Abstract
Study objectives:
Obstructive sleep apnea (OSA) resolves in lateral sleep in 20% of patients. However, the effect of lateral positioning on factors contributing to OSA has not been studied. We aimed to measure the effect of lateral positioning on the key pathophysiological contributors to OSA including lung volume, passive airway anatomy/collapsibility, the ability of the airway to stiffen and dilate, ventilatory control instability (loop gain), and arousal threshold.
Design:
Non-randomized single arm observational study.
Setting:
Sleep laboratory.
Patients/participants:
20 (15M, 5F) continuous positive airway pressure (CPAP)-treated severe OSA patients.
Interventions:
Supine vs. lateral position.
Measurements:
CPAP dial-downs performed during sleep to measure: (i) Veupnea: asleep ventilatory requirement, (ii) passive V0: ventilation off CPAP when airway dilator muscles are quiescent, (iii) Varousal: ventilation at which respiratory arousals occur, (iv) active V0: ventilation off CPAP when airway dilator muscles are activated during sleep, (v) loop gain: the ratio of the ventilatory drive response to a disturbance in ventilation, (vi) arousal threshold: level of ventilatory drive which leads to arousal, (vii) upper airway gain (UAG): ability of airway muscles to restore ventilation in response to increases in ventilatory drive, and (viii) pharyngeal critical closing pressure (Pcrit). Awake functional residual capacity (FRC) was also recorded.
Results:
Lateral positioning significantly increased passive V0 (0.33 ± 0.76L/min vs. 3.56 ± 2.94L/min, P < 0.001), active V0 (1.10 ± 1.97L/min vs. 4.71 ± 3.08L/min, P < 0.001), and FRC (1.31 ± 0.56 L vs. 1.42 ± 0.62 L, P = 0.046), and significantly decreased Pcrit (2.02 ± 2.55 cm H2O vs. −1.92 ± 3.87 cm H2O, P < 0.001). Loop gain, arousal threshold, Varousal, and UAG were not significantly altered.
Conclusions:
Lateral positioning significantly improves passive airway anatomy/collapsibility (passive V0, pharyngeal critical closing pressure), the ability of the airway to stiffen and dilate (active V0), and the awake functional residual capacity without improving loop gain or arousal threshold.
Citation:
Joosten SA, Edwards BA, Wellman A, Turton A, Skuza EM, Berger PJ, Hamilton GS. The effect of body position on physiological factors that contribute to obstructive sleep apnea. SLEEP 2015;38(9):1469–1478.
Keywords: sleep apnea, obstructive, supine position, airway obstruction, lung volume measurements, functional residual capacity
INTRODUCTION
Obstructive sleep apnea (OSA), a medical condition that affects up to 24% of men and 9% of women, is characterized by repetitive upper airway obstruction, oxygen desaturation, and sleep fragmentation. In the long term, OSA predisposes to poor cardiovascular outcome, neurocognitive dysfunction, metabolic dysfunction, and increased risk of motor vehicle accidents.1–6 Current evidence demonstrates that patients with OSA experience varying severities of obstruction depending upon body position. Of all patients who have OSA, up to 60% have a preponderance of respiratory events when sleeping supine7,8; for approximately 20% of patients, upper airway obstruction occurs exclusively in the supine position.8,9 An obvious inference from the existence of positional dependence of OSA is that the pathophysiological causes of upper airway obstruction manifest themselves variably with body position.
Recent evidence has suggested that OSA is not simply due to poor upper airway anatomy,10,11 but has other pathophysiological causes including (1) inability of the pharyngeal muscles to hold open or stiffen the airway during sleep (i.e., impaired upper airway gain),12,13 (2) oversensitive ventilatory control system (i.e., high loop gain),14,15 (3) low respiratory arousal threshold,16,17 and (4) low lung volume.18,19 Surprisingly, the only studies to have investigated the impact of body position on OSA have focused on just one trait—passive upper airway anatomy/collapsibility. These studies, which measured the pharyngeal critical closing pressure (Pcrit), demonstrated a 2.2–2.9 cm H2O increase in Pcrit (i.e., airway more collapsible) when adopting the supine sleeping position.20–22
The current literature fails to assess why 20% of OSA patients have resolution of obstructive events in lateral sleep. Consequently, the aim of the current study was two-fold. Firstly, in patients with severe OSA, we aimed to measure how sleeping position (i.e. lateral versus supine) affects: (i) Veupnea: eupneic ventilatory demand, (ii) passive V0: ventilation off CPAP (pressure = 0 cm H2O) when the upper airway dilator muscles are quiescent, (iii) Varousal: the ventilation at which respiratory arousals begin to occur, (iv) active V0: ventilation off CPAP (pressure = 0 cm H2O) when the upper airway dilator muscles are activated during sleep, (v) loop gain: assessed by the ratio of the ventilatory drive response to a disturbance in ventilation, (vi) arousal threshold: the level of ventilatory drive at which a patient will arouse from sleep, (vii) upper airway gain (UAG) the ability of the upper airway muscles to activate and restore ventilation in response to increases in ventilatory drive, and (vii) functional residual capacity (FRC). Secondly, we aimed to assess the physiological characteristics that differentiate OSA patients who have a reduced apnea and hypopnea index (AHI) in the lateral sleeping position compared to other OSA patients with severe OSA regardless of sleep position. We prespecified the definition of supine OSA based on previously published data from our group.23 Preliminary results of this analysis have been published in abstract form.24
METHODS
Institutional ethics approval was obtained from the Monash Health Human Research Ethics Committee. Patients with severe OSA (AHI > 30 events/h) were identified from a hospital database. To be included, patients were required to have recorded > 30 min supine and > 30 min non-supine NREM sleep on their diagnostic study. Patients were required to be using CPAP for > 2 months and to be adherent for > 4h/night in the 30 days prior to enrolment. Patients were randomly selected from the included list, given written informed consent to participate, and attended the Monash Sleep Centre for 2 overnight studies one week apart in order to measure the OSA traits (detailed below). Anthropomorphic and lung volume measurements were made prior to each overnight study. One overnight study was conducted with the subject in the supine position (with the head also supine). The other study was performed with the patient lying in the right lateral position with the head in the neutral position as comfort allowed. Patients were under continuous video monitoring and were repositioned if they moved from the prescribed position. The order of the position studied was randomized. We defined 2 groups of OSA patients for the purpose of this study based on our previously published work23: patients with a supine AHI to non-supine AHI ratio of > 4:1 on their diagnostic polysomnogram (PSG), whom we refer to as the supine OSA group, and patients with a supine AHI to non-supine AHI ratio of < 4:1, whom we refer to as the position-independent OSA group.
