Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a common sleep disorder in stroke patients and is associated with prolonged hospitalization, decreased functional outcome, and recurrent stroke. Research on the effect of OSA on cognitive functioning following stroke is scarce. The primary objective of this study was to compare stroke patients with and without OSA on cognitive and functional status upon admission to inpatient rehabilitation.
Design:
Case-control study.
Setting and Patients:
147 stroke patients admitted to a neurorehabilitation unit.
Interventions:
N/A.
Measurements:
All patients underwent sleep examination for diagnosis of OSA. We assessed cognitive status by neuropsychological examination and functional status by two neurological scales and a measure of functional independence.
Results:
We included 80 stroke patients with OSA and 67 stroke patients without OSA. OSA patients were older and had a higher body mass index than patients without OSA. OSA patients performed worse on tests of attention, executive functioning, visuoperception, psychomotor ability, and intelligence than those without OSA. No differences were found for vigilance, memory, and language. OSA patients had a worse neurological status, lower functional independence scores, and a longer period of hospitalization in the neurorehabilitation unit than the patients without OSA. OSA status was not associated with stroke type or classification.
Conclusions:
Obstructive sleep apnea (OSA) is associated with a lower cognitive and functional status in patients admitted for stroke rehabilitation. This underlines the importance of OSA as a probable prognostic factor, and calls for well-designed randomized controlled trials to study its treatability.
Citation:
Aaronson JA, van Bennekom CA, Hofman WF, van Bezeij T, van den Aardweg JG, Groet E, Kylstra WA, Schmand B. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. SLEEP 2015;38(9):1431–1437.
Keywords: obstructive sleep apnea, stroke, cognition, functional status, rehabilitation
INTRODUCTION
Stroke is one of the leading causes of death and serious long-term disability worldwide, and is a source of increased health care costs.1 In recent years efforts have been made to improve stroke prevention by early recognition and treatment of well-known modifiable risk factors such as hypertension, diabetes mellitus, and obesity.1 The identification of new, potentially reversible risk factors has also received increased attention. In this context, obstructive sleep apnea (OSA) has been suggested.
OSA is an independent risk factor for stroke and it is very common in the stroke population, with reported prevalence rates between 30% and 70%.2,3 OSA can be effectively treated with continuous positive airway pressure, but it is often left un-diagnosed. When left untreated, OSA is thought to contribute to decreased recovery from stroke.4 In line with this hypothesis, a number of studies have shown that OSA is associated with poor functional recovery, prolonged hospitalization and higher mortality rates.4–7
In the general population, OSA has been found to negatively affect cognitive functioning.8,9 Studies on the effect of OSA on cognitive functioning in stroke patients are scarce, and the results inconsistent.4,5,10 One study found an association between OSA and a cognitive screening instrument (Mini-Mental State Examination [MMSE]),11 whereas two studies reported no relationship. However, the MMSE is not designed to detect subtle cognitive changes, and use of more sensitive neuropsychological tests is recommended.5 We conducted a pilot study (n = 16) examining the effect of OSA on neuropsychological functioning in stroke patients and found that OSA was associated with lower performance in the domains of attention, verbal memory, and visuoperception.12 A larger study in patients with traumatic brain injury showed similar results, with OSA being associated with more impairment of sustained attention and memory.13 In the current study we investigated the association between OSA and both cognitive and functional status in a large sample of stroke patients. We hypothesized that OSA is associated with a lower cognitive and functional status.
METHODS
Participants
Stroke patients admitted to the neurorehabilitation unit of Heliomare Rehabilitation Center between September 2011 and August 2014 were invited to participate if they met the following inclusion criteria: (1) stroke confirmed by a neurologist, (2) age between 18 and 85 y, (3) admission between 1 and 16 w after stroke, (4) able to participate in the sleep study and neuropsychological assessment, and (5) sufficiently fluent in Dutch. Exclusion criteria were: (1) severe, unstable medical conditions, respiratory failure, or history of severe congestive heart failure, (2) traumatic brain injury, (3) severe aphasia, confusion, or severe psychiatric comorbidity, or (4) central sleep apnea or previously diagnosed OSA. Inclusion of patients in the control group was ended at n = 100.
This study is part of the prospective Treatment of OSA and Rehabilitation Outcome in Stroke (TOROS) study (Dutch Trial Register NTR3412).14 The institutional review board of the Academic Medical Centre in Amsterdam approved the study and all subjects provided written consent before participation.
