Abstract
Study Objectives:
Sleep disordered breathing (SDB) is a highly prevalent condition in high-income countries, with major consequences for cardiopulmonary health, public safety, healthcare utilization, and mortality. However, its prevalence and effect in low- and middle-income countries are less well known. We sought to determine the prevalence, risk factors, and comorbidities of SDB symptoms in four resource-limited settings.
Design:
Cross-sectional analysis of the CRONICAS Cohort, a population-based age- and sex-stratified sample.
Setting:
Four resource-limited settings in Peru varying in altitude, urbanization, and air pollution.
Participants:
There were 2,682 adults aged 35 to 92 y.
Measurements and Results:
Self-reported SDB symptoms (habitual snoring, observed apneas, Epworth Sleepiness Scale), sociodemographics, medical history, anthropometrics, spirometry, blood biomarkers were reported. We found a high prevalence of habitual snoring (30.2%, 95% confidence interval [CI] 28.5–32.0%), observed apneas (20.9%, 95% CI 19.4–22.5%) and excessive daytime sleepiness (18.6%, 95% CI 17.1–20.1%). SDB symptoms varied across sites; prevalence and adjusted odds for habitual snoring were greatest at sea level, whereas those for observed apneas were greatest at high altitude. In multivariable analysis, habitual snoring was associated with older age, male sex, body mass index (BMI), and higher socioeconomic status; observed apneas were associated with BMI; and excessive daytime sleepiness was associated with older age, female sex, and medium socioeconomic status. Adjusted odds of cardiovascular disease, depression, and hypertension and total chronic disease burden increased progressively with the number of SDB symptoms. A threefold increase in the odds of having an additional chronic comorbid disease (adjusted odds ratio 3.57, 95% CI 2.18–5.84) was observed in those with all three versus no SDB symptoms.
Conclusions:
Sleep disordered breathing symptoms were highly prevalent, varied widely across four resource-limited settings in Peru, and exhibited strong independent associations with chronic diseases.
Citation:
Schwartz NG, Rattner A, Schwartz AR, Mokhlesi B, Gilman RH, Bernabe-Ortiz A, Miranda JJ, Checkley W, CRONICAS Cohort Study Group. Sleep disordered breathing in four resource-limited settings in Peru: prevalence, risk factors, and association with chronic diseases. SLEEP 2015;38(9):1451–1459.
Keywords: sleep disordered breathing, chronic diseases, population-based study
INTRODUCTION
Sleep disordered breathing (SDB) is a highly prevalent condition with major consequences for cardiopulmonary health, public safety, healthcare utilization, and mortality.1 SDB is characterized by repeated apneas and hypopneas causing frequent arousals, oxyhemoglobin desaturations, and excessive daytime sleepiness (EDS).2 Clinical and epidemiologic studies in high-income countries (HICs) have demonstrated strong independent associations between SDB and cardiovascular outcomes, including prevalent and incident hypertension, coronary artery disease, heart failure, and incident stroke.1,3 Moreover, treatment of SDB can substantially decrease fatal and nonfatal cardiovascular events4,5 and may diminish the severity of metabolic dysfunction and cardiovascular disease.6 SDB also produces daytime hypersomnolence and neurocognitive dysfunction,7,8 which contribute to increased motor vehicle accidents, workplace injuries, and psychiatric disturbances.3 Physical and mental sequelae of SDB account for a twofold increase in healthcare utilization9–11 and loss of economic productivity3,12 in HICs. However, SDB prevalence, risk factors and association with chronic diseases in low- and middle-income countries (LMICs) are less well known.
Regional variations in social and biologic factors may have substantial effects on the prevalence of SDB in specific populations. In HICs, major risk factors for SDB are obesity, age, and male sex.1 With the growing worldwide epidemic of obesity, SDB prevalence is also increasing in LMICs. In addition to obesity, racial and ethnic differences may be associated with an increased SDB prevalence.1,3 Moreover, a recent, large population-based study identified a high SDB prevalence in the United States Hispanic population (25.8% mild SDB, 9.8% moderate SDB, 3.9% severe SDB) but a very low rate of diagnosis (1.3%).13 SDB prevalence and symptoms varied significantly across Hispanic/Latino subgroups; United States-based South Americans had slightly lower prevalence of SDB compared to other Hispanic/Latino groups but shorter sleep duration and more daytime sleepiness. Data from Latin America are limited to PLATINO, which observed a high prevalence of SDB symptoms and undiagnosed obstructive sleep apnea in four major Latin American metropolitan areas outside Peru.14 Specifically, snoring was observed in 60%, witnessed apneas in 12%, excessive daytime sleepiness (EDS) in 16%, and the combination of nocturnal symptoms and EDS in 3%. Altitude-related hypoxia and underlying obstructive lung disease from household and ambient air pollution may also contribute to the relatively high prevalence of SDB in Latin America.15,16 Nevertheless, SDB in Latin America remains largely underdiag-nosed and untreated.
We sought to characterize the prevalence and risk factors of SDB symptoms in four resource-limited settings in Peru. Because SDB symptoms are also associated with nocturnal hemodynamic,17 metabolic,18 and inflammatory stress,19 we hypothesized SDB symptoms conferred an increased risk for comorbid chronic diseases.
METHODS
Study Setting
We investigated the prevalence and risk factors of SDB in adults aged 35 y or older across four Peruvian settings characterized by differences in altitude, urbanization, and air pollution: Pampas de San Juan de Miraflores, a periurban community of 60,000 people in Lima; Tumbes, on the northern coast of Peru, encompassing both agricultural and urbanizing communities totaling about 20,000 people; and Puno, a city and surrounding villages of approximately 150,000 people located in southeastern Peru at 3,825 m above sea level. Within Puno there are two separate sites: an urban setting located at the city center and a rural setting made up of surrounding communities.
All participants provided verbal informed consent after our research team read the entire informed consent document to them and any questions were answered. Informed consent was verbal because of high illiteracy rates. The study was approved by the Institutional Review Boards of Johns Hopkins University in Baltimore, MD, USA, and Universidad Peruana Cayetano Heredia and A.B. PRISMA in Lima, Peru.