Anthropomorphic Measurements
Weight was measured with electronic scales (Seca 703, Hamburg, Germany) and height with a stadiometer (Seca 264, Hamburg, Germany) in order to determine body mass index (BMI). Circumferential measurements were carried out upright with the tape measure in a plane parallel to the ground, completely surrounding the body without compressing the subcutaneous tissues; the neck was measured just inferior to the cricoid cartilage, the chest at the level of the third inter-costal space, the waist at the smallest girth between the iliac crest and the costal margin, and the hips at the level of the largest dimension over the buttocks.
Lung Volume
Awake measurements of FRC were performed 4 times in each of the seated, supine, and lateral positions (with the order of measurement randomized in each patient) using a nitrogen gas washout method,25 with the average of the 4 measurements used subsequently. The full description of the method used can be found in section 1 of the supplemental material.
Overnight Phenotyping
On the study night, patients were instrumented for a standard clinical PSG montage with electroencephalogram, submental and leg electromyogram, electrocardiogram, arterial oxygen saturation, and CPAP mask pressure. Additional respiratory measurements were made using a pneumotachograph, capnograph, and an oxygen analyzer. Exhaled CO2 (NICO Cardiopulmonary Management System, Respironics Novametrix, Wallingford, CT) and exhaled O2 (Ametek S-3A/I, Ametek Process Instruments, Pittsburgh, PA) were sampled via a cannula inserted through a port into the CPAP mask and under constant 0.1 L/ min suction. The CPAP mask was sealed and connected to a pneumotachograph (model 3700A, Hans Rudolph, Kansas City, MO) with a vent inserted in the circuit distal to the pneumotachograph (i.e., with the pneumotachograph closer to the CPAP mask and the vent closer to the pressure source). The circuit was connected to a positive/negative pressure source (Resmed, New South Wales, Australia) that was used to control the level of CPAP delivered and was capable of delivering +20 cm H2O to −20 cm H2O pressure. Sleep state and arousals were scored by an experienced sleep scientist in accordance with standard criteria.26 The scientist was blinded to the respiratory measurements and the position state of the patient. All signals were recorded and displayed overnight using Compumedics Profusion PSG 3 (Compumedics, Abbotsford, Australia). Data were exported from Profusion PSG3 in the European Data Format (EDF) and analyzed in Spike2 (Cambridge Electronic Design, Cambridge, UK) and MatLab (Mathworks, Natick, MA).
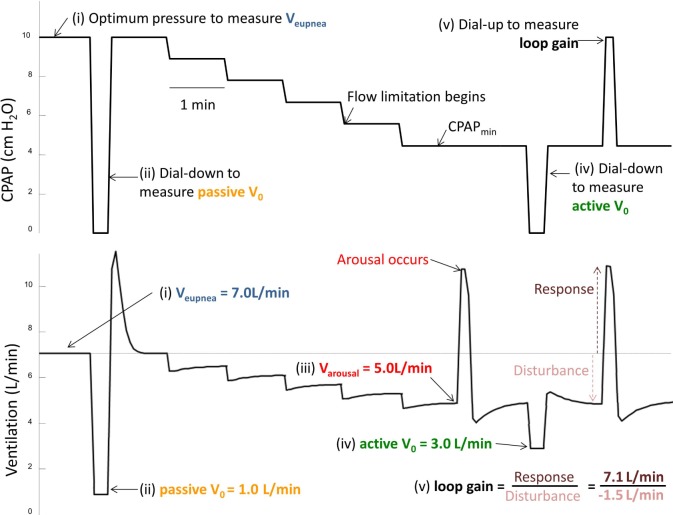
The method for measuring the contributory mechanisms for OSA has been described in detail previously27 and is summarized in Figure 1 (with subsequent numbering corresponding to the numbering in Figure 1). In brief, CPAP was altered during sleep to measure 4 different ventilations and loop gain. Ventilation was determined from flow on a breath-by-breath basis. Subsequent analysis involved averaging of these breaths as described below. The ventilation measurements included (i) Veupnea: the eupneic ventilatory demand or the subject's asleep ventilatory requirement (which is determined by dead space ventilation and ventilatory requirement), (ii) passive V0: ventilation off CPAP (pressure = 0 cm H2O) when the upper airway dilator muscles are quiescent, (iii) Varousal: the ventilation at which respiratory arousals begin to occur, and (iv) active V0: ventilation off CPAP (pressure = 0 cm H2O) when the upper airway dilator muscles are activated during sleep, (v) loop gain: assessed by the ratio of the ventilatory drive response to a disturbance in ventilation.
Figure 1.
CPAP dial-down method for measuring phenotypic traits. (i) Veupnea, ventilation at optimal CPAP with no evidence of snoring or flow limited breathing; (ii) passive V0, ventilation at CPAP = 0 cm H2O with completely relaxed pharyngeal muscles; (iii) Varousal, the ventilation just prior to a respiratory-induced arousal; (iv) active V0, ventilation at CPAP = 0 cm H2O with maximally activated pharyngeal muscles; (v) loop gain, the ratio of ventilatory overshoot (response) above Veupnea when returning to optimal CPAP pressure from a period of sub-optimal CPAP with reduced ventilation (disturbance).
Initially, the CPAP pressure was increased to eliminate snoring and flow limited breathing to obtain the measurement of Veupnea (i). Then the mask pressure was rapidly dialled down to 0 cm H2O for 5 breaths to obtain a measurement for passive V0 (ii). The CPAP was then returned to optimal pressure and subsequently decreased slowly according to the algorithm presented in section 2 of the supplemental material. The pressure was decreased to achieve flow-limited breathing and then to determine the level of ventilation at which arousals begin to occur Varousal (iii). This level of pressure was termed the CPAPmin. At CPAPmin, during periods of relatively arousal-free breathing, a series of dial downs to 0 cm H2O was performed to measure active V0 (iv). An additional maneuver which involved a series of dial ups to the optimal CPAP level from the CPAPmin was then performed in order to determine loop gain (v). If the patient experienced awakening (i.e., an increase in EEG activity > 15 sec) at any time such as at CPAPmin, during the slow decreases in CPAP or subsequent to a dial up or dial down from CPAPmin, the CPAP was returned to the optimal pressure, and once sleep was reinstated, the sequence was repeated. Several reduction sequences such as that demonstrated in Figure 1 were achieved across the night.