Sleep Studies
Within the first weeks of hospitalization, patients underwent a sleep examination using standardized pulse oximetry (WristOx; Nonin Medical, Plymouth, MN, USA) and ambulatory overnight cardiorespiratory polygraphy (Embletta; Embla, Ottawa, Canada). The oxygen desaturation index (ODI) was calculated from pulse oximetry using automated analyses. The ODI was defined as the mean number of oxygen desaturations of ≥ 3% per hour. Patients with an ODI of five or higher were further tested for OSA by polygraphy. Polygraphy included recordings of airflow by oronasal thermistor, oxygen saturation and heart rate by pulse oximetry, and respiratory effort by abdominal wall and thoracic wall motion recording. The data were recorded with a multichannel digital polygraphic system. Trained staff manually scored the polygraph recordings for apnea and hypopnea events. Apnea was defined as a reduction of airflow of ≥ 90% for at least 10 sec and hypopnea was defined as a reduction of airflow of ≥ 50% for at least 10 sec followed by an oxygen desaturation of ≥ 3%. Apneas with thoracic motion, without thoracic motion, or with initial lack of motion followed by respiratory effort, were classified as obstructive, central, or mixed, respectively. The apnea-hypopnea index (AHI) was defined as the mean number of apneas and hypopneas per hour in bed. In patients with an AHI of 15 or higher on polygraphy, the diagnosis of sleep apnea (moderate to severe) was made. OSA was diagnosed when at least 50% of the respiratory events were of the obstructive type, whereas central sleep apnea was diagnosed when more than 50% of respiratory events were of the central type. Patients with a normal ODI (< 5) or AHI < 15 were enrolled in the control group of this study. In the current analyses, however, we excluded patients with mild OSA (AHI between 5–15) to reduce possible misclassifications. Patients with ODI (< 5) or AHI < 5 are further referred to as non-OSA patients.
Outcome Measurements
The primary outcome measures of this study were cognition and functional status. For cognition the following nine domains were assessed: vigilance, attention, memory, working memory, executive functioning, language, visuoperception, psychomotor ability, and intelligence. A trained psychology technician administered the battery of standardized neuropsychological tests within 4 w of admission. The assessment battery consisted of the following tests: (1) Psychomotor Vigilance Task to test of vigilance and reaction time, (2) D-KEFS Trail Making Test for visual attention and mental flexibility, (3) d2 Test of Attention, a measure of sustained and selective attention; (4) Rey Auditory Verbal Learning Test for verbal memory; (5) WAIS-III Letter-Number Sequencing to test working memory, (6) Tower of London for the assessment of executive functioning, (7) Category Fluency to assess verbal fluency, (8) Bells Test, a test for visuoperception and visual neglect, (9) Finger Tapping Test to assess psychomotor ability and motor speed, and (10) WAIS-III Matrix Reasoning, a measure for nonverbal abstract reasoning. For a number of cognitive domains, nonverbal alternative tests were administered to for patients with aphasia: Color Trails Test for visual attention and mental flexibility, Location Learning Test to test visual-spatial memory, and WMS-IV Symbol Span for visual working memory. Categorization of tests into cognitive domains was based on conventional classification as described in the standard textbook of neuropsychological assessment.15 The classification of neuropsychological tests per cognitive domain and a short description of the tests are given in Table S1 (supplemental material).
The obtained test scores were transformed into demographically corrected z-scores using reference data. All tests were corrected for age and the Color Trails Test, Location Learning Test, and Rey Auditory Verbal Learning Test were corrected for both age and education. For three tests (Psychomotor Vigilance Task, Bells Test, and Finger Tapping Test) no reference data were available and age-adjusted z-scores were calculated using a linear regression based approach including age as independent variable. We calculated the regression weights for the non-OSA group and subsequently applied them to all patients. If patients were unable to complete a task, the overall lowest obtained z-score for that test was given. In case of multiple tests within one domain, the average z-score for the domain was calculated. All nine domain scores were averaged into one overall cognition score (Cronbach α = 0.82).
Functional status was assessed by measures of neurological status and functional independence. At hospital admission, a rehabilitation physician administered two scales of neurological status, the Canadian Neurological Scale (CNS)16 and the National Institutes of Health Stroke Scale (NIHSS).17 The obtained scores were transformed into z-scores and averaged into one score for neurological status. Within the first week of admission, a trained nurse practitioner scored the level of functional independence on the physical functioning subscales (mobility and self-care) of the Utrecht Scale for Evaluation of Rehabilitation (USER).18 The obtained scale scores were transformed into z-scores and averaged into one score for functional independence. The score for overall functional status was calculated by averaging the neurological status and functional independence score (Cronbach α = 0.84).
Secondary outcome measures were sleepiness (Stanford Sleepiness Scale),19 fatigue (Checklist Individual Strength),20 anxiety and depression (Hospital Anxiety and Depression Scale),21 and subjective sleep quality (Sleep Quality Scale).22 A trained psychological technician administered these measures at the time of the neuropsychological assessment.
Demographic, clinical, and neurological data (age, sex, education level, body mass index [BMI], stroke type, stroke localization, stroke classification, time after onset of stroke, single versus recurrent stroke) were obtained from the medical files. The level of education was classified into seven categories according to the International Standard Classification of Education.23 Stroke classification at the time of hospital presentation was scored according to the Oxfordshire Community Stroke Project criteria.24 A full description of the assessment procedures has been published previously.13
Statistical Analysis
Data analyses were performed using SPSS (version 19.0, IBM, Armonk, NY). We used descriptive statistics to characterize the sample in terms of demographics, clinical, sleep, and stroke variables. To compare values between groups we used parametric (Student t test) and nonparametric tests (chi-square test or Mann-Whitney U test) as appropriate.