Study Design
Recruitment began in September 2010 and was conducted until about 1,000 participants per site were enrolled. We enrolled a sex- and age-stratified random population-based sample in each site as previously described.20 Eligibility criteria were: age 35 y or older, full-time resident of the study site, able to understand procedures and provide informed consent, not pregnant, without physical disability and without active pulmonary tuberculosis. Only one participant was enrolled per household. Trained field workers conducted a standardized questionnaire to assess sociodemographics and medical history, including cardiopulmonary risk factors and SDB symptoms, followed by a clinical assessment including blood pressure, weight, height, heart rate, spirometry, and pulse oximetry. Certified phlebotomists collected blood for processing in a centralized testing facility for serum lipids (including low-density lipoprotein [LDL] and high-density lipoprotein [HDL]), fasting glucose, fasting insulin, hemoglobin A1c (HbA1c), hemoglobin, and highly sensitive C-reactive protein (hs-CRP). Fasting blood samples were obtained and analyzed in a single facility and the quality of assays was checked with regular external standards and internal duplicate assays monitored by BioRad (www.biorad.com). Plasma glucose was measured using an enzymatic colorimetric method (GOD-PAP, Modular P-E/ Roche-Cobas, Mannheim, Germany) and hs-CRP using Tina-quant CRP-HS (Roche Diagnostics/Hitachi analyzer, Tokyo, Japan). Spirometry was conducted using the Easy-On-PC spirometer (ndd, Zurich, Switzerland) before and after 200 mcg of inhaled salbutamol via a spacer. Acceptability and reproducibility of spirometry followed standard guidelines.20 Detailed information about measurement techniques and evaluation are reported elsewhere.20
Modified Epworth Sleepiness Scale
A Spanish-language version of the Epworth Sleepiness Scale (ESS) was administered. Our questionnaires also included frequency and loudness of snoring and the occurrence of observed apneas. As previously noted, certain ESS questions are not applicable to the daily activities of many Peruvians.21 In our cohort, large proportions of respondents reported sleepiness while driving (88.4%), sitting and reading (10.3%), and watching television (12.3%) as not applicable responses, which likely resulted from low rates of car and television ownership and high rates of illiteracy in Peru. In contrast, nearly all participants responded to the remaining Epworth questions: sitting inactive in a public place (99.1%), as a passenger in a motor vehicle (99.1%), afternoon nap (99.1%), sitting and talking (99.4%), and sitting quietly after lunch (99.4%). Accordingly, we constructed a modified ESS score (mESS) by adding the five Epworth questions with large response rates.
Definitions
We defined SDB symptoms as follows: habitual snoring was defined as self-reported snoring ≥ 3 nights per week; observed apneas were defined as pauses in breathing or choking during sleep reported by a spouse or bed partner to the participant; and excessive daytime sleepiness (EDS) was defined as mESS > 6 (out of 15 maximum possible points), which is proportional to the cutoff of 10 of 24 ESS points used in many studies.22
We defined cardiovascular disease (CVD) as a self-reported diagnosis of heart disease or stroke; chronic obstructive pulmonary disease (COPD) as a postbronchodilator forced expiratory volume/forced vital capacity (FEV1/FVC) < 70%23; depression as a Center for Epidemiologic Studies of Depression (CES-D) scale of ≥ 2324; diabetes as a fasting plasma glucose > 126 mg/ dL or self-reported physician diagnosis and current use of antihyperglycemic medications25; and hypertension as systolic blood pressure ≥ 140, diastolic blood pressure ≥ 90, or self-reported physician diagnosis and current use of antihypertensive medications.26 Metabolic syndrome was defined according to the Joint Interim Statement criteria.27 A chronic disease index was constructed as the sum of each of the following chronic conditions: CVD, COPD, depression, diabetes, and hypertension. Socioeconomic status (SES) was assessed using a wealth index based on current occupation, household income, assets, and household facilities.28
Biostatistical Methods
Our primary objectives were to estimate the prevalence of SDB symptoms, identify risk factors for each symptom, and determine whether SDB symptoms were associated with comorbid chronic diseases. The unadjusted prevalence of three SDB symptoms (habitual snoring, observed apneas, EDS) was calculated for each study setting. To compare characteristics of study participants with differing numbers of SDB symptoms, we conducted a nonparametric test for trend across ordered groups for continuous variables and chi-square tests for categorical variables.29 For comparisons between unordered groups, we used Mann-Whitney U tests for continuous variables and chi-square tests for categorical variables. We used multivariable logistic regression to identify potential risk factors for each SDB symptom (habitual snoring, observed apneas, and EDS) including study site, altitude (sea-level sites in Lima and Tumbes versus high-altitude sites in Puno), sociodemographics (age, sex, BMI, and SES) and a priori selected cardiometabolic biomarkers (HbA1c, HDL/LDL ratio, and hs-CRP). To explore bivariate associations between SDB symptoms and chronic disease, we calculated the unadjusted prevalence of five chronic diseases (CVD, COPD, depression, diabetes, and hypertension) by number of SDB symptoms (0, 1, 2, or 3). We used multivariable logistic regression to calculate adjusted odds ratios (ORs) for each chronic disease by number of SDB symptoms, controlling for study site, age, sex, BMI, and SES. We used multivariable ordinal logistic regression to model chronic disease index as a function of SDB symptoms, study site, age, sex, BMI, and SES. We conducted statistical analyses in Stata 12.1 (StataCorp, College Station, TX, USA) and R (www.r-project.org).
Role of the Funding Source
This study was supported in full by the National Institutes of Health. The sponsor had no role in the study design, data analysis or interpretation, or writing of the report.