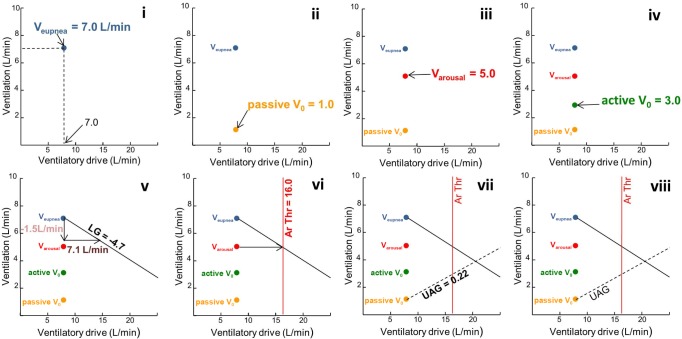
In order to model the interaction between the various traits to determine the predisposition to OSA, the 5 “ventilations”: (i) Veupnea, (ii) passive V0, (iii) Varousal, (iv) active V0, and (v) loop gain; were plotted on a graph of ventilation (L/min) versus ventilatory drive (L/min). One advantage of this method is that it enables a normalization of the trait measurements to ventilatory drive. The graph also allows the calculation of (vi) arousal threshold, and (vii) upper airway gain (see Figure 2 with the sequential numbering matching that of Figure 1). First, Veupnea was determined by averaging several minutes of ventilation on optimal CPAP, was plotted (Figure 2i and Figure 1i). The value was placed along the line of identity between ventilation and ventilatory drive, as it indicates that the patient's ventilatory demand is being fully met with the airway completely patent on optimal CPAP. Second, the passive V0, which is the ventilation at CPAP = 0 cm H2O when the upper airway muscles are passive, was plotted (Figure 2ii and Figure 1ii). This value was determined by averaging the ventilation of breaths 3 and 4 (without arousal) following a rapid dial down from optimal CPAP to 0 cm H2O. Third, Va rousal, which is the ventilation that leads to a respiratory arousal, was plotted (Figure 2iii and Figure 1iii). This value was determined from the mean ventilation of the 5 breaths prior to a respiratory-induced arousal. Fourth, active V0, which is the ventilation at CPAP = 0 cm H2O when the pharyngeal muscles are active, was plotted (Figure 2iv and Figure 1iv). This value was determined by averaging the ventilation of breaths 3 and 4 following a dial down in CPAP pressure from CPAPmin to 0 cm H2O (if CPAPmin is a negative pressure then the pressure is dialled “up” to 0 cm H2O). Fifth, the reciprocal of loop gain line was plotted (Figure 2v and Figure 1v). The slope of the line was determined by calculating how much increased ventilatory drive (horizontal vector of the line) was created by a reduction in ventilation (vertical vector of line). That is, loop gain = response (increase or overshoot in ventilation above eupnea) ÷ disturbance (reduction in ventilation below eupnea)—in this case loop gain = 7.1L/min ÷ −1.5L/ min = −4.7 (note that loop gain is dimensionless). The slope of the line plotted is 1/LG or in this case 1/−4.7.
Figure 2.
Plotting the phenotypic trait variables. Lower case roman numerals correspond with numbering in Figure 1.
Once the loop gain is known, the arousal threshold, which is the level of ventilatory drive at which a patient will arouse from sleep, could be determined from the intersection of a horizontal line through Varousal and the loop gain line (Figure 2vi). Lastly, the upper airway gain (UAG) was determined as follows (Figure 2vii): A horizontal line was drawn through the active V0 and its intersection with the arousal threshold line. The passive V0 point was then connected with this intersection point. The slope of this line is called the upper airway gain (UAG) (UAG = change in ventilation/change in ventilatory drive). The UAG represents the ability of the upper airway muscles to activate and restore ventilation in response to increases in ventilatory drive. Figure 2viii depicts visually how the traits interact and illustrates whether the combination predicts the presence or absence of OSA.27 The generic phenotype diagram depicted in Figure 3 demonstrates how the model predicts airway obstruction. If a steady state ventilation off CPAP (determined by the intersection of the loop gain line and the upper airway gain line) is achieved below the arousal threshold (i.e., if the loop gain and UAG lines intersect to the left of the arousal threshold line [Figure 3A]), the patient will be able to achieve stable breathing, whereas if this intersection occurs to the right of the arousal threshold line (Figure 3B), the patient will experience a respiratory arousal, and thus OSA.
Figure 3.
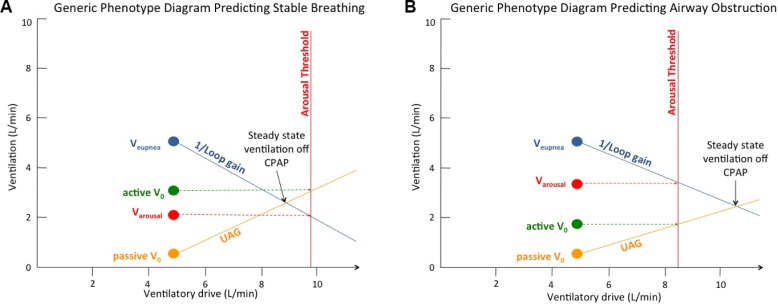
Generic phenotype diagram. (A) Model predicting stable breathing with loop gain line and UAG line intersecting to the left of the arousal threshold line. (B) Model predicting airway obstruction with the loop gain line and UAG line intersecting to the right of the arousal threshold line. UAG, upper airway gain.
In addition to the measurements reported above, for consistency with previous data, we also measured the passive critical collapsing pressure or Pcrit.28 In brief, while the patient was in NREM sleep receiving optimal CPAP, the mask pressure was rapidly reduced for 5 breaths before being returned to optimal CPAP. The reduction in mask pressure was performed in sequenced drops to a level of CPAP (positive or negative) that produced flow limitation. Each run of pressure drops included ≥ 3 drops that produced flow limitation. Peak inspiratory flow was taken from breaths 3–5 in a drop in which flow limitation was observed. The data points were plotted on a flow vs mask pressure graph and a linear regression line was plotted. The intersection of the regression line with the x-axis gives the Pcrit.
Statistics
Data were collated on an Excel spreadsheet (2010, Version 14.0.6129.5000, Microsoft Corporation) and analyzed using IBM SPSS 22 (2013, Release 22.0.0, IBM Corporation). Positional changes on the traits were analyzed using a paired t-test. Mean values between 2 groups (i.e., supine vs. position-independent OSA) were analyzed using an unpaired t-test. We examined both the absolute lung volumes and the positional lung volume expressed as a percentage of the seated lung volume in order to standardize the magnitude of change. All results are given as mean ± standard deviation unless otherwise stated. A P value < 0.05 was considered statistically significant.
RESULTS
The patient demographics are listed in Table 1. The mean value for each phenotypic trait measurement was compared from the supine night to the lateral night, with the results presented in Table 2 and Figure 4.
Table 1.
Patient demographics.

Table 2.
Phenotype values from supine to lateral.