To evaluate differences between groups on cognitive and functional status, we performed a multivariate analysis of covariance (MANCOVA) using the mean z-scores of cognitive and functional status as dependent variables and recurrent stroke and level of education as covariates. Next, we performed univariate analyses of covariance (ANCOVAs) for the individual cognitive and functional domains with Benjamini-Hochberg correction for multiple comparisons.25 Effect sizes were calculated with Cohen d statistic. An effect size of 0.2 was considered small, 0.5 moderate and 0.8 large. We replaced missing values by the group mean of each domain (1 data point was missing for functional independence, 5 for psychomotor ability, 3 for intelligence, 1 for visuoperception). In the cognitive domain of vigilance 19 data points (13%) were missing. We imputed the estimated values using a linear regression equation based on the overall cognition score without the vigilance domain. We performed the main analyses with and without the imputed values and this did not change the results. For secondary outcome measures Student t tests were performed. In case of missing values we imputed the group mean of each measure (7 data points were missing for sleepiness and sleep quality, 5 for fatigue, anxiety and depression).
For all statistical tests, significance was set at P < 0.05.
RESULTS
Characteristics of the Participants
The flow of participants through the study is illustrated in Figure 1. In total, we screened 654 patients and found 449 patients eligible. One hundred twenty-one patients were discharged before they could be included and 122 patients declined participation. We removed one patient from the sample analyses due to changes in AHI after prescription of sleep medication, and six patients were removed because of technical problems with the pulse oximetry data (more detailed information is available upon request). Our initial sample comprised 199 patients, of whom 80 (40%) received a diagnosis of moderate to severe OSA (AHI ≥ 15). Nineteen patients were excluded from the control group because they were referred after the target sample size of 100 controls was reached (May 2014). Additionally, we excluded 33 patients with mild OSA (AHI 5–15) from the control group.
Figure 1.
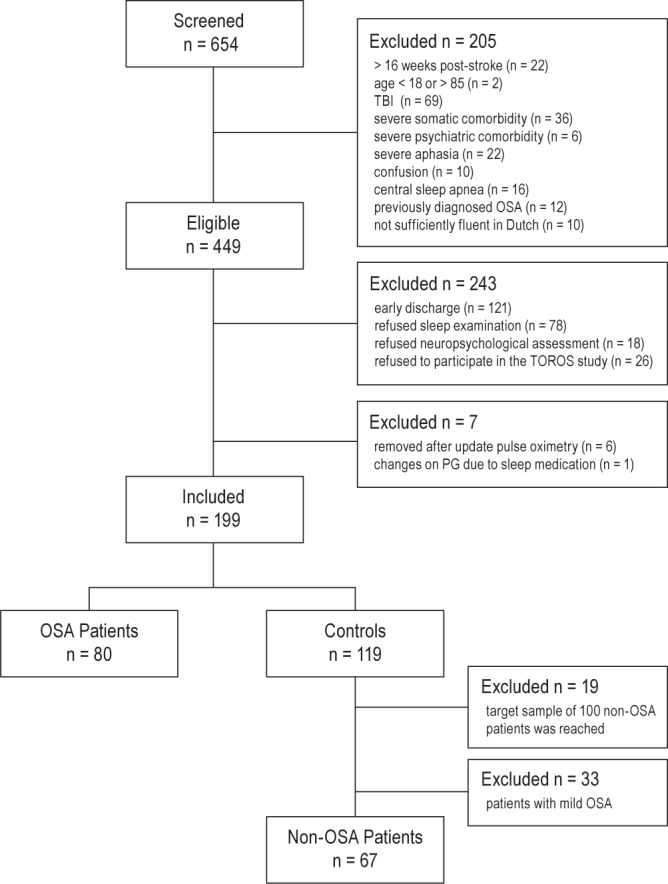
Patient flow chart.
Thus, our final sample comprised 147 patients. Eighty-three patients (57%) were male and the mean age was 57.8 y (± 10.1; range, 24–76). Fifty-one patients (35%) were overweight (BMI 25–30) and 22 patients (15 %) were obese (BMI ≥ 30). The majority of patients had a first-ever stroke (83%). Seventy-one percent of the patients had an ischemic stroke, 22% had a hemorrhagic stroke, and 7% a subarachnoid hemorrhage. Cortical strokes were predominant (88%) and the majority of strokes were classified as partial anterior circulation stroke (60%). On average, patients were admitted to the rehabilitation unit 17.2 days (± 16.0; range 3–98) after the stroke and were discharged after 69.0 days of inpatient rehabilitation (± 32.6; range 13–169). There was no significant difference between stroke types (ischemia, hemorrhage, or subarachnoid hemorrhage) on the primary outcome measures cognitive status (F(2, 144) = 0.79, P = 0.46) and functional status (F(2, 144) = 0.99, P = 0.37), which justifies combining these stroke subtypes in our further analyses.
