Abstract
Objective:
Twin modeling was used to conduct a genetically informative longitudinal analysis of insomnia symptoms in both sexes.
Method:
Data from the Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (n = 7,500) were used. Past-month insomnia symptoms were assessed at two time points with the shortened version of the Symptom Checklist-90. A composite score for the insomnia items (trouble falling asleep, restless or disturbed sleep, early morning awakenings) was computed. Twin modeling on the composite score was conducted in OpenMx to decompose the phenotypic variance, to examine the longitudinal stability of etiologic influences on insomnia symptoms, and to test for sex differences.
Results:
Insomnia symptoms were most commonly endorsed at a mild severity level (composite score mean = 2.24, standard deviation = 2.51). There was no evidence for sex effects in either of the univariate models, and insomnia symptoms were found to be modestly heritable (∼25% at Time 1 and ∼22% at Time 2). In the longitudinal measurement model, which accounts for error of measurement, the heritability for the latent factor of insomnia symptoms increased substantially, and demonstrated quantitative sex differences. The heritability of the latent insomnia factor was ∼59% in females and ∼38% in males.
Conclusions:
Genetic factors influence insomnia symptoms in adults, moreso for females than males, and these influences are largely stable over time. When taking into account measurement error, heritability estimates are substantial, but unique environmental factors continue to account for a large amount of variance in insomnia symptoms.
Citation:
Lind MJ, Aggen SH, Kirkpatrick RM, Kendler KS, Amstadter AB. A longitudinal twin study of insomnia symptoms in adults. SLEEP 2015;38(9):1423–1430.
Keywords: genetics, insomnia symptoms, longitudinal, sleep, sex, twins
INTRODUCTION
Sleep problems are common, with approximately one third of adults reporting at least one of the main nighttime insomnia symptoms from the fourth edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM) criteria.1–3 These symptoms include difficulty falling asleep, difficulty staying asleep, or nonrestorative sleep.4 The new guidelines in the fifth edition of the DSM (DSM-5) define insomnia as sleep dissatisfaction (with quality or quantity) associated with the symptoms of difficulty initiating or maintaining sleep, or early morning awakenings, present for at least 3 nights a week for a minimum of 3 mo, and causing clinically significant distress or impairment.5 The prevalence of insomnia when a full DSM or International Classification of Diseases definition is used is approximately 6% to 10%,3 and the disorder varies with age: insomnia is more common in the middle aged and the elderly than in young adults.3 Although young adults are less likely to experience difficulty sleeping, they more commonly report problems with sleep initiation.3 Older adults tend to experience sleep maintenance problems, and early morning awakenings are most common in the elderly.3 Sex differences exist in that the prevalence of insomnia is higher in women than in men.3 Results from a meta-analysis of insomnia studies report a risk ratio of 1.41 for females versus males.6
Insomnia has many negative consequences that can be both short term and long term.3 Chronic insomnia is a precursor for new onset psychiatric disorders,3 demonstrating the importance of adequate sleep in maintaining mental health. In fact, upward of 40% of people with insomnia are estimated to have a comorbid psychiatric condition.2,7,8 Furthermore, the presence of a psychiatric disorder is a known risk factor for insomnia2,3,7,8; thus, the extant data suggest a bidirectional relationship between sleep and psychopathology.9 Additionally, insomnia is associated with negative physical health outcomes, with evidence supporting a higher prevalence and/or increased risk of hypertension, diabetes, metabolic syndrome, and cardiovascular disease, among other negative health outcomes, in insomnia sufferers.3 Research also indicates that poor sleep is associated with a higher mortality rate.10,11 One suggested mechanism for the relationship between insomnia and physical health outcomes is via immune system alterations; individuals with chronic insomnia have been shown to have lower counts of certain lymphocytes (e.g., CD3+) than normal sleepers.12 These risks underscore the need for more research into the underlying causes of sleep problems.
Both family studies13 and twin studies14 have provided evidence for a genetic influence on insomnia. Twin studies have contributed to our understanding of the etiology of sleep disorders, resulting in more than 100 publications in the past 50 y on the heritability of sleep and sleep related disorders, such as insomnia.13 Previous estimates of the heritability of insomnia are moderate, with additive genetic effects ranging from 0.25 to 0.57 in adults.14 There is some evidence that the genetic influences on insomnia symptoms may vary by sex,15 but this has yet to be formally tested in a twin model for both quantitative (if the same genes influence each sex but in different amounts) and qualitative (if there is evidence for different genes influencing each sex) sex effects.16 The etiologic contributions of genetic and environmental influences may also differ across development, and this has been documented in the adolescent sleep literature.13
Longitudinal studies have reported evidence in support of the stability of genetic influences on sleep problems over time in children.17 However, no genetically informative adult longitudinal studies including both sexes exist. Thus, questions remain with regard to the stability of the genetic influence on this phenotype across time, as well as the potential for genetically based sex differences. Thus, this study seeks to expand the twin literature on the genetics of insomnia to fill these two critical limitations using longitudinal data from the Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (VATSPSUD).16 Aims include: (1) Determining the heritability of insomnia symptoms at the univariate level in this sample using a composite score; (2) Evaluating the stability of insomnia symptoms over time to better understand the stability of both the genetic and environmental contributions via a measurement model; and (3) Fitting all models to evaluate both qualitative and quantitative sex differences.