RESULTS
Participant Characteristics
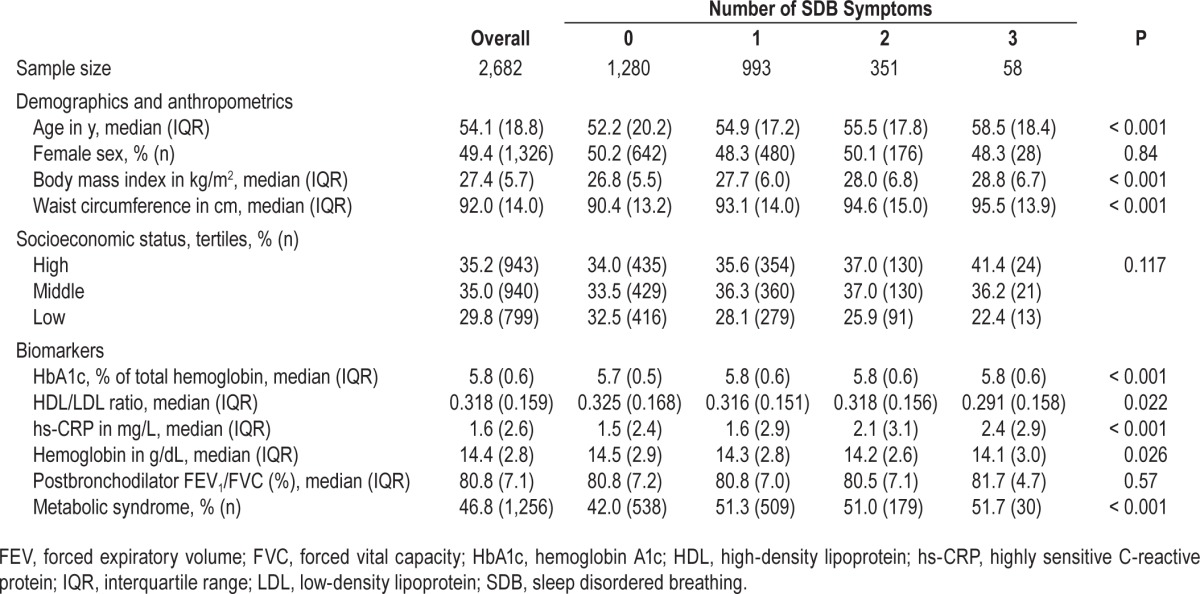
Of 3,601 participants who were enrolled and completed questionnaires, 2,682 (74.5%) had complete data for analysis. There were 919 participants who were missing one or more of the following: 277 (7.7%) were missing information on SDB symptoms, 382 (10.6%) were missing sociodemographics or anthropometrics, 697 (19.4%) were missing chronic disease measures, and 699 (19.4%) were missing biomarkers. Participants with missing data were older (median 58.2 versus 54.1 y; P < 0.001), were more likely to be female (57.5% versus 49.4%; P < 0.001), had a lower median BMI (26.8 versus 27.4 kg/m2; P = 0.003) and were more likely to have low SES (43.7% versus 29.8%; P < 0.001) than those with complete data. Among the 2,682 participants with complete data, those with the greatest number of SDB symptoms had a greater median age, BMI, waist circumference, HbA1c % and hs-CRP concentration and a greater proportion with metabolic syndrome; and had a lower median HDL/LDL ratio and hemoglobin concentration compared to participants with fewer symptoms (Table 1).
Table 1.
Characteristics of study population by number of sleep disordered breathing symptoms.
Prevalence of SDB Symptoms
We found a high overall prevalence of habitual snoring (30.2%, 95% confidence interval [CI] 28.5–32.0%), observed apneas (20.9%, 19.4–22.5%) and EDS (18.6%, 17.1–20.1%). The prevalence of SDB symptoms varied markedly across settings (Figure 1, Table S1, supplemental material). For example, the unadjusted prevalence of habitual snoring was higher in sea-level populations than in those at high altitude (37.3% versus 16.1%, P < 0.001). In contrast, the unadjusted prevalence of observed apneas was higher at altitude than at sea level (28.6% versus 17.0%, P < 0.001). A different pattern was observed in the unadjusted prevalence of EDS: Lima had the highest prevalence, Puno was intermediate, and Tumbes the lowest.
Figure 1.
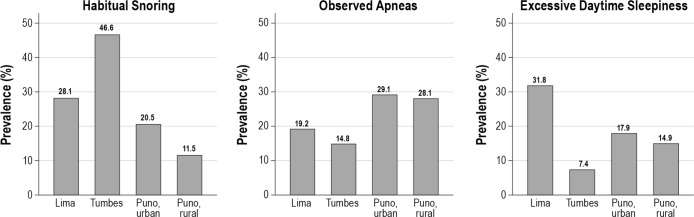
Unadjusted prevalence of sleep disordered breathing symptoms by study site.
Risk Factors for SDB Symptoms
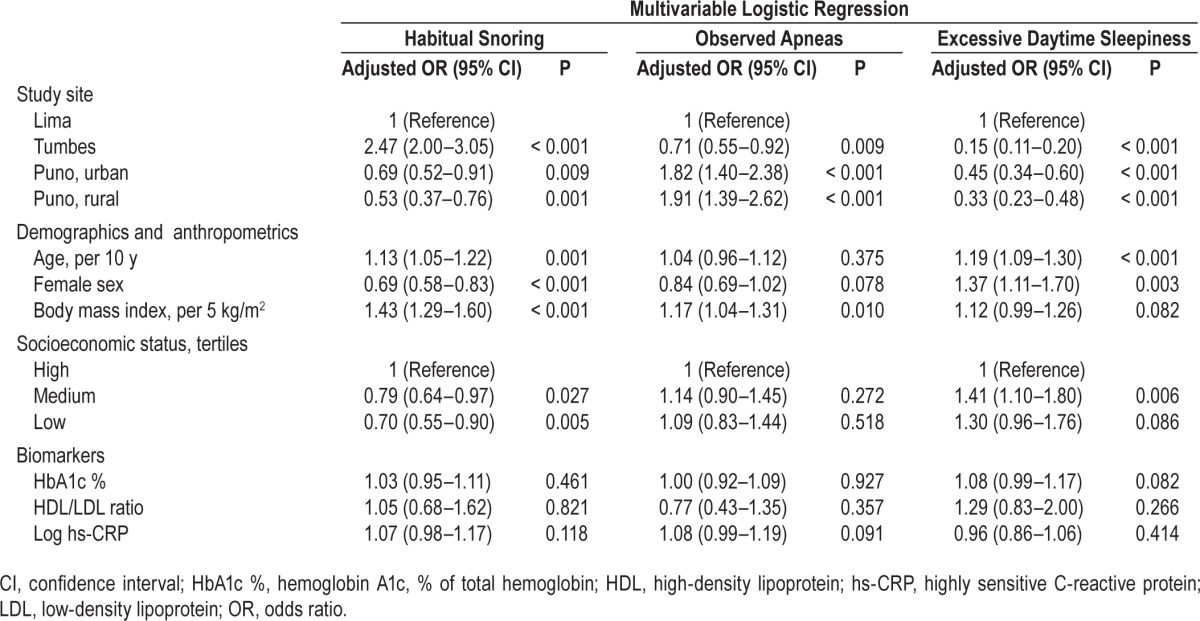
Variation in SDB symptoms across sites persisted when controlling for sociodemographics and biomarkers (Table 2). Consistent with the pattern of unadjusted prevalences, adjusted ORs for habitual snoring were greatest at sea level (Lima and Tumbes), whereas adjusted ORs for observed apneas were greatest at high altitude (urban and rural Puno). Adjusted ORs for EDS were highest in Lima, intermediate in Puno, and lowest in Tumbes. In addition, SDB symptoms varied with sociodemographic factors, with distinct factors associated with each symptom. Habitual snoring was associated with older age, male sex, BMI, and high SES. Observed apneas were associated with BMI. EDS was associated with older age, female sex, and medium SES. None of the SDB symptoms were associated with any of our selected chronic disease biomarkers (HbA1c, HDL/LDL ratio, hs-CRP).