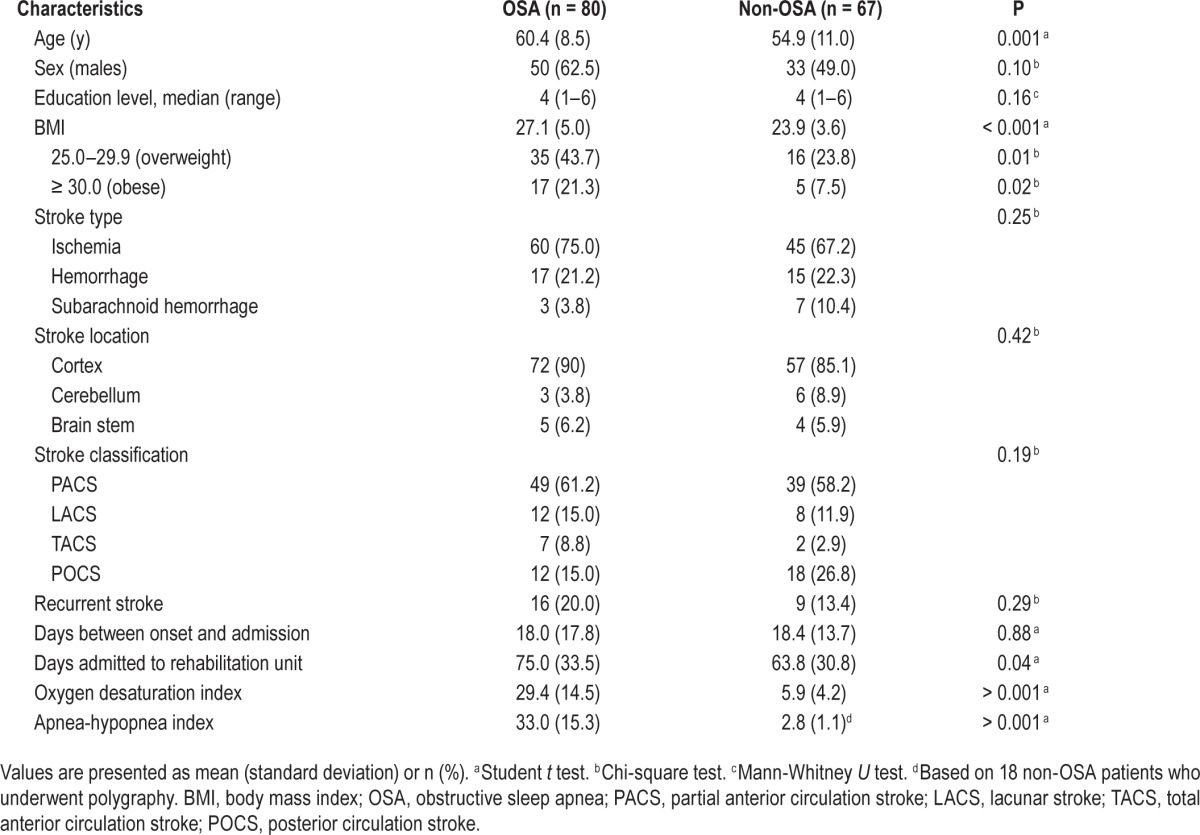
Background characteristics of the OSA and non-OSA groups are presented in Table 1. The mean ODI and AHI in the OSA group were 29.4 and 33.0, respectively, compared to 5.4 and 2.8, respectively, in the non-OSA group (AHI was only available for 18 non-OSA patients because only a subgroup underwent polygraphy). The OSA group was significantly older and had a higher BMI than the non-OSA group. The groups did not differ on sex, education level, or any of the stroke characteristics. There was no difference in time between stroke onset and admission to the rehabilitation center. The OSA patients, however, spent a significantly longer period (average of 11 days) in inpatient rehabilitation than the patients without OSA. This difference in time spent in inpatient rehabilitation was not related to the age of the patients (F(2, 144) = 0.28, P = 0.60).
Table 1.
Patient characteristics of the groups.
We included recurrent stroke and level of education as covariates in our main analyses for cognitive and functional status, because there were group differences on these measures, albeit not significant. We did not include age and BMI, because the cognitive measures were already adjusted for age and we did not find significant correlations between cognitive and functional status, and age or BMI (functional: age r = 0.08, P = 0.32; BMI r = −0.02, P = 0.79; cognitive: age r = −0.01, P = 0.90; BMI r = 0.05, P = 0.55).