METHODS
Sample
Participants were derived from two interrelated Virginia Adult Twin Studies of Psychiatric and Substance Use Disorders (VATSPSUD) studies of Caucasians,16 ascertained from the birth certificate-based Virginia Twin Registry. Female-female (FF) twin pairs, born 1934–1974, were eligible if both members responded to a mailed questionnaire in 1987–1988. Data were collected at the first (FF1) interview wave conducted in 1987–1989, second (FF2) interview wave conducted in 1989– 1991, third (FF3) interview wave conducted in 1992–1995, and fourth (FF4) interview wave conducted in 1995–1997, with cooperation ranging from 88% to 92%. Data on the male-male and male-female pairs (MMMF) came from a sample (birth years 1940–1974) initially ascertained with a 72% cooperation rate from registry records containing all twin births. The first interview (MMMF1) was completed by phone in 1993– 1996, and the second interview (MMMF2) was conducted in 1994–1998. Response rates ranged from 72% to 83%. Our analyses used data from FF1 and MMMF1 as Time 1, and FF3 and MMMF2 as Time 2; for females (FF) the average number of years between Time 1 and 2 was 5.11 (standard deviation [SD] = 0.42), and for males (MMMF) the average number of years between Time 1 and 2 was 1.59 (SD = 0.73).
Zygosity was determined by discriminant function analyses using standard twin questions validated against DNA geno-typing in 496 pairs.18 The mean (SD) age of the twins was 29.3 (7.7) at the FF1 interview, 35.1 (7.5) at the FF3 interview, 35.5 (9.1) at the MMMF1 interview, and 37.0 (9.1) at the MMMF2 interview, whereas the mean (SD) years of education were 13.5 (2.1) at FF1, 13.4 (2.6) at MMMF1, and 13.6 (2.6) at the MMMF2 interview. No data on years of education was obtained at FF3. The VATSPSUD contains data from approximately 7,500 twins, including both members of 3,084 pairs (503 monozygotic (MZ) FF, 346 dizygotic (DZ) FF, 703 MZ MM, 485 DZ MM, and 1,047 opposite sex DZ pairs) and 1,325 twins without their cotwin. (These numbers do not sum because all possible pairings for triplet and quadruplet sets are included in this total). Our specific analyses used this data but excluded triplets and quadruplets.
Insomnia Measurement
Participants completed a shortened version of the Symptom Checklist-90 (SCL-90)19 at every interview wave except for FF2. The SCL items are asked using a past month timeframe and include three symptoms of insomnia: trouble falling asleep, sleep that is restless or disturbed, and awakening in the early morning. All items had five response options (scored as 0–4): not at all, a little bit, moderately, quite a bit, and extremely. A composite score for these SCL-90 sleep items was calculated by adding up the scores for each individual item across participants at both Wave 1 and Wave 2. Scores ranged between 0 and 12. The composite sum score was reorganized into four categories to facilitate ordinal twin data analysis: 0 [0], 1 [1,2], 2 [3,4], and 3 [5–12]. The sleep items showed sufficient internal reliability (Cronbach's alpha = 0.80).
Twin Modeling
In the classic twin model, phenotypic variation can be decomposed into additive genetic factors (A), which contribute twice as much to the correlations between MZ twins as they do for DZ twins; common environmental factors (C), which are the shared factors (e.g., parental attitudes, economic disadvantage) that make twins reared together more similar and contribute equally to the correlation between MZ and DZ twins; and individual specific environmental (E) sources, which reflect environmental experiences not shared by twins and therefore contribute to differences between the twins. This component also includes random errors of measurement.
The insomnia symptom composite score was used in all analyses. We tested for qualitative sex differences (i.e., whether or not the same genetic factors influenced liability to insomnia symptoms for males and females using opposite sex twin pairs, quantified and tested by rg), and for quantitative sex differences (i.e., if there is equality in the estimates of the genetic contribution in males and females) by fitting three models that were compared to the saturated model. First, we constrained rg to 1.0 to test for qualitative sex effects. Second, we tested for quantitative sex effects by constraining the estimates of A, C, and E to be equal in males and females. Finally, we computed a model to test for both qualitative and quantitative sex effects. To evaluate the fit of the different twin model specifications, a full information maximum likelihood approach for raw data as implemented in the freely available OpenMx software20,21 was used. The information-based Akaike Information Criterion (AIC) is also used to evaluate different twin models. AIC is an index balancing goodness of fit; i.e., balance of explanatory power and parsimony.22 Parsimony is an important consideration because with maximum likelihood estimation, log likelihoods will continue to decrease as more parameters are estimated and result in “overfitting.” AIC penalizes models with increasing numbers of parameters, thereby providing an appropriate balance between model complexity and explanatory power as manifest by the degree of misfit.23
Univariate Models
We conducted separate univariate models of the insomnia symptom composite score for Time 1 (FF1, MMMF1) and Time 2 (FF3, MMMF2). Beginning with a saturated model as the baseline, models were fit and compared that included constraints for qualitative and quantitative sex effects. After choosing the best-fit ACE model from the sex-specific testing from each time point, we then fitted subsequent models in which variance component parameters were dropped to determine the most parsimonious model (AE and CE models).