Table 2.
Risk factors associated with sleep disordered breathing symptoms in multivariable logistic regressions including all listed variables.
Nocturnal SDB symptoms and EDS were strongly associated with each other, controlling for sociodemographics and biomarkers, with a progressive increase in the odds for EDS with increasing number of nocturnal symptoms (Table S2, supplemental material).
Associations between SDB Symptoms and Chronic Diseases
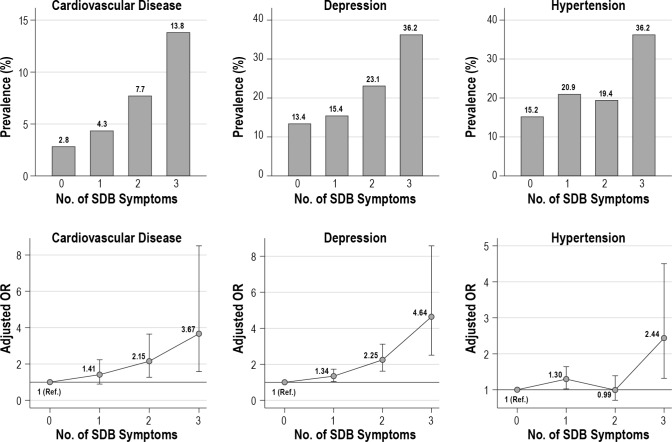
SDB symptom severity was strongly associated with co-morbid chronic diseases. We observed a progressive increase in the unadjusted prevalence of CVD, depression, and hypertension with the number of SDB symptoms (Figure 2, upper panels). Similar trends were observed in adjusted ORs of CVD (3.67, 95% CI 1.58–8.50, all three versus no SDB symptoms), depression (4.64, 2.50–8.58), and hypertension (2.43, 1.32–4.51), controlling for study site, age, sex, BMI, and SES (Figure 2, lower panels). We also observed a step increase in unadjusted COPD prevalence and a trend toward increased adjusted odds of COPD in participants with all three versus no SDB symptoms (adjusted OR 1.42, 95% CI 0.55–3.68). No trend was observed in the unadjusted prevalence or adjusted odds of diabetes (Figure S1, supplemental material). The un-adjusted prevalence and adjusted odds of any chronic disease increased progressively with the number of SDB symptoms (adjusted OR 4.24, 95% CI 2.27–7.90, all three versus no SDB symptoms).
Figure 2.
Unadjusted prevalence and adjusted odds ratios of selected chronic diseases by number of sleep disordered breathing symptoms. Upper panels: unadjusted prevalence. Lower panels: ORs adjusted for study site, age, sex, body mass index, socioeconomic status. Bars represent 95% confidence intervals. Reference level is defined as the adjusted odds for participants with no SDB symptoms. OR, odds ratio; Ref., reference; SDB, sleep disordered breathing.
Both EDS and nocturnal SDB symptoms (i.e., habitual snoring and observed apneas) were independently associated with the overall risk of chronic disease. The overall burden of chronic disease increased progressively with both the severity of daytime sleepiness and with the number of nocturnal SDB symptoms, independent of study site and sociodemographics (Table 3). Moreover, an increase in the total number of SDB symptoms was positively associated with the burden of chronic disease, controlling for study site and sociodemographics (Table 4). The presence of both nocturnal SDB symptoms and EDS was associated with a threefold increase in the odds of having an additional chronic comorbid condition (adjusted OR 3.57, 95% CI 2.18–5.84) versus participants with no SDB symptoms. Study site did not modify the association between SDB symptoms and chronic disease (P = 0.35 by likelihood ratio test).
Table 3.
Associations between nocturnal and daytime sleep disordered breathing symptoms and chronic disease index in multivariable ordinal logistic regression including all listed variables.
