Abstract
Study Objectives:
To evaluate the sleep pattern and the motor activity during sleep in a cohort of patients affected by Huntington disease (HD).
Design:
Cross-sectional cohort study.
Setting:
Sleep laboratory.
Patients:
Thirty HD patients, 16 women and 14 men (mean age 57.3 ± 12.2 y); 30 matched healthy controls (mean age 56.5 ± 11.8 y).
Interventions:
Subjective sleep evaluation: Epworth Sleepiness Scale (ESS); Berlin's Questionnaire, interview for restless legs syndrome (RLS), questionnaire for REM sleep behavior disorder (RBD). Clinical evaluation: disease duration, clinical severity (unified Huntington disease motor rating scale [UHDMRS]), genetic tests. Laboratory-based full-night attended video-polysomnography (V-PSG).
Measurements and Results:
The duration of the disease was 9.4 ± 4.4 y, UHMDRS score was 55.5 ± 23.4, CAG repeats were 44.3 ± 4.1. Body mass index was 21.9 ± 4.0 kg/m2. No patients or caregivers reported poor sleep quality. Two patients reported symptoms of RLS. Eight patients had an ESS score ≥ 9. Eight patients had high risk of obstructive sleep apnea. At the RBD questionnaire, two patients had a pathological score. HD patients, compared to controls, showed shorter sleep, reduced sleep efficiency index, and increased arousals and awakenings. Four patients presented with sleep disordered breathing (SDB). Periodic limb movements (PLMs) during wake and sleep were observed in all patients. No episode of RBD was observed in the V-PSG recordings, and no patients showed rapid eye movement (REM) sleep without atonia. The disease duration correlated with ESS score (P < 0.02). UHMDRS correlated positively with the ESS score (P < 0.005), and negatively with the percentage of REM sleep.
Conclusions:
Patients with Huntington disease showed a severe sleep disruption and a high prevalence of periodic limb movements, but no evidence of sleep disordered breathing or REM sleep behavior disorder.
Citation:
Piano C, Losurdo A, Della Marca G, Solito M, Calandra-Buonaura G, Provini F, Bentivoglio AR, Cortelli P. Polysomnographic findings and clinical correlates in Huntington disease: a cross-sectional cohort study. SLEEP 2015;38(9):1489–1495.
Keywords: Huntington disease, periodic limb movements, polysomnography, REM eye movement sleep behavior disorder, sleep
INTRODUCTION
Huntington disease (HD) is a progressive, neurodegenerative disorder caused by an abnormal expansion of a CAG repeat sequence in the gene encoding the protein huntingtin on chromosome 4.1 HD affects approximately four to eight individuals per 100,0002 and typically presents between 35 and 45 y of age, with an average survival of 15–25 y after disease onset.3 Asymptomatic offspring of patients with HD, and individuals who are at risk of inheriting the expanded CAG nucleotide, can know their genetic status by predictive genetic testing. Clinical features of Huntington disease include progressive motor dysfunction, cognitive decline, and behavioral and psychiatric disorders.4
Sleep disturbances are frequent in patients with HD. In a self- or caregiver-completion questionnaire, 87.8% of patients reported sleep related problems, rated as important by 61.7%; these disorders consisted mainly in restless limb movements, periodic jerks, nocturnal awakenings, daytime sleepiness, and early awakening.5 Disturbed sleep was a prominent feature of the advanced disease, substantially impairing the quality of life of both patients and caregivers.5 Recently, Goodman et al.6 reported asymptomatic sleep changes since early stages, often long before motor disturbances. In this study, subjects remained significantly longer in bed, spent more time awake, and had a marked reduction of deep sleep and rapid eye movement (REM), but none reported excessive daytime sleepiness. Moreover, patients had alterations in rest-activity cycles similar to those described in more advanced stages of the disease, suggesting that sleep abnormalities start early in the disease course and might represent crucial therapeutic target.
Polysomnography and actigraphy in small groups of patients with HD have documented an increased sleep onset latency,7 sleep fragmentation and frequent nocturnal awakenings, reduced sleep efficiency, delayed and shortened REM sleep, increased periodic leg movements, as well as circadian rhythm disturbances.8–16 Moreover, apart from affecting alertness, attention, memory and executive control, lack of sleep may also be considered a risk factor for developing depression.17 However, sleep alterations as well as their association with other symptoms and signs of the disease have not been systematically studied in large groups of patients with HD or premanifest mutation carriers.