Measurement Models
The insomnia symptom composite scores from each assessment wave were used as indicators in a common factor measurement model. This latent factor model assumes that there is an unobserved liability that is common across the two insomnia phenotype time points. Each of the insomnia phenotypes is considered to be a fallible indicator of the true latent liability. The paths λ1 and λ2 (i.e., factor loadings) represent the strength of the relationship between the observed insomnia variables and the common factor. The product of these paths reflects the common reliable and stable covariation of insomnia across time. The latent common liability, as well as time-specific liability, for insomnia was modeled in a standard ACE twin design, as outlined previously. Two departures from the standard twin model and the measurement model are noteworthy. First, the model allows for separate estimates of occasion specific influences (including measurement error) and true or enduring individual specific environmental effects. Second, an estimate of the degree to which the time specific measurements of insomnia are reflective of the true latent liability for insomnia symptoms is produced.
RESULTS
Descriptive Statistics
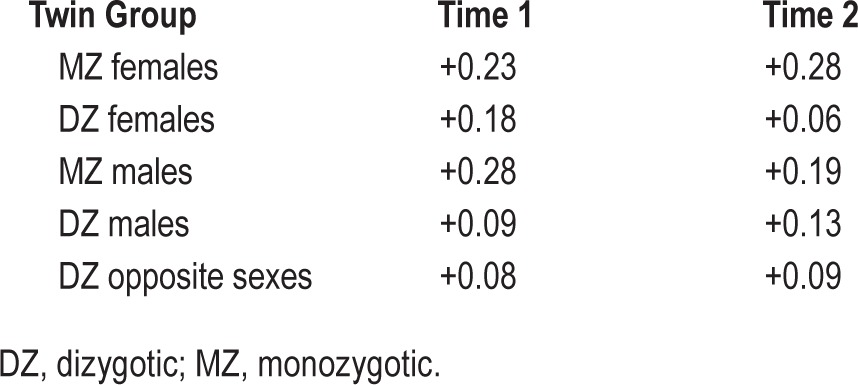
Insomnia symptoms were most commonly endorsed at a mild severity level (mean composite score = 2.24, SD = 2.51, range = 0–12). Correlations between the individual sleep items and age were low (polyserial correlations ranging from +0.02 to +0.06 across the time points). Thus, age was not further considered. In order to determine if the construction of a composite score was justified, a phenotypic factor analysis was run. All three sleep items loaded highly onto a single common factor: estimated factor loadings were 0.78 (trouble falling asleep), 0.99 (restless or disturbed sleep), and 0.71 (early morning awakenings), suggesting that the aggregation of the three SCL sleep items into a single composite score is reasonable. The composite score average endorsement (after recoding into four categories to facilitate analysis) was 1.19 (SD = 1.07, range = 0–3). Mean (± SD) sleep composite score across both time points was 1.21 ± 1.09 in females and 1.17 ± 1.05 in males (t (df = 16158) = 2.74, P = 0.01). The correlations between the sleep composite score and age at the time of interview were also low (∼ +0.04 for both time points). Table 1 reports the polychoric correlations across twin groups for each time point. Overall, point estimates of MZ correlations were higher than DZ correlations for both sexes, and MZ female correlations were higher on average than those for MZ males.
Table 1.
Polychoric correlations for insomnia symptom composite score.
Univariate Twin Models
Table 2 presents the twin models that were fitted for insomnia symptoms at Time 1. The saturated model (Model I) served as a reference for comparisons with the subsequent models. We began by testing for qualitative sex effects (Model II), and then continued to test for quantitative effects (Model III) and a model that was fully constrained for both types of sex effects (Model IV). There was no evidence for qualitative or quantitative sex effects, as constraining rg to 1 or forcing male and female parameters to be equal did not significantly degrade the model misfit or AIC change. These results indicated Model IV to be the best-fitting model, with equality constraints for both sex effects. Using this model we fit the sub-models that set to zero all C paths (AE model) or all A paths (CE model). Dropping C from Model IV did not negatively affect model fit, whereas dropping A resulted in deterioration in fit. Thus, the best-fitting model for Time 1 was an AE model with no sex effects.
Table 2.
Results of model fitting (univariate) to determine the influence of genetic and environmental factors on insomnia symptom composite score for individual time points.