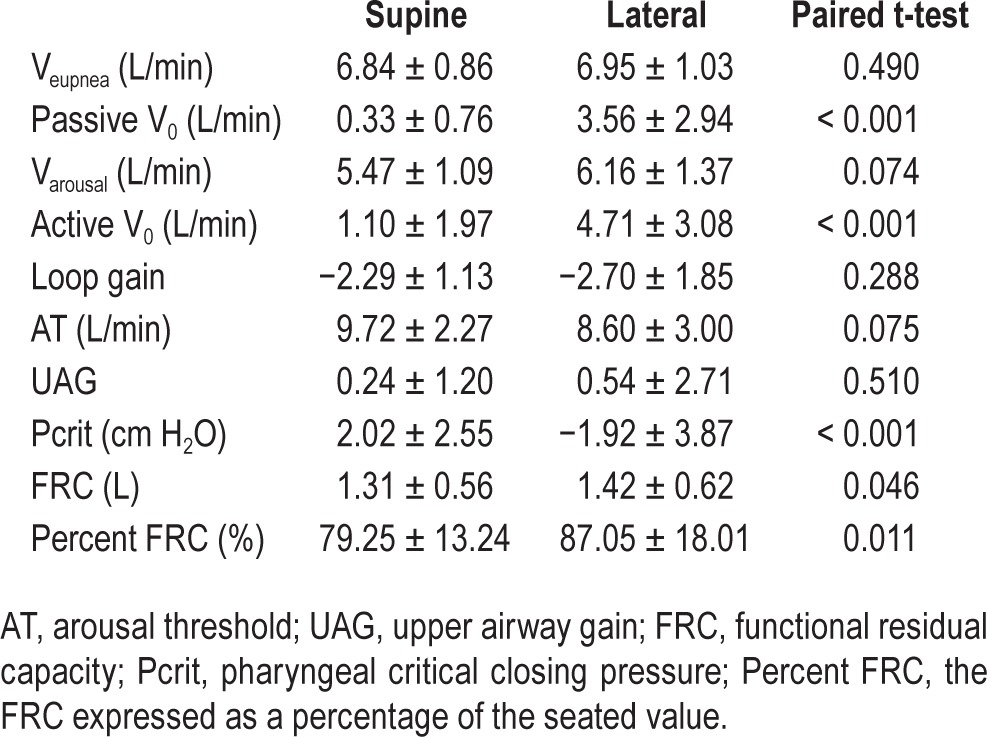
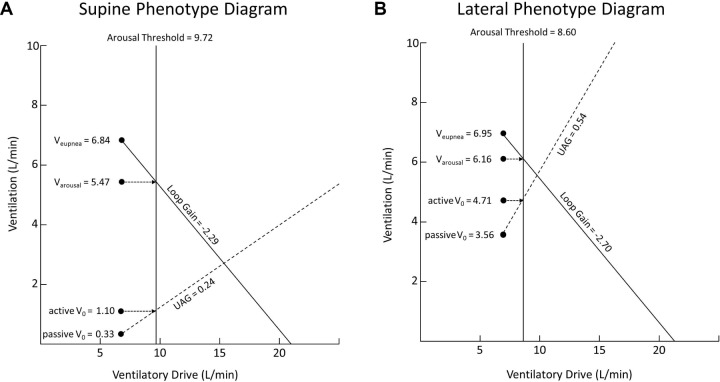
Figure 4.
Summary phenotype diagrams for patients with severe OSA when moving from the supine sleeping position to the lateral sleeping position. UAG, upper airway gain.
There was a similar number of phenotype measurements made in each condition. There was a significant increase when moving from supine to lateral in the passive V0 and active V0, and a significant decrease in Pcrit. The remaining traits (Veupnea, Varousal, loop gain, AT, and UAG) were unaltered by a change in position. The awake FRC was also significantly increased in the lateral position by a mean of 110 mL (8%). The model of the group data (Figure 4) demonstrated that although there was an increase in passive V0 and active V0, the loop gain line and UAG line still intersected to the right of the arousal threshold line when the patients lay in the lateral position. Therefore the model predicted that although lateral sleep increased both the passive anatomy (passive V0, Pcrit) and the achievable ventilation once the upper airway muscles are activated (active V0), it still did not resolve OSA as the intersection of the loop gain and UAG lines lies to the right of the arousal threshold line. Such a prediction is in line with the mean lateral AHI being 36.7 ± 31.9 events/h. The individual phenotype diagrams for each of the 20 patients are included in section 3 of the supplemental material.
We compared the phenotype trait values for patients who had supine OSA with those patients with position-independent OSA; the full results are listed in section 4 of the supplemental material.
There were 7 patients in the supine OSA group and 13 in the position-independent group. There were no significant differences in any of the anthropomorphic or demographic features between the 2 groups. The lateral UAG was significantly increased and the lateral Pcrit significantly decreased in the supine OSA group compared to the lateral UAG and Pcrit in the position-independent group (2.14 ± 2.53 vs −0.32 ± 2.48, P = 0.049 and −4.63 ± 3.33 cm H2O vs −0.50 ± 3.40 cm H2O, P = 0.02, respectively). There was also a strong trend to a significantly increased active V0 in the lateral position in the supine OSA group compared to the position-independent group (6.30 ± 0.74 vs 3.85 ± 0.91, P = 0.052).
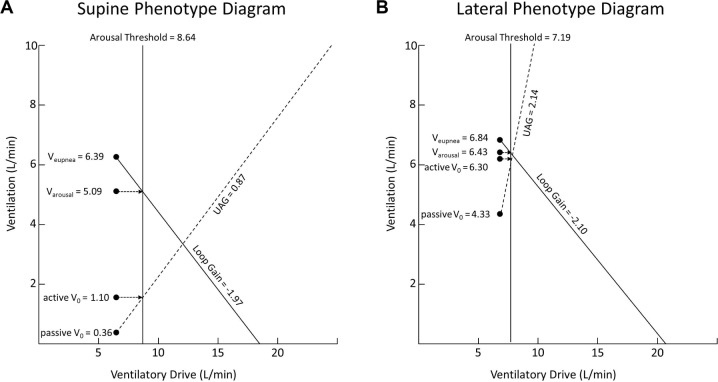
A within-group analysis of the supine OSA group demonstrated a significant improvement when moving from supine to lateral in passive V0 (0.36 ± 0.94 L/min vs 4.33 ± 2.83 L/min, P = 0.007), active V0 (1.56 ± 2.37 L/min vs 6.30 ± 0.74 L/min, P = 0.01), and Pcrit (3.00 ± 2.32 cm H2O vs −4.63 ± 3.33 cm H2O, P = 0.001). For patients with supine OSA, the increase in active V0, passive V0, and UAG brought the intersection of the loop gain line and UAG line very close to the arousal threshold (Figure 5). This is in keeping with the known low lateral AHI in this group of patients of 4.52 events/h (reported with an interquartile range of 12.13, as these data were not normally distributed).
Figure 5.
Phenotype diagrams for supine and lateral position in patients who have a supine to non-supine AHI ratio of > 4:1. UAG – upper airway gain.