The aim of this study was to evaluate sleep features in a population of patients with HD by means of nocturnal, laboratory based video-polysomnography (V-PSG), and to correlate PSG findings with clinical parameters.
PATIENTS AND METHODS
Patients
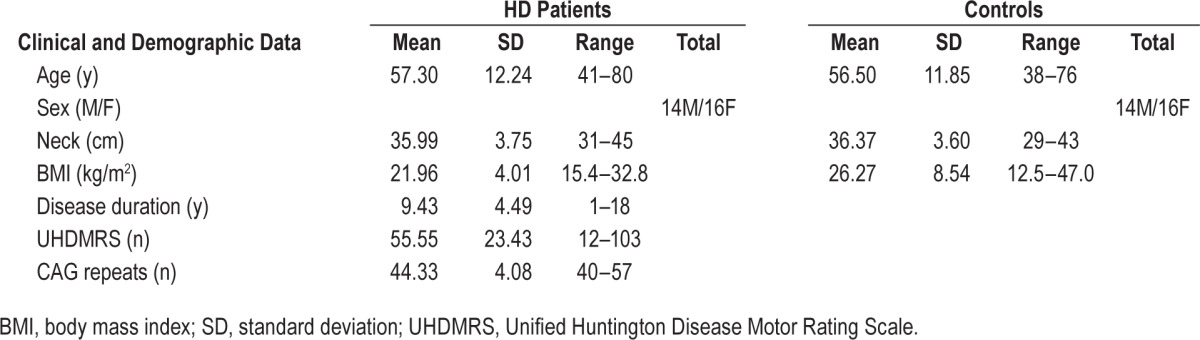
A cohort of 30 consecutive adult patients with HD was enrolled, 16 women and 14 men, mean age was 57.3 ± 12.2 y (range: 41–80 y). Patients were recruited at the Movement Disorders Center of the Catholic University in Rome from February to June 2014. The inclusion criteria were age > 18 y, and diagnosis of HD. Diagnosis of HD was based, according to the established criteria,18 on clinical criteria and confirmed by genetic testing in 21/30 (70%). The nine patients who refused to undergo genetic testing were definitely clinically affected by HD and had a familiar history of HD with at least one family member genetically confirmed. Demographic and clinical data of the study cohort are summarized in Table 1. The study was approved by the local ethics committee, and all patients gave their written consent to participate.
Table 1.
Clinical and demographic data.
Methods
All patients underwent a medical and neurological examination, including measures of weight, height, body mass index (BMI), and neck circumference. The following clinical features were evaluated: disease duration, clinical severity (measured by means of the unified Huntington disease motor rating scale (UHDMRS),19 number of CAG repeats, and pharmacological treatments. In the current study the motor and behavioral symptoms did not allow the withdrawal of pharmacological therapies, which were kept unmodified during the evaluation period. Sleep evaluation included a detailed sleep anamnesis, collected by a sleep specialist, from the patient and the caregivers. For the evaluation of excessive daytime sleepiness (EDS), the validated Italian version of the Epworth Sleepiness Scale (ESS)20 was applied. For diagnosis of restless legs syndrome (RLS) the validated four-item RLS criteria was used21; fulfillment of all four criteria was required for diagnosis. The International RLS Study Group (IRLSSG) scale was administered to patients with positive RLS screening, in order to measure the severity of the disturbance. Moreover, in all subjects an assessment of the symptoms and clinical signs predictors of obstructive sleep apnea syndrome (OSAS) was performed by means of the Berlin Questionnaire (BQ).22 In order to evaluate the presence of rapid eye movement (REM) sleep behavior disorder (RBD), the RBD questionnaire23 was used.
Control Group
Sleep scores and V-PSG findings in patients with HD were compared with a control group of 30 healthy subjects matched for age and sex (14 men, 16 women, mean age 56.5 ± 11.8 y, range 38–76). Control subjects were healthy volunteers, without subjective complaint of sleep disorders or diurnal symptoms consistent with sleep disruption. Controls underwent a full medical and neurological evaluation, and a hypnological interview to rule out present or previous history of sleep disorders. No subject in this group was taking central nervous system active drugs at the time of the study. All control subjects were fully informed and all gave a written consent to participate. Sleep questionnaires (ESS and RLS) were administered to all control subjects.