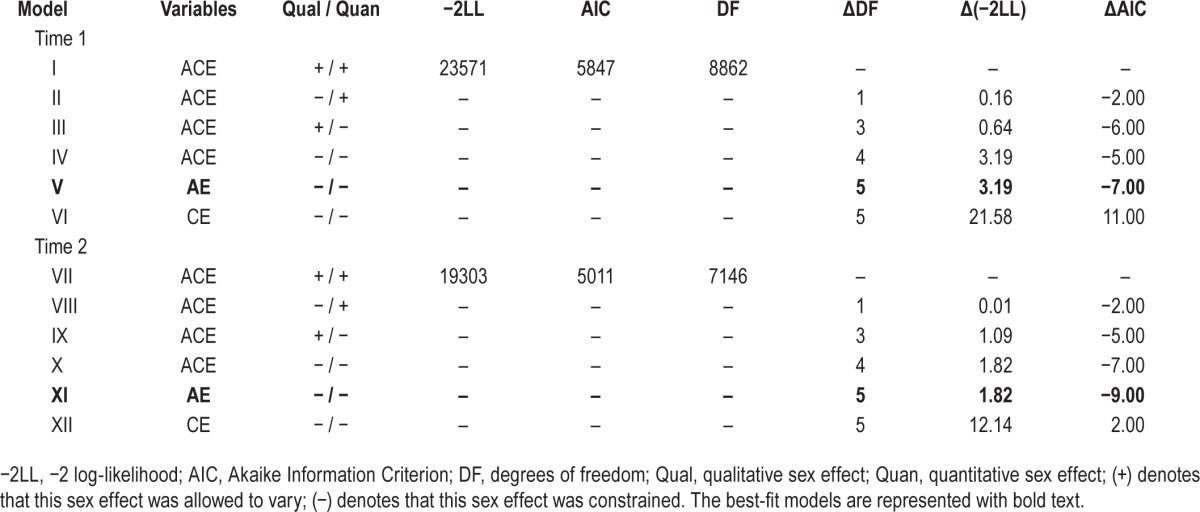
Table 2 also presents the model fitting results for Time 2. Similar to Time 1, there was no evidence for qualitative or quantitative sex effects (Models VIII and IX) and the initial best-fit model constrained for both types of sex differences (Model X). C could also be dropped from this model (Model XI), whereas A (Model XII) could not, resulting in Model XI as the overall best-fit model at Time 2.
Figure 1 presents the parameter estimates for the univariate models. Additive genetic components contributed 25.3% to the variance in insomnia symptoms at Time 1, and 22.2% at Time 2.
Figure 1.
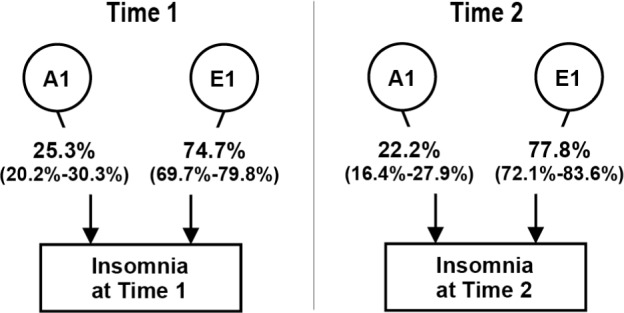
Contributions (percent variance; 95% confidence interval) of genetic and environmental factors to insomnia symptoms at Time 1 and Time 2; best-fit univariate models.
Measurement Models
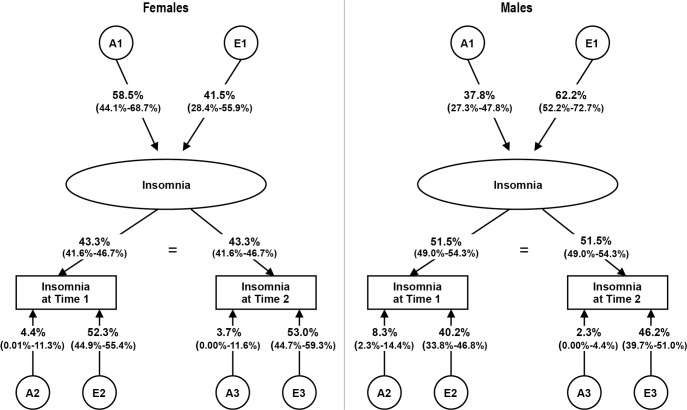
We began by fitting a form of a longitudinal ACE measurement model for the insomnia composite score (see Table 3, Model I). From this model we tested for both qualitative (Model II) and quantitative (Model III) sex effects. As shown in Table 3, there was no evidence for qualitative sex effects. However, there was evidence for quantitative sex effects, as constraining the path coefficient estimates (common, lambdas, and specifics) across the sexes resulted in a deterioration in fit. Thus, the best-fit model for sex differences did not contain qualitative sex effects but required allowing path estimates to differ across the sexes (i.e., quantitative sex effects). From this model, we then fit the AE and CE submodels. As shown in Table 3, dropping C resulted in an improvement in model fit (Model IV), whereas dropping A (Model V) showed deterioration in fit. From the AE model we also tested if the time-specific A estimates (A2 and A3) could be fixed to 0 for Times 1 and 2 (Model VI). Model fit deteriorated for these constraints, making Model IV our final model. Parameter estimates for the final measurement models can be seen in Figure 2.