Primary Outcomes
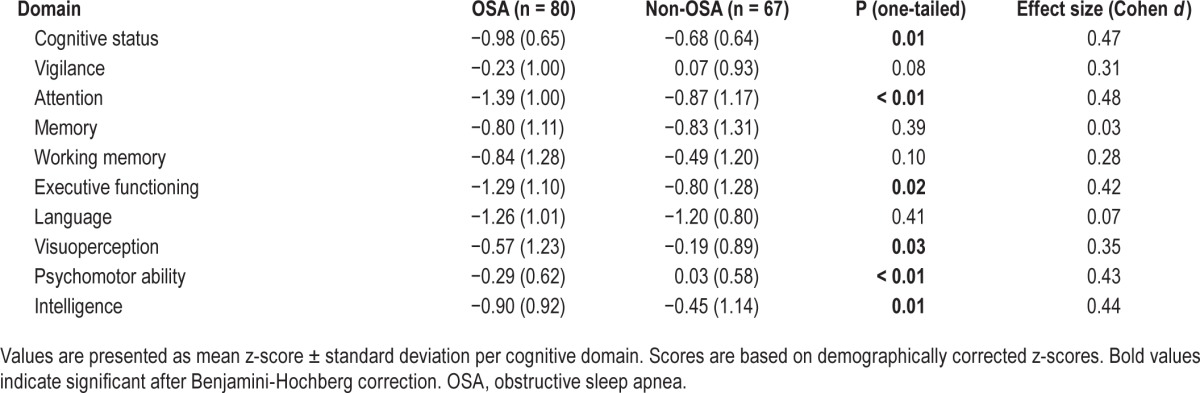
We found a significant difference between the OSA and the non-OSA group on both cognitive and functional status (MANCOVA; Pillai's trace = 0.08, F(2, 142), P = 0.001). OSA patients had a lower overall cognitive status than patients without OSA (Table 2). ANCOVAs showed that OSA patients performed significantly worse in the domains of attention, executive functioning, visuoperception, psychomotor ability, and intelligence. The performance in the domains of vigilance, memory, working memory, and language did not differ between groups. For all domains with significant group differences, Cohen d was small to moderate (0.35–0.48).
Table 2.
Cognitive outcomes.
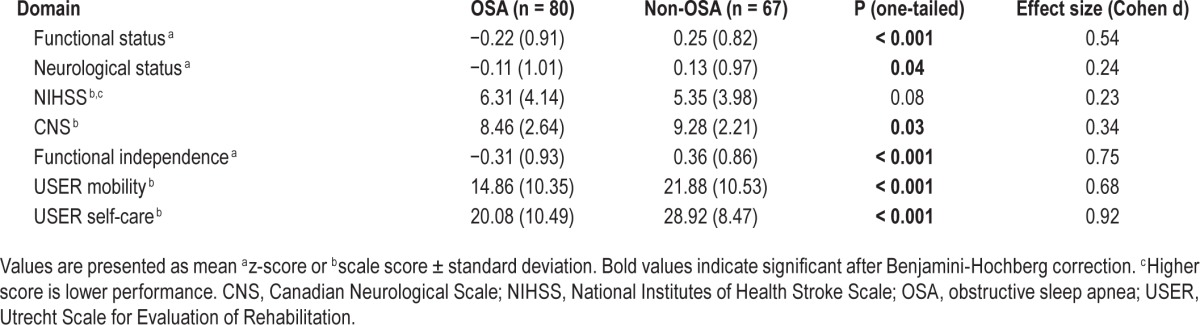
We found that OSA patients had a significantly lower functional status than non-OSA patients (Table 3). ANCOVAs showed that OSA patients had a significantly worse neurological status and significantly lower functional independence, both on self-care and mobility. The effect sizes for the functional domains ranged from small to large (0.24–0.92), with the largest effect sizes for measures of functional independence.
Table 3.
Functional outcomes.
Secondary Outcomes
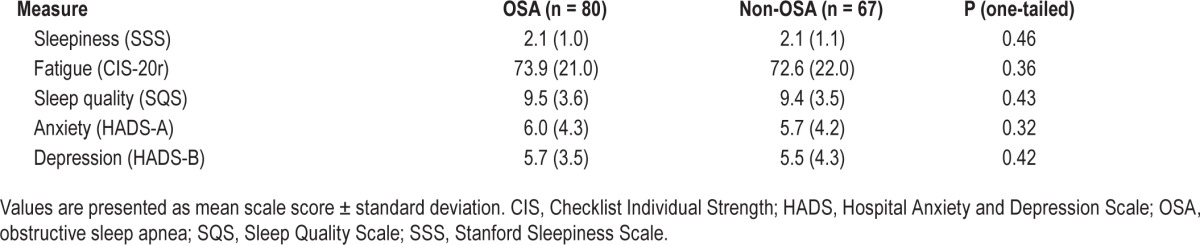
We found no significant differences between the OSA patients and non-OSA patients on sleepiness, fatigue, or sleep quality (Table 4). Moreover, the OSA group did not differ significantly from the non-OSA group on reported symptoms of anxiety or depression.
Table 4.
Secondary outcomes.
DISCUSSION
We found that OSA patients had significantly more cognitive and functional impairment than stroke patients without OSA. They were more impaired in attention, executive functioning, visuoperception, psychomotor ability and intelligence, and had poorer neurological status and a lower level of functional independence upon admission. We also found that OSA patients on average had an 11-day longer hospitalization in the neurorehabilitation than patients without OSA. OSA patients did not differ from the stroke patients without OSA on reported levels of sleepiness, fatigue, and sleep quality or on reported symptoms of depression and anxiety.