Polysomnography
Full-night, attended, laboratory-based V-PSGs were recorded in acclimatized, sound-proof rooms. Time of PSG recording was fixed (between 23:00 and 07:00). Recording montage included electroencephalography (EEG) leads applied to following locations: Fp1, Fp2, F3, Fz, F4, F7, F8, C3, Cz, C4, T3, T4, T5, T6, P3, Pz, P4, O1, O2; reference electrodes applied to the left (A1) and right (A2) mastoids; two electrooculography (EOG) electrodes applied to the outer ocular cantus and referred to the contra-lateral mastoid, surface electromyography (EMG) of submental and intercostal muscles, right and left anterior tibialis, right and left extensor communis carpi, airflow measured by thermocouple transducers, thoracic and abdominal effort, electrocardiography (EKG) and peripheral hemoglobin saturation measured by a sensor placed on a finger. Continuous audio and video recording was performed by means of infrared cameras. Sleep recordings were analyzed on computer monitor, and sleep stages were visually classified according to the criteria of the American Academy of Sleep Medicine (AASM).24 Sleep stage percentages were calculated related to total sleep time (TST). The arousal indexes25 (number of arousals per hour) were calculated for total sleep, nonrapid eye movement (NREM) and REM stages.
The scoring of sleep related respiratory events was performed visually, according to the criteria established by the AASM (2007).24 The analysis of the oxygen saturation (SpO2) parameters was made with dedicated software (Rembrandt SleepView version 7.5, Medcare Automation B.V., Amsterdam, NE). Oxygen desaturation events were defined as a fall in SpO2 ≥ 3%. In the analysis of saturation, the following parameters were considered: baseline SpO2, lowest SpO2, oxygen desaturation indexes (ODIs) in total sleep, in NREM, and in REM. OSA was defined by the presence of an apnea-hypopnea index (AHI) > 5 events per hour of sleep (including obstructive and mixed events); central sleep apnea (CSA) was defined by the presence of a central apnea-hypopnea index (CAHI) > 5 events/h of sleep. Periodic limb movements in wake (PLMW) and periodic limb movements in sleep (PLMS) were scored according to established criteria,26 and periodic limb movement (PLM) indexes were calculated for upper and lower limbs, and for wake, the entire sleep period, NREM and REM sleep. The cutoff value for abnormal PLM indexes during wake and sleep was 15 events/h.26 Additionally, the number of intermovement intervals that were 10–90 sec long and all in sequences of at least three, was divided by the total number of intervals to yield the periodicity index (PI); this index can vary between 0 (absence of periodicity, with none of the intervals having a length between 10 and 90 sec) to 1 (complete periodicity, with all intervals having a length between 10 and 90 sec).27
Statistical Analysis
PSG data obtained in the patient group were compared to those obtained from controls. All sleep parameters and PSG measures were compared in these two groups by means of a non-parametric test (Mann-Whitney U test). The threshold for significance was P = 0.05. In case of multiples comparison, in order to avoid family-wise type I errors, a formal Bonferroni correction was applied to each family of comparisons, by dividing the limit of significance by the number of comparisons (for the ODI, three comparisons were made, in the conditions “sleep,” “NREM” and “REM,” therefore the threshold level for significance was P = 0.05/3 = 0.017; for the PLM indexes, four comparisons were made, in the conditions “wake,” “sleep,” “NREM” and “REM,” therefore the threshold level for significance was P = 0.05/4 = 0.0125). Moreover, within the HD group, we calculated the correlation indexes between sleep parameters and the clinical findings (UHDMRS, CAG repeats, disease duration). These correlations were evaluated by means of the Spearman correlation index: the critical value of the Spearman ranked correlation coefficient was ρ = 0.362, corresponding to a significance level P < 0.05. Correlations were controlled for age differences.
RESULTS
Clinical Evaluation
The mean duration of the disease was 9.4 ± 4.4 y, UHDMRS score was 55.5 ± 23.4, CAG repeats were 44.3 ± 4.1. Average BMI was 21.9 ± 4.0 kg/m2, neck circumference was 36.0 ± 3.7 cm. Fifteen patients were taking tetrabenazine, 18 were on neuroleptic treatment, 15 assumed antiepileptic drugs as mood stabilizers (prevalently valproic acid). Most patients (23/30) were assuming more than one drug. In the control group, mean age was 56.5 ± 11.8 y (range: 38–76), average BMI was 26.3 ± 8.5 kg/m2, neck circumference was 36.4 ± 3.6 cm. Clinical and demographic data of study populations are illustrated in Table 1.