Table 3.
Results of model fitting to determine the genetic and environmental influences on the variance in insomnia symptoms across multiple time points (measurement model)
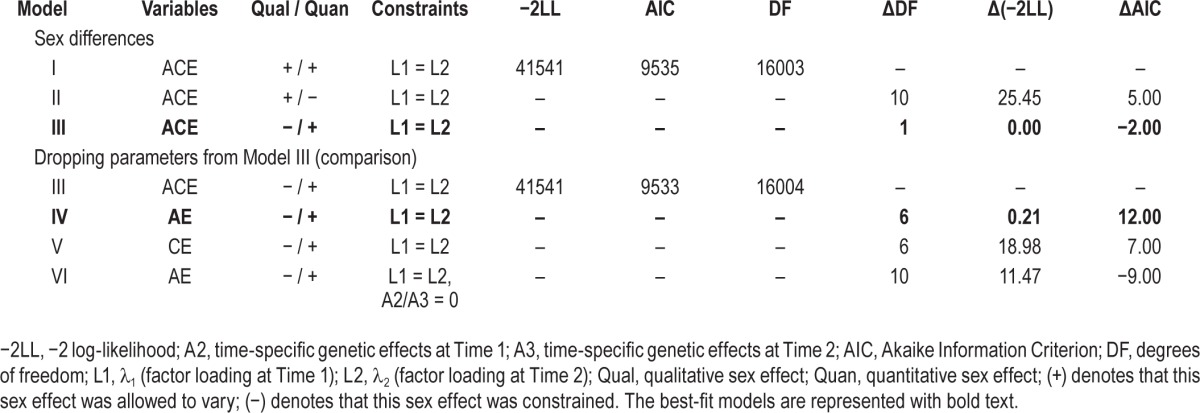
Figure 2.
Contributions (percent variance; 95% confidence interval) of genetic and environmental factors to insomnia symptoms at Time 1 and Time 2 (best-fit longitudinal model) across the sexes.
There are five key findings to be drawn from the final model. First, we found evidence for quantitative sex effects in the longitudinal model, suggesting that the heritability of insomnia symptoms is different/larger for females than males. Second, heritability for the latent factor for insomnia was in the moderate range when unconfounded by measurement error (58.5% in females and 37.8% in males; see Figure 2). Third, the two time points provided a good index of the true level of insomnia symptoms, with the predicted stability of our insomnia construct across time points equal to 0.43 for females and 0.52 for males. Fourth, the overall heritability at the individual time points was in the mild range, with most of the genetic influences attributable to common, enduring effects: for Time 1, the overall heritability was 29.7% for females, with 85.3% of this associated with the latent common insomnia construct and 14.7% specific to Time 1. For males, the overall heritability was slightly lower at Time 1, 27.7%, with 70.2% of this coming from the latent construct and 29.8% time-specific. Results were similar at Time 2, with the overall heritability for females at 29.1%, with 87.2% from the latent factor and the remaining 12.8% time-specific. Overall heritability at Time 2 was once again lower for males, 21.7%, with 89.6% common and 10.4% time-specific. Fifth, time-specific environmental effects and/ or measurement error had a greater effect on the etiology of insomnia symptoms than enduring environmental effects for females, making up approximately 75% of the total E at both Time 1 and Time 2. For males, the environmental effects were more evenly split between specific and latent, with 56% of all E at Time 1 and 59% of all E at Time 2 due to time-specific environmental effects.
DISCUSSION
This study has two innovative features: first, it is the only study to date that examines the genetic and environmental influences on insomnia symptoms in adults in a longitudinal, representative twin sample; and second, this is the first study to formally test for sex differences, as well as estimate and test the stability of the etiologic influences on both the common/stable aspect of insomnia over time in addition to influences specific to the different assessments. These methodological advances help fill critical gaps in the literature. The longitudinal measurement model allowed us to account for the unreliability of measurement by separating measurement error and occasion-specific effects from the common, enduring environmental effects. In doing so, our estimate of the heritability of the latent construct of insomnia was substantially higher than estimates obtained from the time-specific analyses. Also, quantitative sex effects were detected within the longitudinal model.
Univariate Analyses
Univariate analyses of insomnia symptoms found moderate heritability estimates (∼25% at Time 1 and ∼22% at Time 2), which are consistent with, but on the lower end of, previously reported heritability estimates of insomnia.14 No sex differences (qualitative or quantitative) were detected for the univariate case. These results suggest that although genetic influences cannot be ignored in the etiologic study of insomnia symptoms, unique environment accounts for a greater proportion of variance in this phenotype. The lack of evidence for sex differences was somewhat surprising despite the fact that twin models do not address mean differences, given that the composite score was on average higher for females than males, and the between-twin correlations were also higher for females than males. However, confidence intervals around our point estimates were large and we may be underpowered to detect these differences at the univariate level.