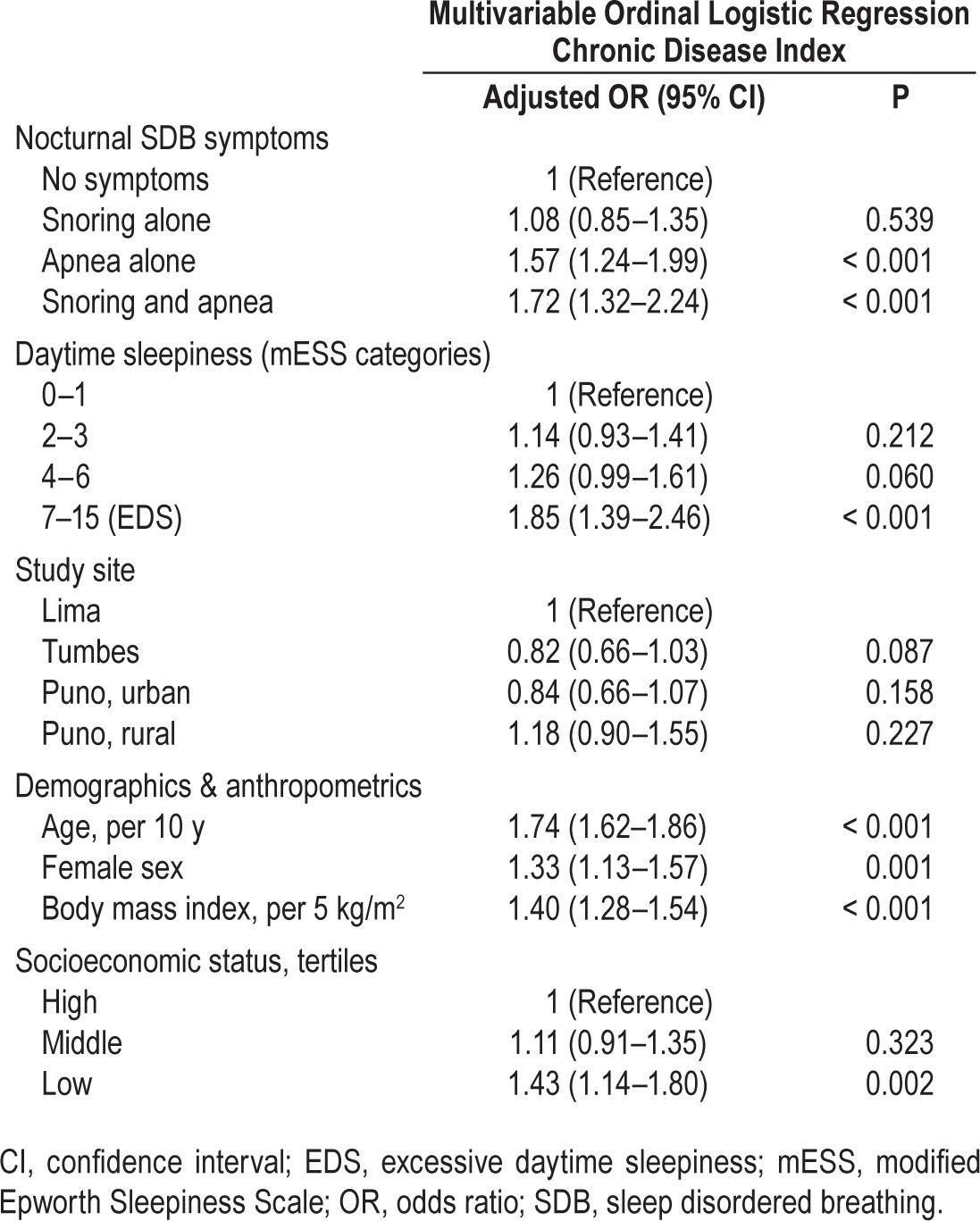
Table 4.
Associations between sleep disordered breathing symptoms and chronic disease index in multivariable ordinal logistic regression including all listed variables.
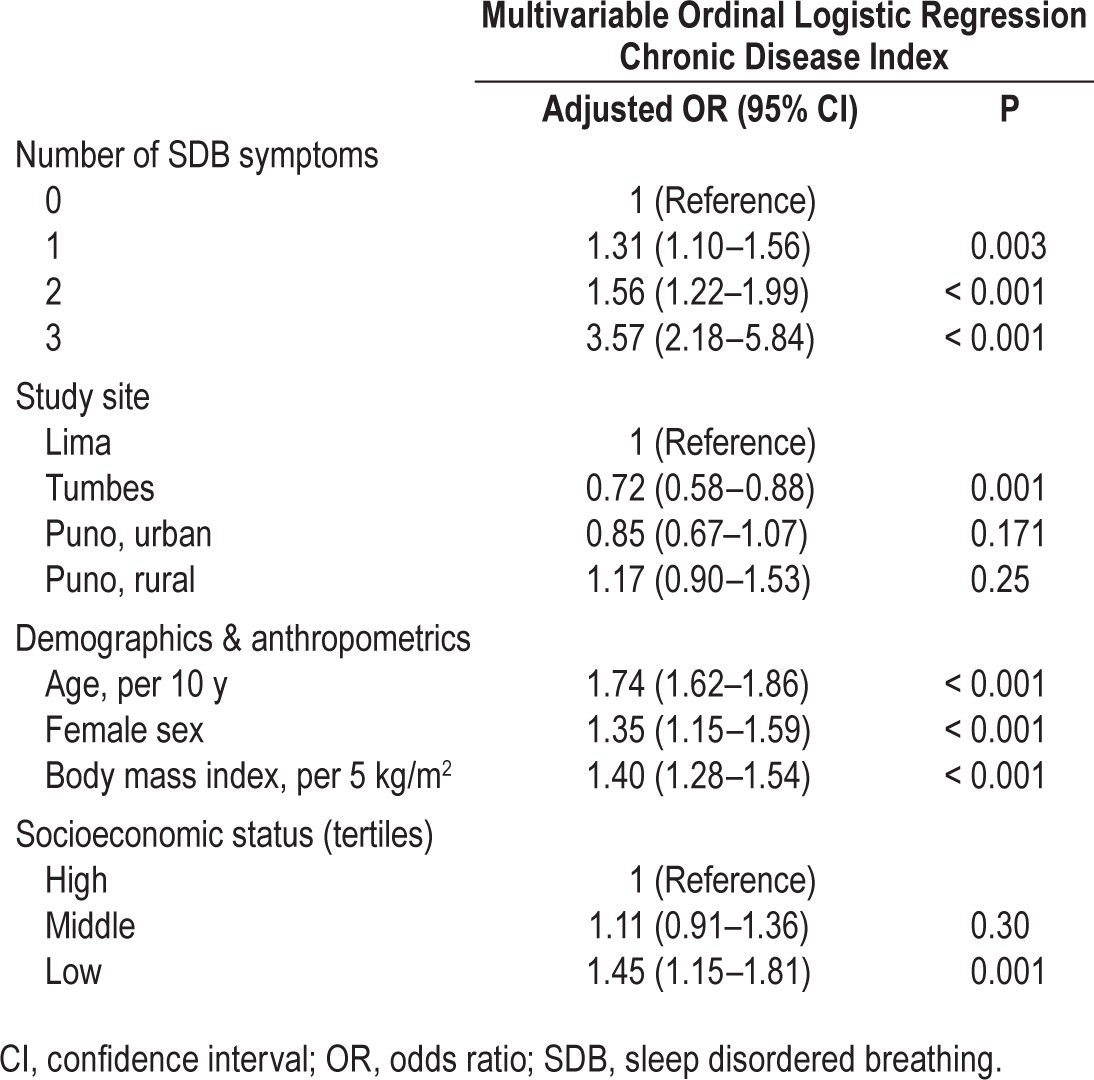
To examine associations of comorbid conditions with nocturnal SDB symptoms, we conducted sensitivity analyses excluding EDS from the count of SDB symptoms. We found that the odds of having an additional comorbid condition increased by a factor of 1.23 (95% CI 1.03–1.46) for one nocturnal symptom and by a factor of 2.00 (95% CI 1.51–2.65) for two nocturnal symptoms versus no nocturnal symptoms.