Subjective Sleep Evaluation
As noted in the preliminary anamnestic interview, none of the patients, relatives, or caregivers complained about poor quality of sleep. At the clinical sleep evaluation, two patients reported symptoms consistent with RLS (all four IRLSSG criteria were fulfilled). Symptoms were relatively mild (IRLSSG scores were 9 and 11, respectively). The mean ESS score was 6.3 ± 5.0; eight patients had an ESS score ≥ 9, suggesting EDS. The BQ suggested a high risk of OSAS in eight patients. At the RBD questionnaire, the mean score was 2.3 ± 2.0; and two patients had scores above the cutoff value = 5. In the control group, the mean ESS score was 4.0 ± 2.4, no subject reported snoring, witnessed apneas, symptoms of RBD or RLS.
Polysomnography
PSG: Sleep Structure
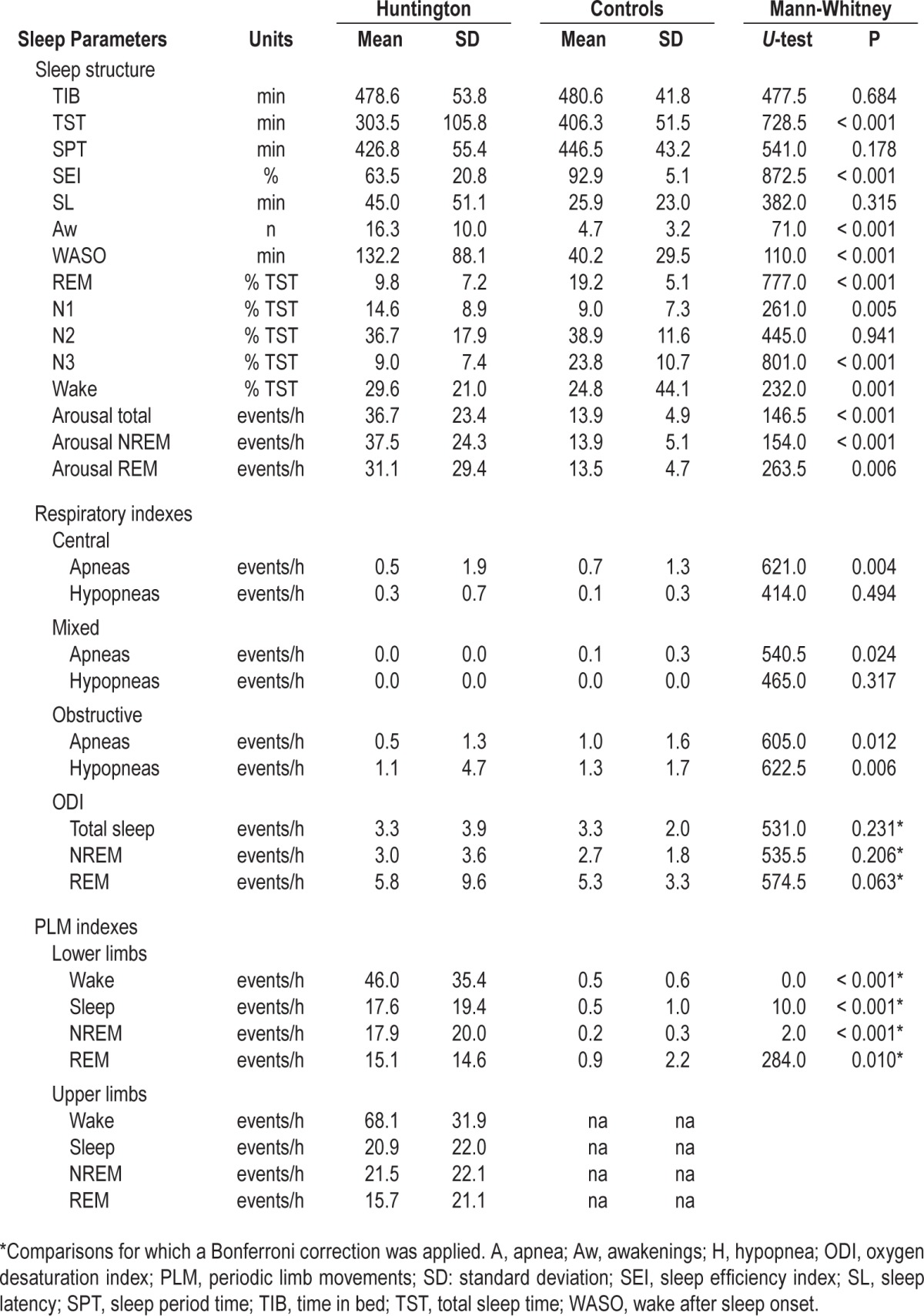
Useful sleep recordings were obtained in all patients and controls. One patient showed an almost total insomnia (TST = 15 min). Patients with HD, as compared to controls (C), showed shorter TST (HD: 303 ± 105 min, C: 406 ± 51 min, P < 0.001), reduced sleep efficiency index (SEI; HD: 63 ± 21%, C: 93 ± 5%, P < 0.001), increased number of awakenings (HD: 16 ± 10, C: 5 ± 3, P < 0.001), and consequently augmented wake after sleep onset (WASO; HD: 132 ± 88 min, C: 40 ± 29 min, P < 0.001). Stages