Measurement Model
With measurement error and occasion-specific effects explicitly partitioned off, the heritability of the latent construct of insomnia representing the common stable aspect of insomnia across time noticeably increased from the univariate heritability estimates. Quantitative sex differences were also detected, although no evidence for qualitative sex effects was found. In our final measurement model genetic influences contribute ∼59% to the variance of the latent, underlying insomnia trait for females and ∼38% for males. For females, this point estimate is on the higher end of previous heritability estimates,14 and falls into the moderately high range.16 For males the heritability is in the moderate range16 and is consistent with past estimates.14 To our knowledge, this is the first twin study to formally test for sex differences in adult insomnia, demonstrating via quantitative sex effect testing that genetic influences are more important in the etiology of insomnia symptoms for females than for males. It is important to note the absence of qualitative sex effects, which suggests that although genes contribute relatively more to the common stable portion of insomnia symptoms in females versus males over time, it appears that the same genes are operative for both sexes. Given that the prevalence of insomnia is higher for females than males,3,6 it was not surprising to find evidence for quantitative sex effects. Our results are somewhat consistent with prior analyses of major depression in the VATSPSUD data, which has also been shown to be more heritable for females than males.16 However, note that qualitative sex effects have been shown for major depression.16 Our results suggest that clinicians should pay close attention to a family history of sleep problems in patients, and should educate patients, especially women, about proper sleep hygiene and consequences of disturbed sleep. Females are also at an increased risk for other forms of psychopathology such as major depression,24,25 and it is thought that insomnia itself is a risk factor for the development of new-onset psychopathology.3 Given this information, targeted interventions for sleep disturbances in females may be beneficial. Current research shows that treating sleep problems in individuals with both insomnia and depression can help to improve symptoms of both disorders.26,27
Compared to the univariate estimates, the time-specific heritability estimates were generally higher in our longitudinal model (with the exception of the male estimate at Time 2), but still in the mild range, which is consistent with the literature.14 The use of this longitudinal model is unique in that it allows us to obtain better heritability estimates, because we are using a latent insomnia variable and have partitioned out measurement error. Most of the genetic influences at the individual time points can be explained in our model by common, enduring effects. The loadings onto the latent factor for insomnia symptoms were high (as estimated by the λ parameters, which represent the degree to which the assessments of sleep problems obtained at the two assessments reflect the true liability), suggesting that the estimates of both genetic and environmental effects are reasonably stable across time. Approximately 85% of the total genetic influences at Time 1 and 87% at Time 2 could be attributed to the common, enduring component in females. Results were similar in males, with the majority of the heritable influence associated with the latent factor (∼70% and 90% at Times 1 and 2, respectively). Prior longitudinal studies of sleep have been done in children and adolescents,13 but not in adults. Thus, this study is one of the first to show that the majority of genetic influences appear to be on the common stable insomnia component across time and that there appears to be much less genetic innovation specific to early and mid adulthood. However, it should be noted that the time-specific genetic components could not be constrained to zero, suggesting that though small in magnitude, they do contribute to the overall etiologic picture. Although our results suggest that additive genetic influences seem to be much stronger for the stable aspect of insomnia covariation over time, further investigation into the specific genes involved in the development of insomnia symptoms is clearly needed.
This study also quantifies the degree to which environmental events are involved in insomnia symptoms across time, as cross-sectional studies are limited in how well errors of measurement can be handled. All environmental effects on insomnia symptoms could be attributed to unique environmental sources: no evidence for shared environmental influences was found. After partitioning out measurement error, unique environmental effects accounted for 42% of the variance of the common stable latent insomnia component in females and 62% in males. Of the total environmental effects at both Time 1 and Time 2 for females, approximately 75% was coming from time-specific effects that included measurement error, with the remaining variance being contributed by environmental influences on the latent insomnia variable. For males, close to half of the total environmental effect at Time 1 and Time 2 was accounted for by time-specific effects (56% and 59% at Time 1 and Time 2, respectively). These occasion-specific environmental effects comprise a much larger portion of the variance at individual time points than the occasion-specific genetic effects, which only contribute ∼13% to 30% across both sexes. These findings highlight the importance of considering the environment when addressing insomnia symptoms. Specific short-term experiences, such as feeling anxious due to problems at work or having a baby who cries all night, can have just as much, if not more, of an effect on current sleep symptoms as do more persistent influences. Stress is known to affect sleep, and the systems involved in the stress response (primarily the hypothalamic-pituitary-adrenal [HPA] axis) are also important in sleep and arousal.28 HPA-axis changes and hyperarousability are thought to be biological risk factors for insomnia,3 which could contribute to the specific or enduring environmental influences, depending on the nature of the stressor. For example, adverse childhood experiences, such as abuse or neglect, have been linked to poor sleep in adulthood.29–31 Additionally, other characteristics of childhood such as socioeconomic difficulty32 and family conflict33 have been shown to contribute to adult insomnia. Poor sleep early on may persist and influence sleep problems in adults.34
Limitations
The current results should be interpreted in light of a number of limitations. There was a limited number of sleep related items available in our twin assessment battery, as the VATSPSUD interviews did not specifically include items for insomnia that directly map onto DSM criteria or existing formal sleep questionnaires. Additionally, there are no data available on other comorbid sleep disorders (e.g., circadian rhythm disorders, restless legs syndrome), and thus these could possibly contribute to and inflate the endorsement of the insomnia items used in our phenotype. It should be noted that the three SCL-90 sleep items that we used to create the insomnia symptom composite score (difficulty falling asleep or staying asleep and early morning awakenings) do have some similarities with the main symptoms described in the DSM-5 criteria for insomnia. All three items also loaded highly onto one single common factor, indicating that they represent a latent insomnia construct. However, given that our checklist assessed symptoms present in the past month whereas the current DSM definition of insomnia requires symptoms to be present for at least 3 mo, and that we do not have information on daytime (or other clinically significant) impairment that results from experiencing the symptoms described, we cannot say that our phenotype meets the definition of insomnia disorder.5 It has been noted that inconsistent phenotyping is a problem in insomnia genetics research and that a wide range of phenotypes have been used, many of which focus primarily on symptoms, not the full disorder.35 Despite this, consistent heritability estimates have been produced across twin studies and the results of our study are similar to these estimates.35 Our insomnia phenotype has similarities with others found in the literature.14 Additionally, our insomnia symptom composite score provides a dimensional perspective and the use of a quantitative trait in this way provides greater power.