DISCUSSION
This study offers a novel examination of SDB symptoms and chronic disease in a large cohort in four resource-limited settings in Peru. The overall prevalence of habitual snoring, observed apneas, and EDS were high, and symptoms varied by site and sociodemographic factors. Subjects living at altitude had greater odds of having observed apneas, whereas those at sea level were more prone to habitual snoring. Habitual snoring was associated with age, BMI, male sex, and high SES; observed apneas with BMI; and EDS with age, female sex, and medium SES, suggesting distinct SDB pheno-types in this population. We also demonstrated that nocturnal and daytime SDB symptoms are independently associated with increased risk of chronic disease. Specifically, the risk of CVD, depression, hypertension, and the overall burden of chronic disease increased progressively with the number of nocturnal and daytime SDB symptoms, independent of location and sociodemographic factors, suggesting a strong association between chronic disease and SDB.
Our findings suggest that multiple social and medical factors were associated with SDB symptoms including location and sociodemographic and anthropometric factors. As expected, recognized SDB risk factors such as age, BMI, and male sex1 conferred increased risk of habitual snoring, whereas nocturnal apneas were primarily associated with BMI and altitude in our population. Unlike nocturnal SDB symptoms, the odds of EDS were diminished at altitude, greater in women, and elevated in lower socioeconomic strata. These findings suggest that EDS in Latino populations may be chiefly related to differences in total sleep time rather than to SDB.13 For example, women could have reported greater degrees of daytime sleepiness than men due to greater sleep requirements30 or disturbances in sleep and mood.14,31 Consistent with our findings, ESS score has been found to be elevated in Peruvian transport workers who work additional nighttime hours or suffered from depression.32 In addition to sleep restriction, sleep disruption in older participants can also contribute to EDS, particularly in those with SDB.7,33,34 Thus, distinct nocturnal and daytime SDB symptom profiles in Peruvians likely reflect disturbances in sleep quantity and quality and related sociodemographic and biologic risk factors.
We also found that both nocturnal SDB symptoms and EDS were independent predictors of comorbid chronic disease. These symptoms have been demonstrated to predict sleep apnea in both clinical35–37 and epidemiologic38 populations in high-income countries as well as low- to middle-income Latin American countries.14 The spectrum of nocturnal SDB symptoms, however, differed by altitude in our cohort. Residents at low altitude demonstrated a substantially greater prevalence of snoring, whereas those at high altitude exhibit a higher prevalence of apneas. These findings suggest that ambient hypoxia influences the expression of SDB at high altitude with a preponderance of central rather than obstructive sleep apnea,39,40 and that survey instruments should assess both snoring and apnea symptoms to capture the spectrum of SDB across altitudes. Furthermore, the high prevalence of nocturnal SDB symptoms in combination with EDS in our cohort suggests that SDB remains largely underdiagnosed and under-treated in resource-limited settings.
The burden of SDB is further highlighted by striking increases in chronic disease with progressive increases in SDB symptoms in our cohort. Of note, the combination of nocturnal and daytime symptoms was associated with a threefold increased risk of chronic disease. These findings are consistent with prior studies demonstrating similar associations between sleep apnea and cardiovascular morbidity and mortality.1,3,41–43 Excess risk of chronic disease in sleep apnea may be mediated by cytokines44 known to be pathogenic for sleep apnea,45,46 EDS,45,47,48 metabolic syndrome,49–52 and cardiovascular disease.53–56 Alternatively, sleep apnea can trigger systemic inflammation56,57 leading to EDS and an increased risk of comorbid chronic disease.57–59 Current evidence suggests that improvements in hypertension and cardiovascular risk profile with continuous positive airway pressure (CPAP) therapy may be confined to apneic patients with significant apnea and concomitant EDS.60–62 Thus, the group with both nocturnal and daytime SDB symptoms has the greatest burden of chronic disease and will likely benefit most from early SDB recognition and treatment.
Several limitations should be considered in interpreting the findings of the current study. First, self-reported symptoms rather than objective measurements of SDB were used. Nevertheless, these symptoms are known to be associated with objective SDB in sleep study data in US Latinos13 and Latin America14; and we have confirmed population studies demonstrating that habitual snoring in the absence of nocturnal apneas or EDS has been associated with intermediate levels of comorbidity.1 Moreover, the identical SDB symptom survey was applied across all sites, thereby minimizing any potential bias from differential classification. Any remaining nondifferential misclassification of participants' symptoms would likely lead us to underestimate the magnitude of the observed differences between sites (i.e., biasing results toward the null hypothesis of no difference). Second, our cross-sectional study design has delineated important links between nocturnal SDB symptoms, EDS, and chronic disease, but it cannot demonstrate causality. The CRONICAS cohort, however, is drawn from stratified random population-based samples in four locations, enhancing the generalizability of our findings and potential implications for public health. Moreover, longitudinal information collected will allow for future investigation of causal relationships between nocturnal SDB symptoms, EDS, and chronic disease. Finally, it is possible that cultural or linguistic barriers may have contributed to ascertainment bias, censored data and residual confounding, potentially diluting the strength of associations observed in our cohort. Nonetheless, we detected robust and stable effects across single variable and multivariable models.