DISCUSSION
Our study demonstrates for the first time that when patients with severe OSA shift from the supine to lateral position, they experience a significant increase in awake FRC, passive V0, and active V0, and a significant decrease in Pcrit. On their own, these improvements are not large enough to prevent OSA in the lateral position in all patients because two other traits that predispose to OSA, loop gain and arousal threshold, are not altered by changes in body position. Furthermore, we demonstrate for the first time that the subgroup of OSA patients who have a supine predominant OSA (defined by a supine to non-supine AHI ratio of > 4:1) have significantly more favorable UAG and Pcrit, and a trend to significant improvement in active V0 in the lateral position compared to other patients with OSA. This improvement is large enough to almost completely avert OSA in the lateral position in these patients. Our findings demonstrate that, in a subpopulation of OSA patients with supine OSA, the preservation of airway function in the lateral position arises from an ability to stiffen and dilate the airway more effectively (improved UAG and active V0) than patients with position-independent OSA. Additionally, our findings suggest that patients with supine OSA exhibit a dynamic change in their collapsibility with positional changes. In a subset of patients who display a relatively less collapsible airway in the lateral position, we speculate that these individuals will be more likely to respond to therapies targeting the non-anatomical traits (i.e., loop gain and arousal threshold)— as those with position-independent OSA will need to have their anatomy altered before any non-anatomical therapy is likely to be of benefit.
The observed improvements in the passive and active V0 observed in all patients in the lateral sleeping position likely result from a combination of: (1) a more effective airway dilatation in the lateral position, (2) improved caudal traction of the trachea secondary to improved lung volume, (3) the inherent folding qualities of the lateral pharyngeal walls, and (4) a change in the direction of gravity through upper airway structures.
The improvements observed in active V0 in the lateral position indicate that the airway is able to stiffen or dilate more effectively in that position. In addition to the improvements of lateral positioning on passive characteristics of the airway listed above (i.e., improvement in passive V0 and Pcrit), it may be that the function or effectiveness of pharyngeal dilator muscles is also improved in this position. Previous studies have demonstrated that the genioglossus muscle is at its most active when OSA patients and normal subjects lie in the supine sleeping position.29,30 This is likely the result of the muscle having to work harder to overcome the unfavorable passive anatomy in the supine position (i.e., a higher Pcrit when supine). With the improvement in passive qualities and change in the direction of gravity through soft tissue structures it may be that the genioglossus has to work less to achieve greater stiffening and dilating in the lateral position. In our study, the subgroup of patients with supine-predominant OSA demonstrated a significant improvement in UAG and a trend towards a significant improvement (P = 0.052) in active V0 in the lateral position, despite no significant differences in passive V0. In this group of patients, the function of pharyngeal dilator muscles appears to be the main difference determining almost complete resolution of OSA in the lateral position.
In our patients, the awake FRC increased when they shifted from supine to lateral—an effect already reported31 and shown to be associated with a reduced upper airway collapsibility that may result from an increase in caudal tracheal traction and reduced tissue pressures around the upper airway.32–34 Whether the size of the volume change we observed (110 mL) is sufficient to explain an improved Pcrit of 3.9 cm H2O (from 2.0 ± 2.5 cm H2O supine to −1.9 ± 3.9 cm H2O lateral) is questionable in view of earlier studies. For instance, Jordan et al. demonstrated that increasing end-expiratory lung volume by 500 mL improved the Pcrit from 2.2 ± 0.7 cm H2O to −1.0 ± 0.5 cm H2O. Of note, the improvement in Pcrit observed in our study of −3.9 cm H2O in the lateral position is larger than that observed in previous studies (2.2–2.9 cm H2O).20–22 The most likely explanation for the larger Pcrit improvement observed in lateral sleep compared to previous reports is that the proportion of supine-related OSA patients in our cohort (7/20) is greater than in other cohorts of severe OSA patients.9 A larger proportion of patients with supine-related OSA will bias the results to a greater improvement in Pcrit in the lateral position compared to previous studies.
The folding characteristics of the lateral pharyngeal airway may also play an important role in determining collapse in the supine sleeping position. With the airway adopting a laterally oriented ellipsoid shape with the patient supine,35 collapse is most likely to occur initially at the lateral walls before propagating medially.36,37 Modeling of the human airway, using both physical38 and mathematical39 models, indicates that the folding geometry of the lateral airway is critically important in determining collapsibility of the airway. When a patient with OSA lies in the lateral position, there is an opening up of the lateral portions of the airway, so that the overall shape of the velopharynx becomes more circular.35 Coupled with a change in the direction of gravity through the upper airway soft tissues, the altered geometry of the lateral airway when the patient lies in the lateral position may explain part of the improvement seen in passive V0 in that position.
When lying in the supine position, the bulk of the soft tissue structures such as the tongue and soft palate lie anterior to the velopharyngeal airway.40 In this position, gravitational pull favors posterior collapse of the bulky soft tissue structures. With the patient lying in the lateral position, the tongue and soft palate now lie perpendicular to the gravitational pull (i.e., they now constitute the lateral wall of a 90° rotated airway). It is now the smaller volume of soft tissue that constitutes the lateral pharyngeal wall that lies in the anterior position, resulting in an airway that is less likely to collapse when passive and in the lateral position.40
Implication for an Alternative to CPAP Treatment of OSA
A number of strategies may be employed to prevent patients with OSA from sleeping in the supine position.41 In certain patients, where the lateral AHI is low from night to night, and the propensity for supine airway collapse is repeatable,23 positional therapy could normalize the AHI.42 Our study demonstrates why positional therapy does not work for all OSA patients. Only some patients improve their active (active V0) and passive (passive V0 and Pcrit) anatomy sufficiently when moving to the lateral position, particularly when considering the interaction between airway anatomy and the other physiological traits predisposing to OSA. We show for the first time that arousal threshold and loop gain are not significantly affected by moving to the lateral position and continue to predis-pose the airway to collapse.
Several studies have addressed the contribution of a high loop gain and low arousal threshold to OSA severity.43–45 From these studies, it has been estimated that a low arousal threshold contributes to OSA in up to 50% of patients with the disease,46 and that the administration of a non-muscle relaxant sedative can elevate the arousal threshold by 28% to 48%.44,47 In addition, the administration of acetazolamide reduces the loop gain in OSA sufferers by 41% without altering any of the other pathophysiological traits measured.45 Altering these traits has been shown to lead to a partial improvement in OSA.
