovascular response to a HUT has previously been suggested to reflect immaturity of cerebrovascular control.32 This suggests that immature cerebrovascular control persists across the first 6 mo of infancy in preterm infants. The response of cerebral blood flow to a HUT has been shown to become more uniform with increasing gestational age in preterm infants assessed at < 26 w, 27–32 w, and > 32 w of gestation, with the pattern in older preterm infants being similar to that in term infants.32 We found no significant correlation between gestational age at birth and the TOI response to a HUT, suggesting that postconceptional age influences cerebrovascular control regardless of gestational age at birth in preterm infants.
Implications for SIDS
Preterm infants are at significantly increased risk for SIDS,13,33 the underlying mechanism for which is commonly believed to be due to immaturity of cardiovascular control.34 We have recently identified that preterm infants have significantly reduced cerebral oxygenation compared to term infants, particularly in the prone sleeping position during QS.12 Greater variability in the cerebral vascular response to a HUT suggests that cerebral vascular control is immature in preterm compared to term infants across the first 6 mo after term-equivalent age. Furthermore, in preterm infants, the presumed protective increase in cerebral oxygenation immediately after a postural change in the prone position9 is delayed in QS at 2–4 w and 2–3 mo CA. We suggest that these factors could denote a greater risk of cerebral hypoxia during sleep in preterm infants, particularly during QS, which could contribute to their heightened risk for SIDS.
CONCLUSIONS
To our knowledge this is the first study to assess cerebrovascular control in preterm infants beyond term-equivalent age. We have shown that control of cerebral oxygenation in response to a cardiovascular challenge is similar to that of term infants at matched postconceptional ages across the first 6 mo postterm. However, preterm infants display greater variability in their responses, suggesting immaturity of cerebrovascular control persists beyond term-equivalent age. Consistent with findings in term infants, the prone sleeping position is associated with an altered cerebrovascular response to HUT, possibly to protect against cerebral hypoxia in a condition when baseline cerebral oxygenation is low.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded by the National Health and Medical Research Council of Australia project grant number: APP1006647 and the Victorian Government's Operational Infrastructure Support Program. Dr Fyfe wrote the first draft of the manuscript and no payment was given to anyone to produce the manuscript. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
We would like to thank the parents and infants involved in this study and the staff at the Melbourne Children's Sleep Centre.
REFERENCES
- 1.Murphey SL, Xu J, Kochanek KD. Deaths: final data for 2010. Hyattsville, MD: National Centre for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2.Harper RM. Sudden infant death syndrome: a failure of compensatory cerebellar mechanisms? Pediatr Res. 2000;48:140–2. doi: 10.1203/00006450-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kato I, Franco P, Groswasser J, Scaillet S, Kelmanson I, Togari H, et al. Incomplete arousal processes in infants who were victims of sudden death. Am J Respir Crit Care Med. 2003;168:1298–1303. doi: 10.1164/rccm.200301-134OC. [DOI] [PubMed] [Google Scholar]
- 4.Moon RY, Horne RS, Hauck FR. Sudden infant death syndrome. Lancet. 2007;370:1578–87. doi: 10.1016/S0140-6736(07)61662-6. [DOI] [PubMed] [Google Scholar]
- 5.Halloran DR, Alexander GR, Halloran DR, Alexander GR. Preterm delivery and age of SIDS death. Ann Epidemiol. 2006;16:600–6. doi: 10.1016/j.annepidem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Yiallourou SR, Walker AM, Horne RS. Effects of sleeping position on development of infant cardiovascular control. Arch Dis Child. 2008;93:868–72. doi: 10.1136/adc.2007.132860. [DOI] [PubMed] [Google Scholar]
- 7.Yiallourou SR, Walker AM, Horne RS. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep. 2008;31:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 8.Wong FY, Witcombe NB, Yiallourou SR, Yorkston S, Dymowski AR, Krishnan L, et al. Cerebral oxygenation is depressed during sleep in healthy term infants when they sleep prone. Pediatrics. 2011;127:e558–65. doi: 10.1542/peds.2010-2724. [DOI] [PubMed] [Google Scholar]