We observed a relatively high prevalence of OSA in our sample (40% of the initial sample of 199), which is consistent with previous findings. Johnson and Johnson performed a meta-analysis on the prevalence of sleep apnea in stroke patients.3 They included six studies conducted on stroke rehabilitation units and found prevalence rates between 22–61% (for AHI > 10 and AHI > 20). However, it should be noted that the prevalence rate in our study may not be representative for our neurorehabilitation unit as a whole, as only a selective group of patients participated in the study. The observed lack of association between stroke characteristics and OSA is also consistent with earlier studies.5,10 In our study, OSA was associated significantly with older age and higher BMI. This is not surprising as both are well-known risk factors for OSA.26
One of the main findings of our study is that, at the time of admission to the neurorehabilitation unit, OSA is associated with a poorer neurological status and higher functional dependence. Our findings are supported by a number of earlier studies that found that OSA was associated with worse functional outcome at discharge, and at 3, 6, and 12 mo after stroke onset.4,5,7 The findings on functional status upon admission to stroke rehabilitation are less consistent.5,6,10 Kaneko et al.5 and Sandberg et al.10 found higher functional dependence in OSA patients upon admission, whereas Cherkassky et al.6 did not find a difference in functional dependence. In contrast to our findings, Kaneko et al.5 found that the neurological status measured with the CNS upon admission to the stroke rehabilitation unit did not differ between OSA and non-OSA patients. In their study OSA patients did score slightly lower on the CNS than the OSA patients, although the difference was not significant. This may be explained by the small size of the non-OSA group in their study (n = 17). Furthermore, in accordance with Kaneko et al., we also found that OSA patients spent a longer period of time hospitalized in the neurorehabilitation unit than patients without OSA.
To our knowledge, this is the first study that compared cognitive functioning of stroke patients with and without OSA using a comprehensive neuropsychological assessment. We found that OSA patients are significantly more impaired in a number of cognitive domains. Our results are, to a large extent, comparable to cognitive effects found in the otherwise healthy OSA population, and confirm our earlier pilot study results.12,8 In accordance with our findings, substantial effects on attention and to a lesser degree on executive functioning, visuoperception and motor function are often reported in the general OSA population, whereas language is often spared. However, in contrast to our results, vigilance and memory are also often reported to be affected by OSA in the healthy population.27,28 In our study we did observe a trend for worse vigilance in the OSA group, but we did not find any effects of OSA on memory. In our pilot study,12 we did observe a significant correlation between OSA and verbal memory. This discrepancy in findings is most probably related to methodological differences between the two studies. In the current study we compared two groups (OSA and non-OSA), whereas in the pilot study cognitive measures were correlated to measures of OSA (AHI and ODI). Last, we found that the intelligence domain was more impaired in OSA patients, whereas the more general finding is that intellectual functioning is spared in otherwise healthy OSA patients.8,9 This discrepancy may be explained by the fact that we only used one cognitive test of performance intelligence to measure the intelligence domain (WAIS-III Matrix Reasoning) instead of a complete intelligence quotation (IQ) assessment, as there are some indications in the general population that performance IQ is lower in OSA patients, whereas verbal IQ and total IQ are spared.9
Contrary to general expectations, we did not find higher levels of fatigue or sleepiness in OSA patients. The first could be explained by the fact that fatigue is not only a common sign of OSA, but also the most reported complaint after stroke.29 This explanation is supported by the high levels of fatigue reported by both groups. The lack of difference between patients with and without OSA on reported sleepiness may be caused by the difficulty to differentiate between the concepts of sleepiness and fatigue or alternatively by a lack of awareness or underestimation of their sleepiness as result of the stroke. Although these findings or not in line with the generally held view, they are less surprising in the light of an earlier study we performed on predictive value of self-reported complaints for OSA in stroke patients.30 In this study we found that self-reported symptoms such as fatigue and sleepiness could not adequately predict a high likelihood of OSA in the stroke population.
Several limitations of our study should be noted. First, our findings are only correlational and do not imply causality. It could therefore be argued that the lower cognitive and functional status of OSA patients are not attributable to OSA, but rather to stroke severity. However, we did not observe any significant differences between the OSA and non-OSA groups in stroke type, location, or classification (a measure of stroke severity) at time of admission. Moreover, we included recurrent stroke as covariate in our main analyses. Thus, this suggests that the excess of cognitive and functional impairment observed in the OSA patients is not just a marker of more severe stroke. Nor can the lower performance of OSA patients be attributed to age, educational level, or BMI, as we corrected the cognitive data for age, included education as a covariate in our analyses, and demonstrated that both age and BMI were not correlated to functional status.