composition in HD was characterized by increased amount of N1 (HD: 15 ± 9%, C: 9 ± 7%, P = 0.005), reduction of N3 (HD: 9 ± 7%, C: 24 ± 11%, P < 0.001) and REM (HD: 10 ± 7%, C: 19 ± 5%, P < 0.001). Patients with HD showed higher arousal indexes (number of events/hour) in total sleep (HD: 37 ± 23, C: 14 ± 5, P < 0.001), in NREM (HD: 37 ± 24, C: 14 ± 5, P < 0.001), and REM (HD: 31 ± 29, C: 13 ± 5, P = 0.006). Detailed results of PSG study and comparison with controls are shown in Table 2.
Table 2.
PSG results, scoring of arousal, respiratory events, and periodic limb movements and comparison of sleep parameters in patients and controls (Mann-Whitney U-test).
PSG: Respiratory Findings
Four patients presented PSG evidence of sleep disordered breathing (SDB): two had obstructive events (mild in one case, OAHI = 5.9; and moderately severe in another, OHI = 29.6 events/h) and one had central events (CAHI = 7.1 events/h). One patient presented hemoglobin desaturations during sleep (ODI = 16.7 events/h) in absence of sleep related respiratory events, possibly suggesting sleep related hypoventilation. The patient with CSA and the patients with mild OSA had ESS scores above the cutoff. None of the subjects in the control group presented PSG findings consistent with SDB.
PSG: Motor Activity, PLMS, RBD