Sleep was measured subjectively through self-report questionnaire, which is less detailed than information that comes from objective measurements (e.g., polysomnography, actigraphy). Analysis of more comprehensive sleep data would allow us to more thoroughly understand the genetic and environmental contributions to this phenotype. Given the structure of the assessments, the time between assessments was not equal for all zygosity groups. It should also be noted that we had to constrain λ1 and λ2 to be equal across the two time points so that the model would be properly identified, but this is not a major concern given that the univariate estimates at Time 1 and Time 2 were similar. Although we had a large sample size, it is still possible that we were underpowered to detect qualitative sex effects, as a large number of opposite-sex twin pairs are needed for this analysis. Finally, the generalizability of these data is limited, as the sample is representative of twins in the Mid-Atlantic region of the United States in the 1990s.
CONCLUSIONS AND FUTURE DIRECTIONS
Although not without limitations, our results confirm that both genetic and environmental factors influence insomnia symptoms, and the estimates of genetic influences increase when utilizing multiple time points and a latent variable model that explicitly accounts for measurement error. As the assessment of insomnia symptoms covering a short time span could be more influenced by transient environmental effects (e.g., having a sick child, construction occurring around one's home) instead of tapping more enduring sleep problems, the ability to separate errors in measurement from lasting etiologic influences is a benefit for understanding insomnia as a phenotype. To our knowledge, this is the first time that this approach has been used for an insomnia phenotype in an adult population. We have also shown that these etiologic influences are largely stable across time in adults and have provided the first formal evidence that insomnia symptoms are more heritable in females than males. Future research should aim to investigate the specific genetic and environmental components that contribute to this prevalent complaint. Although our sleep items loaded highly onto a single common factor and we used a composite score, it is possible that the genetic influences may differ across these sleep items. As insomnia is by definition heterogeneous, further research into the genetics of individual symptoms is needed. More specifically, difficulty staying asleep (sleep maintenance insomnia, which also includes waking up too early) may be more heritable than difficulty falling asleep (sleep onset insomnia).36 A prior twin study of insomnia symptoms found evidence that there were no genetic contributions to trouble falling asleep,15 but this has not been replicated in an independent study. The degree to which the genetic and environmental influences on insomnia symptoms overlap with those of other psychopathology, such as major depressive disorder and generalized anxiety disorder (which include sleep complaints in their DSM criteria5), is another important research direction. This has mostly been explored in children and adolescents,37 and there is still much to learn about the relationship between disturbed sleep and psychopathology.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported in part by NIH grants R01 AA020179, P20 AA107828, R37 AA011408. The Mid-Atlantic Twin Registry is supported by NIH grant UL1RR031990. Dr. Amstadter is supported by grants R01AA020179, K02 AA023239, BBRF 20066, R01MH101518, and P60MD002256. The other authors have indicated no financial conflicts of interest. All participants gave informed consent and were treated according to the APA's ethical standards. The Virginia Commonwealth University Institutional Review Board approved the research. The work was performed at: Virginia Commonwealth University, Richmond, VA.