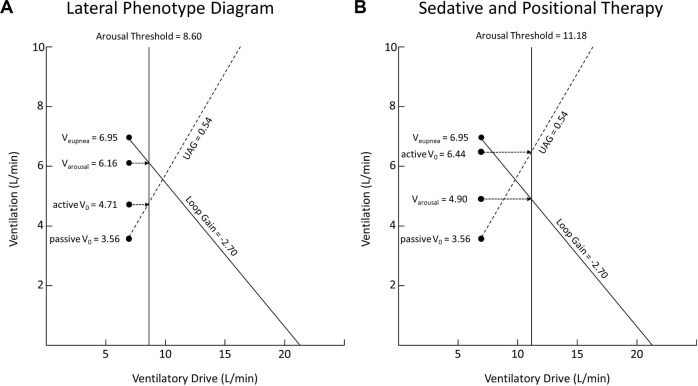
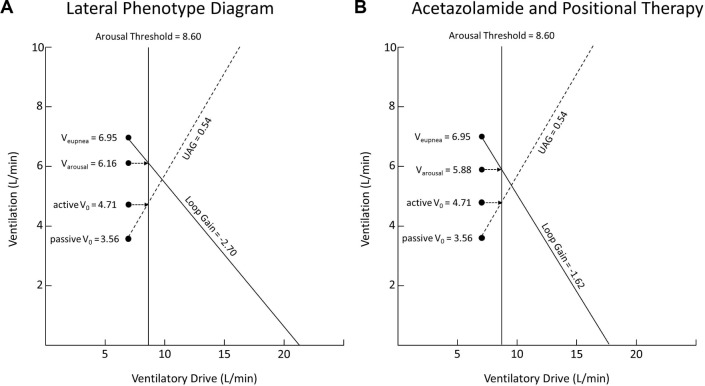
Given that lateral sleep improves passive V0 and active V0 but not loop gain or arousal threshold, we considered the effect combining these treatments would have on patients with OSA (i.e., the effect of lateral positioning combined with treatment of loop gain or arousal threshold). By using our data demonstrating the phenotypic trait values of all 20 patients when lying in the lateral position (see Figure 6A, copied from Figure 4B above) and applying an improvement in arousal threshold of 30% (from previously published data) to our data, we can demonstrate that the model now predicts complete resolution of obstructive events (see Figure 6B). That is, in Figure 6A the model predicts obstructive events with the loop gain line and UAG line intersecting to the right of the arousal threshold line, while in Figure 6B, with the improvement in arousal threshold applied, the model predicts stable breathing with the loop gain line and arousal threshold line intersecting to the left of the arousal threshold line. By contrast, an improvement in loop gain of 40% applied on its own is not enough to resolve obstructive events (Figure 7). Figure 7A is the data from our 20 patients in the lateral position (again, copied from Figure 4B above), with the model predicting obstructive events. In this instance, the model still predicts obstructive events despite the applied improvement in loop gain (see Figure 7B where the loop gain line and UAG line still intersect to the right of the arousal threshold line).
Figure 6.
(A) Data of the 20 patients in our study in the lateral position. (B) Predicted effect of combination therapy with lateral positioning and non-muscle relaxant sedative to improve arousal threshold.
Figure 7.
(A) Data of the 20 patients in our study in the lateral position. (B) Predicted effect of combination therapy with lateral positioning and acetazolamide treatment of ventilatory control instability (loop gain).
This analysis suggests that the potential combination of positional modification and non-muscle relaxant sedatives as an alternative to CPAP therapy in severe OSA sufferers is a fruitful strategy for further investigation.
Limitations
The first limitation of our study is that it included only a small cohort of patients, all of whom had severe OSA, limiting its applicability to the OSA population as a whole, particularly patients of varying OSA severity.
For technical reasons, we limited our measurements of the pathophysiological traits to NREM sleep. The interaction of the variables measured in REM sleep and the effect of lateral position on the variables has not been demonstrated, although we know from previous studies that positional effects on the AHI do occur in REM sleep.22,48
The lung volume measurements we made were during wakefulness. Measurements of both supine and lateral lung volumes have not been made during sleep in individuals with OSA, and no studies have compared these values with awake values in the same postures. We therefore cannot be certain of how our awake measurements of FRC relate to the actual FRC during sleep, and this is a limitation of the data presented.
CONCLUSION
We have demonstrated for the first time that patients with severe OSA who move from the supine to lateral position have a significant improvement in awake FRC, passive upper airway collapsibility (passive V0 and Pcrit), and the ability of the airway to dilate and stiffen (active V0), but there is no significant change in the respiratory arousal threshold or loop gain. In a subgroup of OSA patients with supine OSA (a supine to non-supine AHI ratio > 4:1), the improvement in OSA in the lateral position appears to be the result of an improvement in the ability of the airway to stiffen and dilate in that position, possibly as a function of more effective pharyngeal dilation in that position (i.e., improved UAG and trend to significant improvement in active V0). Our data suggest that, although positional therapy alone will not resolve obstruction in the majority of patients with severe OSA, it could be combined with treatments that improve arousal threshold and loop gain as an alternative to CPAP in patients with severe OSA.
DISCLOSURE STATEMENT
The dial-down CPAP machine used in this project was loaned for use by ResMed. Dr. Joosten is supported by a National Health and Medical Research Council (NHMRC) of Australia's Postgraduate Research Scholarship (1038124) and has been a paid speaker for Astra Zenica. Dr. Wellman is a consultant for Galleon and has received grant/research support from Philips/Respironics. Mr. Turton has consulted for Compumedics. Dr. Hamilton has received equipment for investigator lead research from ResMed. Dr. Edwards is supported by the NHMRC of Australia's CJ Martin Overseas Biomedical Fellowship (1035115). This was not an industry supported study. The other authors have indicated no financial conflicts of interest. Author Contribution: Concept and design: Drs. Joosten, Hamilton, Berger, Edwards, and Wellman. Data collection: Dr. Joosten, Mr. Turton, and Ms. Skuza. Data analysis: Dr. Joosten, Dr. Berger, and Mr. Turton. Manuscript preparation: Drs. Joosten, Hamilton, Berger, and Edwards.
SUPPLEMENTAL MATERIAL
Section 1: Description of Method for Lung Volume Nitrogen Gas Washout Method
The patient's head and neck position were controlled in the supine and lateral position using a series of pillows. Briefly, patients breathed through a mouthpiece that facilitated the measurement of ventilation and carbon dioxide (CO2) (NICO Cardiopulmonary Management System) and oxygen (O2) (Ametek S-3A/I, Ametek Process Instruments, Pittsburgh, PA). Fractional expired nitrogen was calculated from the fractional expired O2 and CO2 levels (expired FN2 = 1- expired FO2- expired FCO2). Once patients were acclimatized (∼2–3 min) to the breathing circuit, we switched the inspired gas from room air (79% nitrogen, N2) to 100% O2 (0% N2), facilitated by a low resistance non-rebreathing valve (Medium T shape 2-Way NRBV, Hans-Rudolph, Kansas City, MO). When a steady-state expired FN2 was achieved, participants were switched back to room air (“wash-in” phase) until a steady state was reached (constant FiN2 within 1.5% between breaths, ∼5–7 min). The time course of the rise in N2 was used to measure FRC according to the equation FRC = ΔVolN2/ΔFN2; where ΔVolN2 is the change in alveolar N2 volume from the start to end of the test (area under the curve of expired FN2 versus cumulative expired volume), and ΔFN2 is the change in alveolar N2 concentration during this time (final FN2 − initial FN2). The “wash-in” phase of the test, rather than the “washout” phase, was used to avoid the transient reduction in ventilation that can occur with a rapid rise in PO2 at the onset of the washout.