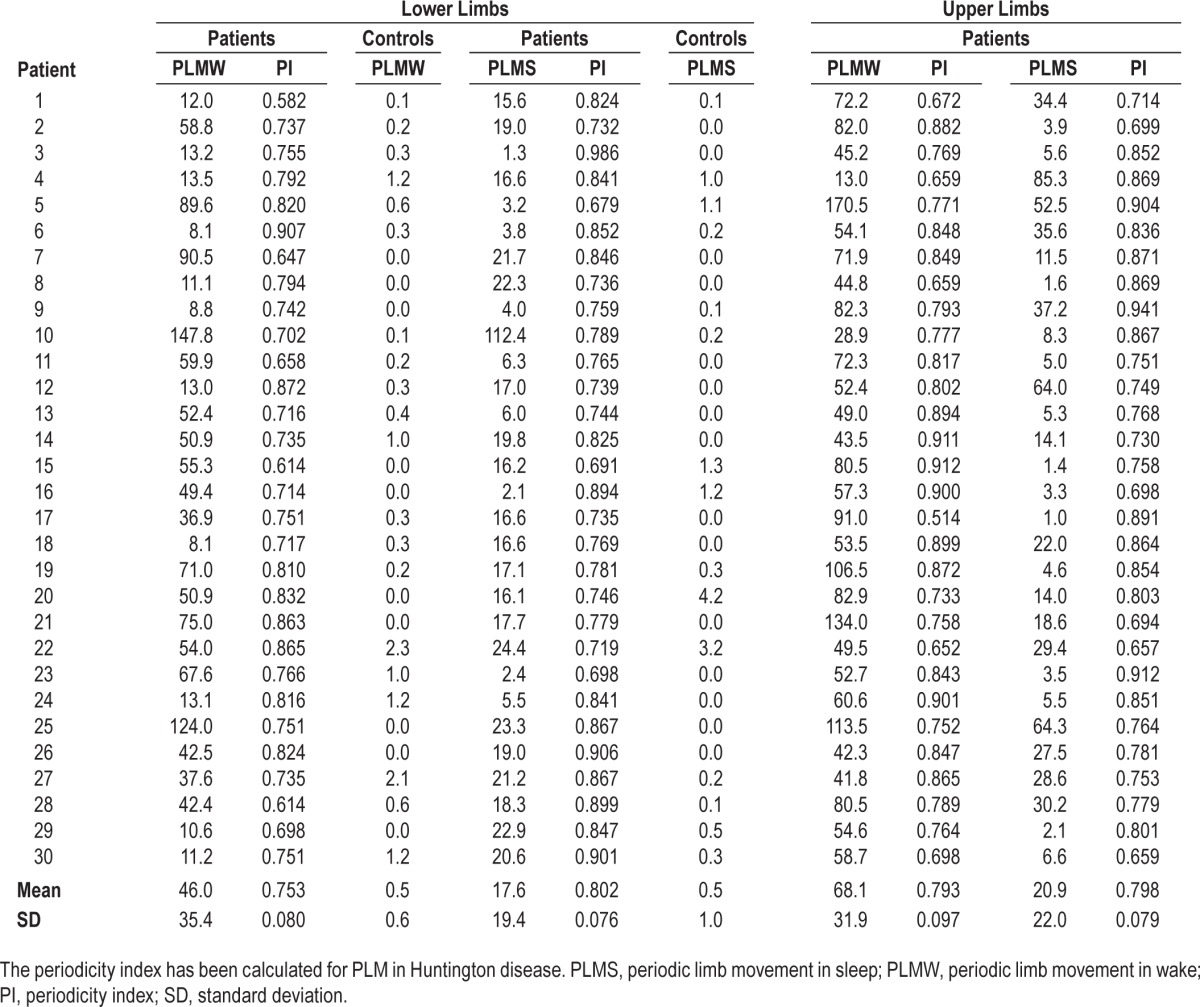
Patients with HD presented an overall increase of motor activity during wake and sleep. PLM were observed in all patients, both during presleep wake and sleep. All patients, compared to controls, presented increased indexes of PLM at the lower limbs, during wake (PLMW; HD: 46 ± 135, C: 0.5 ± 1, P < 0.001), total sleep (PLMS; HD: 18 ± 19, C: 0.5 ± 1, P < 0.001), NREM (PLMS; HD: 18 ± 19, C: 0.2 ± 0.3, P < 0.001) and REM (PLMS; HD: 15 ± 24, C: 0.9 ± 2, P = 0.01). Patients with HD presented high indexes of PLM during wake and all sleep stages also in the upper limbs; recording of upper limbs PLM was not performed in the control group. Both upper and lower limbs PLM persisted during all sleep stages, but were consistently reduced during REM. No episode of RBD was observed in the V-PSG recordings; the two patients who had RBD-Q > 5 did not show PSG evidence of REM sleep Without Atonia (RWA). None of the subjects in the control group presented PSG findings consistent with PLMS or RBD. Detailed results of PLM scoring, in patients and controls, in lower and upper limbs, and values of the periodicity index in HD patients during wake and sleep, are shown in Table 3.
Table 3.
Results of the scoring of periodic limb movements in wake and sleep, lower and upper limbs, patients and controls.