CONCLUSIONS
In summary, our findings in a broad population-based sample across urban and rural Peruvian sites have demonstrated an overall high prevalence of SDB symptoms, and a strong independent association between SDB symptoms and comorbid chronic diseases. Of note, nocturnal symptoms varied by altitude with highlanders demonstrating greater odds of having observed apneas, and lowlanders more prone to habitual snoring. Finally, we found that the number of SDB symptoms was directly associated with chronic disease and with multiple comorbidities across four resource-limited settings in Peru. Our findings imply that in low-resource settings, patients with SDB symptoms should be targeted for early diagnosis and intervention to stem the development of chronic diseases such as cardiovascular disease and depression.
DISCLOSURE STATEMENT
This work was supported in full by the United States National Heart, Lung And Blood Institute, National Institutes of Health, Department of Health and Human Services contract HHSN268200900033C. Dr. Checkley was further supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health. Dr. Schwartz has received research support from inSleep LLC, ResMed, ImThera, Respicardia, and Maxis, and holds stock in Sova, RespEQ, and Discover Medical. Dr. Mokhlesi has received research support from Philips Respironics and participated in a speaking engagement for Zephyr Medical Technologies. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are indebted to all participants who kindly agreed to participate in the study. Special thanks to all field teams for their commitment and hard work, especially to Lilia Cabrera, Rosa Salirrosas, Viterbo Aybar, Sergio Mimbela, and David Danz for their leadership in each of the study sites, as well as Marco Varela for data coordination.
CRONICAS Cohort Study Group: Cardiovascular Disease: Antonio Bernabé-Ortiz, Juan P. Casas, George Davey Smith, Shah Ebrahim, Héctor H. García, Robert H. Gilman, Luis Huicho, Germán Málaga, J. Jaime Miranda, Víctor M. Montori, Liam Smeeth; Chronic Obstructive Pulmonary Disease: William Checkley, Gregory B. Diette, Robert H. Gilman, Luis Huicho, Fabiola León-Velarde, María Rivera, Robert A. Wise; Training and Capacity Building: William Checkley, Héctor H. García, Robert H. Gilman, J. Jaime Miranda, Katherine Sacksteder.
Footnotes
A commentary on this article appears in this issue on page 1349.
SUPPLEMENTAL MATERIAL
Unadjusted prevalence of sleep disordered breathing symptoms (95% confidence intervals) by study site.
Associations between excessive daytime sleepiness and nocturnal sleep disordered breathing symptoms in logistic regression including all listed variables.
(A) Unadjusted prevalence of chronic disease by number of sleep disordered breathing symptoms. (B) Adjusted odds ratios of chronic diseases by number of sleep disordered breathing symptoms. ORs adjusted for study site, age, sex, body mass index and socioeconomic status. Bars represent 95% confidence intervals. Reference level is defined as the adjusted odds for participants with no SDB symptoms. COPD, chronic obstructive pulmonary disease; OR, odds ratio; Ref., reference; SDB, sleep disordered breathing.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1:167–86. doi: 10.1016/0006-8993(66)90117-x. [DOI] [PubMed] [Google Scholar]
- 3.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2:349–64. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young T. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep. 2008;31:1071–80. [PMC free article] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 6.Schahin SP, Nechanitzky T, Dittel C, et al. Long-term improvement of insulin sensitivity during CPAP therapy in the obstructive sleep apnoea syndrome. Med Sci Monit. 2008;14:CR117–21. [PubMed] [Google Scholar]
- 7.Punjabi NM, O'hearn DJ, Neubauer DN, et al. Modeling hypersomnolence in sleep-disordered breathing. A novel approach using survival analysis. Am J Respir Crit Care Med. 1999;159:1703–9. doi: 10.1164/ajrccm.159.6.9808095. [DOI] [PubMed] [Google Scholar]
- 8.Kribbs NB, Pack AI, Kline LR, et al. Effects of one night without nasal CPAP treatment on sleep and sleepiness in patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1162–8. doi: 10.1164/ajrccm/147.5.1162. [DOI] [PubMed] [Google Scholar]
- 9.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22:740–7. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 10.Kryger MH, Roos L, Delaive K, Walld R, Horrocks J. Utilization of health care services in patients with severe obstructive sleep apnea. Sleep. 1996;19:S111–6. doi: 10.1093/sleep/19.suppl_9.s111. [DOI] [PubMed] [Google Scholar]
- 11.Tarasiuk A, Greenberg-Dotan S, Brin YS, Simon T, Tal A, Reuveni H. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128:1310–4. doi: 10.1378/chest.128.3.1310. [DOI] [PubMed] [Google Scholar]
- 12.Hillman DR, Murphy AS, Pezzullo L. The economic cost of sleep disorders. Sleep. 2006;29:299–305. doi: 10.1093/sleep/29.3.299. [DOI] [PubMed] [Google Scholar]
- 13.Redline S, Sotres-Alvares D, Loredo J, et al. Sleep disordered breathing in Hispanic/Latino individuals of diverse backgrounds: the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–44. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouscoulet LT, Vazquez-Garcia JC, Muiño A, et al. Prevalence of sleep related symptoms in four Latin American cities. J Clin Sleep Med. 2008;4:579–85. [PMC free article] [PubMed] [Google Scholar]
- 15.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol. 2010;108:369–77. doi: 10.1152/japplphysiol.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol. 1987;62:2201–11. doi: 10.1152/jappl.1987.62.6.2201. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb JD, Schwartz AR, Marshall J, et al. Hypoxia, not the frequency of sleep apnea, induces acute hemodynamic stress in patients with chronic heart failure. J Am Coll Cardiol. 2009;54:1706–12. doi: 10.1016/j.jacc.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jun JC, Drager LF, Najjar SS, et al. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34:1207–13. doi: 10.5665/SLEEP.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–8. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 20.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosales-Mayor E, Rey de CJ, Huayanay L, Zagaceta K. Validation and modification of the Epworth Sleepiness Scale in Peruvian population. Sleep Breath. 2012;16:59–69. doi: 10.1007/s11325-011-0485-1. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 23.Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2013. Global Initiative for Chronic Obstructive Lung Disease. [Accessed March 28, 2015]. Available from www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.