Section 2: Algorithm For Performing CPAP Dial-Down
In order to model the interaction between the various traits to determine the predisposition to OSA, the five “ventilations” (Veupnea, passive V0, Varousal, active V0, and ventilatory response/ventilatory disturbance) are plotted on a graph of ventilation versus ventilatory drive. This graph also allows the calculation of the arousal threshold and the responsiveness of the upper airway muscles (Figure 2-main manuscript). First, Veupnea, which is the subject's asleep ventilatory requirement, determined by averaging several minutes of ventilation on optimal CPAP, is plotted (Figure 2i and Figure 1i-main manuscript). The value is placed along the line of identity between ventilation and ventilatory drive as it indicates that the patient's ventilatory demand is being fully met with the airway totally patent on optimal CPAP. Second, the passive V0, which is the ventilation at CPAP = 0 cm H2O when the upper airway muscles are passive, is plotted (Figure 2ii and Figure 1ii-main manuscript). This value is determined by averaging the ventilation of breaths 3 and 4 (without arousal) following a rapid dial down from optimal CPAP to 0 cm H2O. Third, Varousal, which is the ventilation that leads to a respiratory arousal is plotted (Figure 2iii and Figure 1iii-main manuscript). This value is determined from the mean ventilation of the 5 breaths prior to a respiratory-induced arousal. Fourth, active V0, which is the ventilation at CPAP = 0 cm H2O when the pharyngeal muscles are active, is plotted (Figure 2iv and Figure 1iv-main manuscript). This value is determined by averaging the ventilation of breaths 3 and 4 following a dial down in CPAP pressure from CPAPmin to 0 cm H2O (if CPAPmin is a negative pressure then the pressure is dialled “up” to 0 cm H2O). Fifth, the reciprocal of loop gain line is plotted (Figure 2v and Figure 1v-main manuscript). The slope of the line is determined by calculating how much increased ventilatory drive (horizontal vector of the line) is created by a reduction in ventilation (vertical vector of line). That is, loop gain = response (increase or overshoot in ventilation above eupnea) ÷ disturbance (reduction in ventilation below eupnea), in this case loop gain = 7.1 L/min ÷ −1.5 L/min = −4.7 (note that loop gain is dimensionless). The slope of the line plotted is 1/LG or in this case 1/−4.7.
Algorithm for measuring phenotypic traits.
Once the loop gain is known, the arousal threshold, which is the level of ventilatory drive at which a patient will arouse from sleep, can now be determined from the intersection of a horizontal line through Varousal and the loop gain line (Figure 2vi-main manuscript). Lastly, the upper airway gain (UAG) can be determined as follows (Figure 2vii-main manuscript): a horizontal line is drawn through the active V0 and its intersection with the arousal threshold line. The passive V0 point is then connected with this intersection point. The slope of this line is called the UAG (UAG = change in ventilation/change in ventilatory drive). The UAG represents the ability of the upper airway muscles to activate and restore ventilation in response to increases in ventilatory drive. Figure 2viii (main manuscript) depicts visually how the traits interact. The model is able to predict OSA in that if a steady state ventilation off CPAP (determined by the intersection of the loop gain line and the upper airway gain line) is achieved below the arousal threshold (if the LG and UAG lines intersect to the left of the arousal threshold line) then the patient will be able to achieve stable breathing, whereas if this intersection occurs to the right of the arousal threshold line, the patient will experience a respiratory arousal, and thus OSA.
Section 3: Individual Phenotype Diagrams
Lateral 1.
Supine 1.
Lateral 2.
Supine 2.
Lateral 3.
Supine 3.
Lateral 4.
Supine 4.
Lateral 5.
Supine 5.
Lateral 6.
Supine 6.
Lateral 7.
Supine 7.
Lateral 8.
Supine 8.
Lateral 9.
Supine 9.
Lateral 10.
Supine 10.
Lateral 11.
Supine 11.
Lateral 12.
Supine 12.
Lateral 13.
Supine 13.
Lateral 14.
Supine 14.
Lateral 15.
Supine 15.
Lateral 16.
Supine 16.
Lateral 17.
Supine 17.
Lateral 18.
Supine 18.
Lateral 19.
Lateral 19.
Lateral 20.
Supine 20.
Section 4: Comparison of Supine OSA Group and Position-Independent OSA Groups
Comparison of patients with supine to non-supine AHI ratio of > 4:1 to all other patients.
Lung volume variability, seated position.
Lung volume variability, lateral position.
Lung volume variability, supine position.
Lung volume variability, seated position: night 1.
Lung volume variability, seated position: night 2.
Lung volume variability, lateral position: night 1.
Lung volume variability, lateral position: night 2.
Lung volume variability, supine position: night 1.
Lung volume variability, supine position: night 2.