PSG: Clinical Correlations
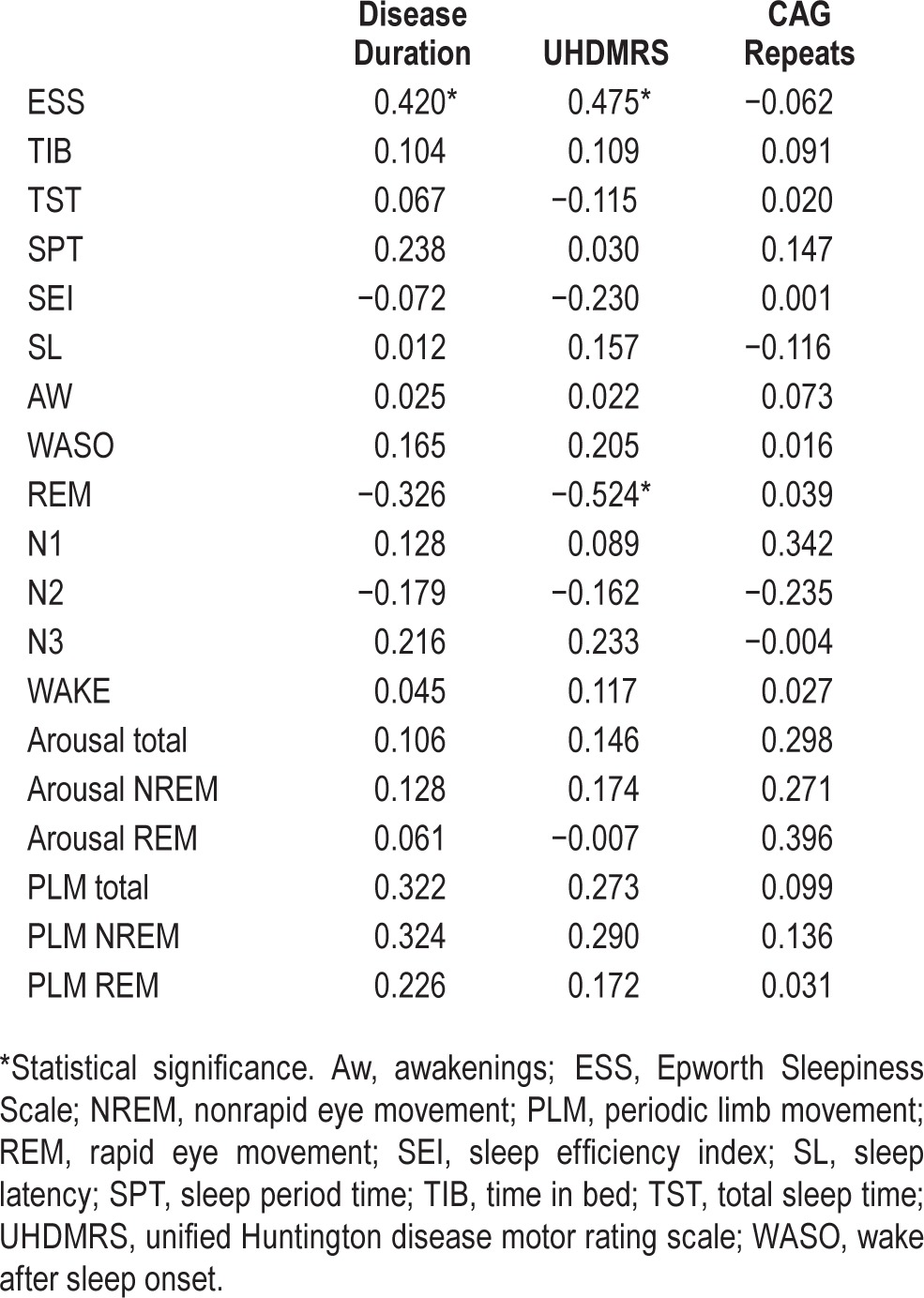
The analysis of correlations between clinical and sleep parameters showed that disease duration was significantly correlated with ESS score (Spearman ρ = 0.420; P < 0.02) but not with PSG scores. UHDMRS correlated positively with the ESS score (Spearman ρ = 0.475; P < 0.005), and negatively with REM percentage (Spearman ρ = −0.524; P < 0.002). No correlation between number of CAG repeats and sleep results was observed. Results of the analysis of correlations are shown in Table 4.
Table 4.
Spearman rank correlations between sleep parameters, disease duration, unified Huntington disease motor rating scale and CAG repeats.
Effect of Drug Treatment
Finally, in order to rule out the effect of pharmacological treatment, the HD group was split into two subgroups: patients taking neuroleptics or benzodiazepines, which can potentially affect sleep measures (n = 18), and patient not assuming these drugs (n = 12). The comparison did not show any significant difference between the subgroups, in any of the clinical and PSG variables. Also, the comparison of PLM indexes between patients taking the different class of drugs did not show any significant difference.