- 24.Ruiz-Grosso P, Loret de MC, Vega-Dienstmaier JM, et al. Validation of the Spanish Center for Epidemiological Studies Depression and Zung Self-Rating Depression Scales: a comparative validation study. PLoS One. 2012;7:e45413. doi: 10.1371/journal.pone.0045413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.Howe LD, Galobardes B, Matijasevich A, et al. Measuring socioeconomic position for epidemiological studies in low- and middle-income countries: a methods of measurement in epidemiology paper. Int J Epidemiol. 2012;41:871–86. doi: 10.1093/ije/dys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuzick J. A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 30.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 31.Krishnan V, Collop NA. Gender differences in sleep disorders. Curr Opin Pulm Med. 2006;12:383–9. doi: 10.1097/01.mcp.0000245705.69440.6a. [DOI] [PubMed] [Google Scholar]
- 32.Risco J, Ruiz P, Marinos A, et al. Excessive sleepiness prevalence in public transportation drivers of a developing country. Traffic Inj Prev. 2013;14:145–9. doi: 10.1080/15389588.2012.692493. [DOI] [PubMed] [Google Scholar]
- 33.Blackman MR. Age-related alterations in sleep quality and neuroendocrine function: interrelationships and implications. JAMA. 2000;284:879–81. doi: 10.1001/jama.284.7.879. [DOI] [PubMed] [Google Scholar]
- 34.Sahlin C, Franklin KA, Stenlund H, Lindberg E. Sleep in women: normal values for sleep stages and position and the effect of age, obesity, sleep apnea, smoking, alcohol and hypertension. Sleep Med. 2009;10:1025–30. doi: 10.1016/j.sleep.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Silva GE, Vana KD, Goodwin JL, Sherrill DL, Quan SF. Identification of patients with sleep disordered breathing: comparing the four-variable screening tool, STOP, STOP-Bang, and Epworth Sleepiness Scales. J Clin Sleep Med. 2011;7:467–72. doi: 10.5664/JCSM.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18:158–66. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 38.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 39.Insalaco G, Romano S, Salvaggio A, Pomidori L, Mandolesi G, Cogo A. Periodic breathing, arterial oxyhemoglobin saturation, and heart rate during sleep at high altitude. High Alt Med Biol. 2012;13:258–62. doi: 10.1089/ham.2012.1035. [DOI] [PubMed] [Google Scholar]
- 40.Julian CG, Vargas E, Gonzales M, et al. Sleep-disordered breathing and oxidative stress in preclinical chronic mountain sickness (excessive erythrocytosis) Respir Physiol Neurobiol. 2013;186:188–96. doi: 10.1016/j.resp.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mooe T, Franklin KA, Holmstrom K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease. Long-term prognosis. Am J Respir Crit Care Med. 2001;164:1910–3. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 42.Peker Y. Growing research evidence for continuous positive airway pressure treatment for sleepy patients with milder obstructive sleep apnea. Am J Respir Crit Care Med. 2012;186:583–4. doi: 10.1164/rccm.201208-1400ED. [DOI] [PubMed] [Google Scholar]
- 43.Peker Y, Carlson J, Hedner J. Increased incidence of coronary artery disease in sleep apnoea: a long-term follow-up. Eur Respir J. 2006;28:596–602. doi: 10.1183/09031936.06.00107805. [DOI] [PubMed] [Google Scholar]
- 44.Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9:1003–12. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 46.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 48.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–64. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Drager LF, Lopes HF, Maki-Nunes C, et al. The impact of obstructive sleep apnea on metabolic and inflammatory markers in consecutive patients with metabolic syndrome. PLoS One. 2010;5:e12065. doi: 10.1371/journal.pone.0012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228–34. doi: 10.1164/rccm.200804-608OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 52.Alam I, Lewis K, Stephens JW, Baxter JN. Obesity, metabolic syndrome and sleep apnoea: all pro-inflammatory states. Obes Rev. 2007;8:119–27. doi: 10.1111/j.1467-789X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 53.Miller MA. Association of inflammatory markers with cardiovascular risk and sleepiness. J Clin Sleep Med. 2011;7:S31–3. doi: 10.5664/JCSM.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arnardottir ES, Maislin G, Schwab RJ, et al. The interaction of obstructive sleep apnea and obesity on the inflammatory markers C-reactive protein and interleukin-6: the Icelandic Sleep Apnea Cohort. Sleep. 2012;35:921–32. doi: 10.5665/sleep.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–1205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 56.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain Behav Immun. 2004;18:298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 58.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnoea syndrome. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1666–70. doi: 10.1152/ajpregu.00401.2007. [DOI] [PubMed] [Google Scholar]
- 59.Testelmans D, Tamisier R, Barone-Rochette G, et al. Profile of circulating cytokines: impact of OSA, obesity and acute cardiovascular events. Cytokine. 2013;62:210–6. doi: 10.1016/j.cyto.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 60.Craig SE, Kohler M, Nicoll D, et al. Continuous positive airway pressure improves sleepiness but not calculated vascular risk in patients with minimally symptomatic obstructive sleep apnoea: the MOSAIC randomised controlled trial. Thorax. 2012;67:1090–6. doi: 10.1136/thoraxjnl-2012-202178. [DOI] [PubMed] [Google Scholar]
- 61.Barbe F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Ann Intern Med. 2001;134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]
- 62.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Unadjusted prevalence of sleep disordered breathing symptoms (95% confidence intervals) by study site.
Associations between excessive daytime sleepiness and nocturnal sleep disordered breathing symptoms in logistic regression including all listed variables.
(A) Unadjusted prevalence of chronic disease by number of sleep disordered breathing symptoms. (B) Adjusted odds ratios of chronic diseases by number of sleep disordered breathing symptoms. ORs adjusted for study site, age, sex, body mass index and socioeconomic status. Bars represent 95% confidence intervals. Reference level is defined as the adjusted odds for participants with no SDB symptoms. COPD, chronic obstructive pulmonary disease; OR, odds ratio; Ref., reference; SDB, sleep disordered breathing.