REFERENCES
- 1.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 2.Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–14. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- 3.Cheshire K, Engleman H, Deary I, Shapiro C, Douglas NJ. Factors impairing daytime performance in patients with sleep apnea/hypopnea syndrome. Arch Intern Med. 1992;152:538–41. [PubMed] [Google Scholar]
- 4.Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502–7. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 5.Coughlin SR, Mawdsley L, Mugarza JA, Calverley PM, Wilding JP. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 7.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 8.Joosten SA, Hamza K, Sands S, Turton A, Berger P, Hamilton G. Phenotypes of patients with mild to moderate obstructive sleep apnoea as confirmed by cluster analysis. Respirology. 2012;17:99–107. doi: 10.1111/j.1440-1843.2011.02037.x. [DOI] [PubMed] [Google Scholar]
- 9.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 10.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol. 1997;82:1319–26. doi: 10.1152/jappl.1997.82.4.1319. [DOI] [PubMed] [Google Scholar]
- 11.Schwab RJ. Upper airway imaging. Clin Chest Med. 1998;19:33–54. doi: 10.1016/s0272-5231(05)70430-5. [DOI] [PubMed] [Google Scholar]
- 12.Eckert DJ, Lo YL, Saboisky JP, Jordan AS, White DP, Malhotra A. Sensorimotor function of the upper-airway muscles and respiratory sensory processing in untreated obstructive sleep apnea. J Appl Physiol. 2011;111:1644–53. doi: 10.1152/japplphysiol.00653.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–7. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–32. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellman A, Malhotra A, Jordan AS, Stevenson KE, Gautam S, White DP. Effect of oxygen in obstructive sleep apnea: role of loop gain. Respir Physiol Neurobiol. 2008;162:144–51. doi: 10.1016/j.resp.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan AS, Eckert DJ, Wellman A, Trinder JA, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med. 2011;184:1183–91. doi: 10.1164/rccm.201106-0975OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–33. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 18.Stanchina ML, Malhotra A, Fogel RB, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–6. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 19.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–8. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudewyns A, Punjabi N, Van de Heyning PH, et al. Abbreviated method for assessing upper airway function in obstructive sleep apnea. Chest. 2000;118:1031–41. doi: 10.1378/chest.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 21.Ong JS, Touyz G, Tanner S, Hillman DR, Eastwood PR, Walsh JH. Variability of human upper airway collapsibility during sleep and the influence of body posture and sleep stage. J Sleep Res. 2011;20:533–7. doi: 10.1111/j.1365-2869.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 22.Penzel T, Moller M, Becker HF, Knaack L, Peter JH. Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea. Sleep. 2001;24:90–5. doi: 10.1093/sleep/24.1.90. [DOI] [PubMed] [Google Scholar]
- 23.Joosten SA, O'Donoghue FJ, Rochford PD, et al. Night-to-night repeatability of supine-related obstructive sleep apnea. Ann Am Thorac Soc. 2014;11:761–9. doi: 10.1513/AnnalsATS.201309-306OC. [DOI] [PubMed] [Google Scholar]
- 24.Joosten SA, Edwards BA, Wellman A, et al. Obstructive sleep apnea phenotypic trait changes from supine to lateral position [abstract] Am J Respir Crit Care Med. 2014;189:A3909. [Google Scholar]
- 25.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 26.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 27.Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol. 2013;114:911–22. doi: 10.1152/japplphysiol.00747.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gold AR, Schwartz AR. The pharyngeal critical pressure. The whys and hows of using nasal continuous positive airway pressure diagnostically. Chest. 1996;110:1077–88. doi: 10.1378/chest.110.4.1077. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka R, Ono T, Ishiwata Y, Kuroda T. Respiratory-related genioglossus electromyographic activity in response to head rotation and changes in body position. Angle Orthod. 2000;70:63–9. doi: 10.1043/0003-3219(2000)070<0063:RRGEAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tagaito Y, Isono S, Remmers JE, Tanaka A, Nishino T. Lung volume and collapsibility of the passive pharynx in patients with sleep-disordered breathing. J Appl Physiol. 2007;103:1379–85. doi: 10.1152/japplphysiol.00026.2007. [DOI] [PubMed] [Google Scholar]
- 32.Kairaitis K, Byth K, Parikh R, Stavrinou R, Wheatley JR, Amis TC. Tracheal traction effects on upper airway patency in rabbits: the role of tissue pressure. Sleep. 2007;30:179–86. doi: 10.1093/sleep/30.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–31. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 34.Rowley JA, Permutt S, Willey S, Smith PL, Schwartz AR. Effect of tracheal and tongue displacement on upper airway airflow dynamics. J Appl Physiol. 1996;80:2171–8. doi: 10.1152/jappl.1996.80.6.2171. [DOI] [PubMed] [Google Scholar]
- 35.Walsh JH, Leigh MS, Paduch A, et al. Effect of body posture on pharyngeal shape and size in adults with and without obstructive sleep apnea. Sleep. 2008;31:1543–9. doi: 10.1093/sleep/31.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab RJ, Pack AI, Gupta KB, et al. Upper airway and soft tissue structural changes induced by CPAP in normal subjects. Am J Respir Crit Care Med. 1996;154:1106–16. doi: 10.1164/ajrccm.154.4.8887615. [DOI] [PubMed] [Google Scholar]
- 37.Kuna ST, Bedi DG, Ryckman C. Effect of nasal airway positive pressure on upper airway size and configuration. Am Rev Respir Dis. 1988;138:969–75. doi: 10.1164/ajrccm/138.4.969. [DOI] [PubMed] [Google Scholar]
- 38.Amatoury J, Kairaitis K, Wheatley JR, Bilston LE, Amis TC. Onset of airflow limitation in a collapsible tube model: impact of surrounding pressure, longitudinal strain, and wall folding geometry. J Appl Physiol. 2010;109:1467–75. doi: 10.1152/japplphysiol.00096.2010. [DOI] [PubMed] [Google Scholar]
- 39.Lucey AD, King AJ, Tetlow GA, et al. Measurement, reconstruction, and flow-field computation of the human pharynx with application to sleep apnea. IEEE Trans Biomed Eng. 2010;57:2535–48. doi: 10.1109/TBME.2010.2052808. [DOI] [PubMed] [Google Scholar]
- 40.Isono S, Tanaka A, Nishino T. Lateral position decreases collapsibility of the passive pharynx in patients with obstructive sleep apnea. Anesthesiology. 2002;97:780–5. doi: 10.1097/00000542-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18:7–17. doi: 10.1016/j.smrv.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–83. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckert DJ, Owens RL, Kehlmann GB, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–14. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edwards BA, Connolly JG, Campana LM, et al. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep. 2013;36:281–5. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards BA, Eckert DJ, McSharry DG, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–12. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oksenberg A, Arons E, Nasser K, Vander T, Radwan H. REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med. 2010;6:343–8. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Algorithm for measuring phenotypic traits.
Lateral 1.
Supine 1.
Lateral 2.
Supine 2.
Lateral 3.
Supine 3.
Lateral 4.
Supine 4.
Lateral 5.
Supine 5.
Lateral 6.
Supine 6.
Lateral 7.
Supine 7.
Lateral 8.
Supine 8.
Lateral 9.
Supine 9.
Lateral 10.
Supine 10.
Lateral 11.
Supine 11.
Lateral 12.
Supine 12.
Lateral 13.
Supine 13.
Lateral 14.
Supine 14.
Lateral 15.
Supine 15.
Lateral 16.
Supine 16.
Lateral 17.
Supine 17.
Lateral 18.
Supine 18.
Lateral 19.
Lateral 19.
Lateral 20.
Supine 20.
Comparison of patients with supine to non-supine AHI ratio of > 4:1 to all other patients.
Lung volume variability, seated position.
Lung volume variability, lateral position.
Lung volume variability, supine position.
Lung volume variability, seated position: night 1.
Lung volume variability, seated position: night 2.
Lung volume variability, lateral position: night 1.
Lung volume variability, lateral position: night 2.
Lung volume variability, supine position: night 1.
Lung volume variability, supine position: night 2.