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3:S7–10. [PMC free article] [PubMed] [Google Scholar]
- 3.Morin CM, Jarrin DC. Epidemiology of insomnia: prevalence, course, risk factors, and public health burden. Sleep Med Clin. 2013;8:281–97. doi: 10.1016/j.jsmc.2022.03.003. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Washington, DC: American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. [Google Scholar]
- 5.American Psychiatric Association. Arlington, VA: American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. [Google Scholar]
- 6.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 7.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? J Am Med Assoc. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 8.McCall WV. A psychiatric perspective on insomnia. J Clin Psychiatry. 2000;62:27–32. [PubMed] [Google Scholar]
- 9.Krystal AD. Psychiatric disorders and sleep. Neurol Clin. 2012;30:1389–413. doi: 10.1016/j.ncl.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hublin C, Partinen M, Koskenvuo M, Kaprio J. Heritability and mortality risk of insomnia-related symptoms: a genetic epidemiologic study in a population-based twin cohort. Sleep. 2011;34:957–64. doi: 10.5665/SLEEP.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Mendoza J, Vgontzas AN. Insomnia and its impact on physical and mental health. Curr Psychiatry Rep. 2013;15:418. doi: 10.1007/s11920-013-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savard J, Laroche L, Simard S, Ivers H, Morin CM. Chronic insomnia and immune functioning. Psychosom Med. 2003;65:211–21. doi: 10.1097/01.psy.0000033126.22740.f3. [DOI] [PubMed] [Google Scholar]
- 13.Barclay NL, Gregory AM. Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013;17:29–40. doi: 10.1016/j.smrv.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Gehrman PR, Byrne E, Gillespie N, Martin NG. Genetics of insomnia. Sleep Med Clin. 2011;6:191–202. [Google Scholar]
- 15.Drake CL, Friedman NP, Wright KP, Jr, Roth T. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34:1179–88. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendler KS, Prescott CA. New York, NY: Guilford Press; 2006. Genes, environment, and psychopathology: understanding the causes of pyschiatric and substance use disorders. [Google Scholar]
- 17.Gregory AM, Rijsdijk FV, Lau JY, Dahl RE, Eley TC. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. J Sleep Res. 2008;17:140–1. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendler KS, Prescott CA. A population-based twin study of lifetime major depression in men and women. Arch Gen Psychiatry. 1999;56:39–44. doi: 10.1001/archpsyc.56.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Derogatis LR, Lipman RS, Covi L. SCL-90: an outpatient psychiatric rating scale--preliminary report. Psychopharmacology Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 20.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th edition. Richmond, VA: Department of Psychiatry; 2003. [Google Scholar]
- 21.Boker S, Neale M, Maes H, et al. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–17. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–32. [Google Scholar]
- 23.Williams L, Holahan P. Parsimony-based fit indicies for multiple-indicator models: Do they work? Structural Equation Modeling. 1994;1:161–89. [Google Scholar]
- 24.Kessler RC, Stang PE, Wittchen H-U, Ustun TB, Roy-Burne PP, Walters EE. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiatry. 1994;51:37852. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 25.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 26.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7:645–52. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han KS, Kim L, Shim I. Stress and sleep disorder. Exp Neurobiol. 2012;21:141–50. doi: 10.5607/en.2012.21.4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koskenvuo K, Hublin C, Partinen M, Paunio T, Koskenvuo M. Childhood adversities and quality of sleep in adulthood: a population-based study of 26,000 Finns. Sleep Med. 2010;11:17–22. doi: 10.1016/j.sleep.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Bader K, Schafer V, Schenkel M, Nissen L, Kuhl HC, Schwander J. Increased nocturnal activity associated with adverse childhood experiences in patients with primary insomnia. J Nerv Ment Dis. 2007;195:588–95. doi: 10.1097/NMD.0b013e318093ed00. [DOI] [PubMed] [Google Scholar]
- 31.Bader K, Schafer V, Schenkel M, Nissen L, Schwander J. Adverse childhood experiences associated with sleep in primary insomnia. J Sleep Res. 2007;16:285–96. doi: 10.1111/j.1365-2869.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 32.Lallukka T, Arber S, Rahkonen O, Lahelma E. Complaints of insomnia among midlife employed people: the contribution of childhood and present socioeconomic circumstances. Sleep Med. 2010;11:828–36. doi: 10.1016/j.sleep.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Gregory AM, Caspi A, Moffitt TE, Poulton R. Family conflict in childhood: a predictor of later insomnia. Sleep. 2006;29:1063–7. doi: 10.1093/sleep/29.8.1063. [DOI] [PubMed] [Google Scholar]
- 34.Dregan A, Armstrong D. Adolescence sleep disturbances as predictors of adulthood sleep disturbances-a cohort study. J Adolesc Health. 2010;46:482–7. doi: 10.1016/j.jadohealth.2009.11.197. [DOI] [PubMed] [Google Scholar]
- 35.Gehrman PR, Pfeiffenberger C, Byrne E. The role of genes in the insomnia phenotype. Sleep Med Clin. 2013;8:323–31. doi: 10.1016/j.jsmc.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarren M, Goldberg J, Ramakrishnan V, Fabsitz R. Insomnia in Vietnam era twins: influence of genes and combat experience. Sleep. 1994;17:456–61. doi: 10.1093/sleep/17.5.456. [DOI] [PubMed] [Google Scholar]
- 37.Gregory AM, Buysse DJ, Willis TA, et al. Associations between sleep quality and anxiety and depression symptoms in a sample of young adult twins and siblings. J Psychosom Res. 2011;71:250–5. doi: 10.1016/j.jpsychores.2011.03.011. [DOI] [PubMed] [Google Scholar]