Second, we performed a two-tiered screening procedure for the diagnosis of OSA. In our study patients were first examined by standardized pulse oximetry, and patients only underwent a full polygraphy when the ODI was elevated (ODI ≥ 5). We chose to use this two-tiered method, because earlier studies showed that an elevated ODI on pulse oximetry is highly sensitive for the diagnosis of mild and moderate to severe sleep apnea (AHI ≥ 5: 86% sensitivity; AHI ≥ 15: 96–100% sensitivity, respectively),31,32 and pulse oximetry is less burdensome and costly than polygraphy. This method, however, may have led to misclassification of subjects. Additionally, the use of polygraphy without electroencephalography may have led to some degree of misclassification, because total recording time was used to calculate AHI, instead of total sleep time, which may have resulted in an artificially lower AHI. Earlier studies have shown that the AHI based on total sleep time is on average 2 to 8 points lower in patients with moderate and severe OSA respectively.33
Third, our patient sample may not have been representative of stroke patients in general. We only included patients admitted to the neurorehabilitation unit. These patients had a level of disability that precluded them from being discharged immediately after hospitalization, but they were not so severely disabled that they did not have rehabilitation potentials. In addition, more than half of the eligible patients did not participate because of early discharge from the rehabilitation unit or declining to undergo sleep examination or neuropsychological assessment. Therefore, we cannot rule out the possibility of a selection bias.
Our study also had a number of important strengths, including the large sample size, the use of both cognitive and functional measures, and the administration of a comprehensive neuropsychological assessment battery for the evaluation of cognitive functioning.
CONCLUSIONS
In summary, our results indicate that OSA is associated significantly with a lower cognitive and functional status in stroke patients admitted for stroke rehabilitation. Our findings underline the importance of OSA as a probable prognostic factor, and call for well-designed randomized clinical trials to investigate whether treatment of OSA could improve cognitive and functional outcome of stroke patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Brita Daniels, Irene Kos, and Mario van Lieshout for their support with data collection.
SUPPLEMENTAL MATERIAL
Neuropsychological tests by cognitive domain.
REFERENCES
- 1.Dinges DF, Powell JW. Microcomputer analysis of performance on a portable, simple visual RT task during sustained operations. Behav Res Met Ins C. 1985;17:652–5. [Google Scholar]
- 2.Delis DC, Kaplan E, Kramer JH. San Antonio, TX: Psychological Corporation; 2001. Delis-Kaplan Executive Function System. [Google Scholar]
- 3.D'Elia LF, Satz P, Uchiyama CL, White T. Odessa, FL: Psychological Assessment Resources; 1994. Color Trails Test 1 & 2. [Google Scholar]
- 4.Brickenkamp R, Zillmer E. Seattle, WA: Hogrefe & Huber Publishers; 1998. The d2 Test of Attention. [Google Scholar]
- 5.Rey A. Paris: Presses Universitaires de France; 1964. L'examen clinique en psychologie. [Google Scholar]
- 6.Schmand B, Houx P, de Koning IM, et al. Normen van psychologische tests voor gebruik in de klinischeneuropsychologie [norms for psychological tests for use in clinical neuropsychology] Published on the website of the section Neuropsychology of the Dutch Institute of Psychology (Nederlandse Instituut van Psychologen; NIP) [Google Scholar]
- 7.Bucks RS, Willison J, Byrne L. Bury, St Edmunds: Thames Valley Test Company; 2000. Location learning test: Manual. [Google Scholar]
- 8.Wechsler D. New York, NY: Psychological Corporation; 1997. Wechsler Adult Intelligence Scale 3rd edition (WAIS-III): Test manual. [Google Scholar]
- 9.Wechsler D. New York, NY: Psychological Corporation; 2009. WMS-IV: Wechsler Memory Scale-Administration and Scoring Manual. [Google Scholar]
- 10.Culbertson CQ, Zillmer EA. North Tonawanda, NY: Multi-Health Systems Incorporated (MHS); 2001. Tower of London Drexel University (TOL DX): Technical manual. [Google Scholar]
- 11.Luteijn F, Barelds DPH. Amsterdam, The Netherlands: Harcourt Assessment BV; 2004. Groningen Intelligence Test 2 (GIT-2): Manual. [Google Scholar]
- 12.Gauthier L, Dehaut F, Joanette Y. The Bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol. 1989;11:49–54. [Google Scholar]
- 13.Reitan RM, Wolfson D. Tucson, AZ: Neuropsychology Press; 1993. The Halstead-Reitan Neuropsychological Test Battery: theory and clinical interpretation. [Google Scholar]
REFERENCES