- 9.Wong F, Yiallourou SR, Odoi A, Browne P, Walker AM, Horne RSC. Cerebrovascular control is altered in healthy term infants when they sleep prone. Sleep. 2013;36:1911–8. doi: 10.5665/sleep.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair PS, Platt MW, Smith IJ, Fleming PJ, Group CSR, Platt MW. Sudden infant death syndrome and sleeping position in pre-term and low birth weight infants: an opportunity for targeted intervention. Arch Dis Child. 2006;91:101–6. doi: 10.1136/adc.2004.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson HL, Walker AM, Horne RSC. Sleep position alters arousal processes maximally at the high-risk age for sudden infant death syndrome. J Sleep Res. 2008;17:450–7. doi: 10.1111/j.1365-2869.2008.00683.x. [DOI] [PubMed] [Google Scholar]
- 12.Fyfe KL, Yiallourou SY, Wong FY, Odoi A, Walker AM, Horne RSC. Cerebral oxygenation in preterm infants. Pediatrics. 2014;134:435–45. doi: 10.1542/peds.2014-0773. [DOI] [PubMed] [Google Scholar]
- 13.Trachtenberg FL, Haas EA, Kinney HC, Stanley C, Krous HF. Risk factor changes for sudden infant death syndrome after initiation of back-to-sleep campaign. Pediatrics. 2012;129:630–8. doi: 10.1542/peds.2011-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106:625–32. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- 15.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–73. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 16.Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121:e604–11. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- 17.Gilmore MM, Stone BS, Shepard JA, Czosnyka M, Easley RB, Brady KM. Relationship between cerebrovascular dysautoregulation and arterial blood pressure in the premature infant. J Perinatol. 2011;31:722–9. doi: 10.1038/jp.2011.17. [DOI] [PubMed] [Google Scholar]
- 18.Wong FY, Barfield CP, Horne RS, Walker AM, et al. Dopamine therapy promotes cerebral flow-metabolism coupling in preterm infants. Intensive Care Med. 2009;35:1777–82. doi: 10.1007/s00134-009-1602-5. [DOI] [PubMed] [Google Scholar]
- 19.Yiallourou SR, Walker AM, Horne RS. Validation of a new noninvasive method to measure blood pressure and assess baroreflex sensitivity in preterm infants during sleep. Sleep. 2006;29:1083–8. doi: 10.1093/sleep/29.8.1083. [DOI] [PubMed] [Google Scholar]
- 20.Grigg-Damberger M, Gozal D, Marcus CL, Quan SF, Rosen CL, Chervin RD, et al. The visual scoring of sleep and arousal in infants and children. J Clin Sleep Med. 2007;3:201–40. [PubMed] [Google Scholar]
- 21.Anders T, Emde RN, Parmelee AH University of California LABIS. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. UCLA Brain Information Service. 1971 [Google Scholar]
- 22.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. [Google Scholar]
- 23.Witcombe NB, Yiallourou SR, Walker AM, Horne RSC. Delayed blood pressure recovery after head-up tilting during sleep in preterm infants. J Sleep Res. 2009;19:93–102. doi: 10.1111/j.1365-2869.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 24.Hoaglin DC, Mosteller F, Tukey JW. Understanding robust and exploratory data analysis. Wiley. 1983 [Google Scholar]
- 25.Boylan GB, Young K, Panerai RB, Rennie JM, Evans DH. Dynamic cerebral autoregulation in sick newborn infants. Pediatr Res. 2000;48:12–17. doi: 10.1203/00006450-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Aaslid R, Newell DW, Stooss R, Sorteberg W, Lindegaard KF. Assessment of cerebral autoregulation dynamics from simultaneous arterial and venous transcranial Doppler recordings in humans. Stroke. 1991;22:1148–54. doi: 10.1161/01.str.22.9.1148. [DOI] [PubMed] [Google Scholar]
- 28.Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, et al. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol. 2006;100:1475–82. doi: 10.1152/japplphysiol.01585.2005. [DOI] [PubMed] [Google Scholar]
- 29.Viskari-Lahdeoja S, Hytinantti T, Andersson S, Kirjavainen T, Viskari-Lahdeoja S, Hytinantti T, et al. Acute cardiovascular responses in preterm infants at 34-39 weeks of gestational age. Early Hum Devel. 2012;88:871–7. doi: 10.1016/j.earlhumdev.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab. 2002;22:335–41. doi: 10.1097/00004647-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Silas R, Sehgal A, Walker AM, Wong FY. Cerebral oxygenation during subclinical seizures in neonatal hypoxic-ischaemic encephalopathy. Eur J Paediatr Neurol. 2012;16:304–7. doi: 10.1016/j.ejpn.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Anthony MY, Evans DH, Levene MI. Neonatal cerebral blood flow velocity responses to changes in posture. Arch Dis Child. 1993;69:304–8. doi: 10.1136/adc.69.3_spec_no.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malloy MH. Prematurity and sudden infant death syndrome: United States 2005-2007. J Perinatol. 2013;33:470–5. doi: 10.1038/jp.2012.158. [DOI] [PubMed] [Google Scholar]
- 34.Horne RSC, Witcombe NB, Yiallourou SR, Scaillet S, Thiriez G, Franco P. Cardiovascular control during sleep in infants: implications for sudden infant death syndrome. Sleep Med. 2010;11:615–21. doi: 10.1016/j.sleep.2009.10.008. [DOI] [PubMed] [Google Scholar]