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart Disease and Stroke Statistics--2013 Update: a report from the American Heart Association. Circulation. 2013;127:e1–241. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KG, Johnson DC. Frequency of sleep apnea in stroke and TIA patients: a meta-analysis. J Clin Sleep Med. 2010;6:131–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Good DC, Henkle JQ, Gelber D, Welsh J, Verhulst S. Sleep-disordered breathing and poor functional outcome after stroke. Stroke. 1996;27:252–9. doi: 10.1161/01.str.27.2.252. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko Y, Hajek VE, Zivanovic V, Raboud J, Bradley TD. Relationship of sleep apnea to functional capacity and length of hospitalization following stroke. Sleep. 2003;26:293–7. doi: 10.1093/sleep/26.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Cherkassky T, Oksenberg A, Froom P, Ring H. Sleep-related breathing disorders and rehabilitation outcome of stroke patients: a prospective study. Am J Phys Med Rehab. 2003;83:452–5. [PubMed] [Google Scholar]
- 7.Turkington PM, Allgar V, Bamford J, Wanklyn P, Elliott MW. Effect of upper airway obstruction in acute stroke on functional outcome at 6 months. Thorax. 2004;59:367–71. doi: 10.1136/thx.2003.005348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beebe D, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 9.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 10.Sandberg O, Franklin K, Bucht G, Gustafson Y. Sleep apnea, delirium, depressed mood, cognition, and ADL ability after stroke. JAGS. 2001;49:391–7. doi: 10.1046/j.1532-5415.2001.49081.x. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician”. J Psych Res. 1975;3:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs J, Groet E, Schmand B. De invloed van het slaapapneusyndroom op het cognitieve functioneren bij CVA-patiënten: Een verkennend onderzoek. Tijdschrift voor Neuropsychologie. 2008;6:131–7. [Google Scholar]
- 13.Wilde MC, Castriotta RJ, Lai JM, Atanasov S, Masel BE, Kuna ST. Cognitive impairment in patients with traumatic brain injury and obstructive sleep apnea. Arch Phys Med Rehabil. 2007;88:1284–8. doi: 10.1016/j.apmr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Aaronson JA, van Bennekom CAM, Hofman WF, et al. The effect of obstructive sleep apnea and treatment with continuous positive airway pressure on stroke rehabilitation: rationale, design and methods of the TOROS study. BMC Neurol. 2004;14:e1471–2377. doi: 10.1186/1471-2377-14-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 4th ed. New York: Oxford University Press; 2012. [Google Scholar]
- 16.Cote R, Battista R, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian neurological scale validation and reliability assessment. Neurology. 1989;39:638–43. doi: 10.1212/wnl.39.5.638. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46:660–2. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 18.Post MW, van de Port IG, Kap B, Berdenis van Berlekom SH. Development and validation of the Utrecht scale for evaluation of clinical rehabilitation (USER) Clin Rehabil. 2009;23:909–17. doi: 10.1177/0269215509341524. [DOI] [PubMed] [Google Scholar]
- 19.Herscovitch J, Broughton R. Sensitivity of the Stanford sleepiness scale to the effects of cumulative partial sleep deprivation and recovery oversleeping. Sleep. 1981;4:83–91. doi: 10.1093/sleep/4.1.83. [DOI] [PubMed] [Google Scholar]
- 20.Vercoulen JH, Swanink C, Fennis JF, Galama J, van der Meer JW, Bleijenberg G. Dimensional assessment of chronic fatigue syndrome. J Psychosom Res. 1994;38:383–92. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond AS, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Visser P, Hofman WF, Kumar A, et al. Sleep and mood: measuring the sleep quality. In: Priest RG, Pletscher A, Ward J, editors. Sleep research. Baltimore, MD: University Park Press; 1979. pp. 135–45. [Google Scholar]
- 23.UNESCO (2006 [1997]) Montreal: UNESCO Institute for Statistics; International Standard Classification of Education: ISCED 1997 (re-edition) [Google Scholar]
- 24.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–6. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B. 1995;57:289–300. [Google Scholar]
- 26.Young T, Skatrud J, Peppard P. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 27.Beebe D, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 28.Aloia MS, Arnedt JT, Davis JD, Riggs RL, Byrd D. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772–85. doi: 10.1017/S1355617704105134. [DOI] [PubMed] [Google Scholar]
- 29.Lerdal A, Bakken LN, Kouwenhoven SE, et al. Poststroke fatigue—a review. J Pain Symptom Manage. 2009;38:928–49. doi: 10.1016/j.jpainsymman.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Aaronson JA, Nachtegaal J, van Bezeij T, et al. Can a prediction model combining self-reported symptoms, sociodemographic and clinical features serve as a reliable first screening method for sleep apnea syndrome in patients with stroke? Arch Phys Med Rehabil. 2014;95:747–52. doi: 10.1016/j.apmr.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Aaronson JA, van Bezeij T, van den Aardweg JG, van Bennekom CAM, Hofman WF. Diagnostic accuracy of nocturnal oximetry for detection of sleep apnea syndrome in stroke rehabilitation. Stroke. 2012;43:2491–3. doi: 10.1161/STROKEAHA.112.665414. [DOI] [PubMed] [Google Scholar]
- 32.Vázquez JC, Tsai WH, Flemons WW, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dingli K, Coleman EL, Vennelle M, et al. Evaluation of a portable device for diagnosing the sleep apnoea/hypopnoea syndrome. Eur Resp J. 2003;21:253–9. doi: 10.1183/09031936.03.00298103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Neuropsychological tests by cognitive domain.