DISCUSSION
The current article describes the largest cohort of patients with HD enrolled so far for a PSG sleep study. The results suggest that sleep is severely disrupted in patients with HD. In particular, our patients with HD had difficulties in initiating sleep (longer sleep latency than controls), and sleep was fragmented by frequent arousal and repeated, long-lasting awakenings. As a result, sleep structure was characterized by increase of light sleep and decrease of deep sleep and REM. These findings, in particular REM sleep reduction, appear to be directly related with the clinical severity, measured by the UHDMRS.
Concomitant sleep disorders were relatively uncommon in our sample. Two patients (6.6%) had symptoms of RLS, of mild-moderate severity; and two (6.6%) had PSG findings consistent with OSA. Daytime somnolence, measured by the ESS, was present in eight patients (26.6%). Somnolence was present in 2/4 (50%) of the patients with PSG findings of SDB. These data do not differ from the prevalence data observed in the general population.21,28 This is in accordance with the result of a previous PSG study by Cuturic et al. (2009).7
In our sample, the prevalence of RBD was, once again, not different from that of the general population.29 No episode of RBD was observed in the V-PSG recordings, and only two patients had, at the RBD-Q, a score > 5, which is considered the cutoff for the clinical suspicion of RBD. In V-PSG, these patients did not show RBD episodes or RWA.
In our patients with HD, nevertheless, motor activity during wake and sleep was considerably increased. All patients presented a variety of PLM during wake and sleep, involving both the upper and the lower limbs. PLMS persisted along all NREM sleep stages, and were markedly reduced during REM. Most PLM were associated with EEG arousals, suggesting that this motor activity may affect the structure of sleep. These data suggest that PLM may be a major pathogenic mechanism of sleep disruption and daytime sleepiness.
The current data are in accordance with some previous reports available in literature, which suggest that sleep is severely affected in HD.5,6,8,9,11,13,14 Conversely, some data do not confirm previous findings. In particular, a previous study by Arnulf et al. (2008)8 reported a higher prevalence of RBD in HD (up to 12%). In our sample, symptoms of RBD were reported in only two cases, and no episode of RBD was recorded in the V-PSG. As concerns the RBD-Q, it is known that this questionnaire, which has a high sensitivity for RBD, poorly discriminated patients with the most challenging differential diagnoses such as sleepwalking or epilepsy. Analogously, it may be conceived that the continuous motor activity of HD patients during sleep might mimic some RBD phenomena. Other relevant differences between our population and that described by Arnulf et al.8 concern the disease severity and the pharmacological treatment. Our patients were older, had longer disease duration, and more severe motor impairment (mean UHDMRS score = 55.5 versus 25.4 in the study by Arnulf et al8). As a consequence of the clinical severity of our patients, we decided to perform the sleep study without any drug withdrawal. Even though this could bias some sleep measures, when we compared patients assuming neuroleptics or benzodiazepines with those assuming only amantadine or tetrabenazine, no difference was observed in clinical and sleep parameters.
We observed in our D patients with HD a very high prevalence of PLM, both during presleep wake and sleep, also involving upper limbs. Also in this respect, the greater clinical severity of our patients may account for the higher indexes of PLM as compared to that reported by Arnulf et al.8 Moreover, we decided to explore, in PSG, also the movements of the upper limbs, which were not recorded in previous studies. This technical issue may further explain the differences in PLM indexes.
Recently, the role of caudate nucleus in sleep pathology has been evaluated.30 Moreover, experimental animal models suggest that caudate lesions may induce behavioral restlessness and hyperresponsivity, consequent to failing of inhibitory modulation of sensory input.31 Electrical stimulation of the caudate enhances cortical synchronization, inhibits behavioural and autonomic nervous system responses to stimuli,32 and suppresses neuronal firing in the reticular formation, thalamus, hypothalamus, and cortex.33 Also in humans, the stimulation of the caudate reduces cortical excitability.34 Taken together, these data may suggest that the caudate degenerative process observed in HD account for the increased arousability, increased motor activity during wake and sleep (originating PLM), reduction of slow wave sleep and in particular of REM sleep and, overall, a general sleep disruption.11,12
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–83. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 2.Harper PS. The epidemiology of Huntington's disease. Hum Genet. 1992;89:365–76. doi: 10.1007/BF00194305. [DOI] [PubMed] [Google Scholar]
- 3.Foroud T, Gray J, Ivashina J, Conneally PM. Differences in duration of Huntington's disease based on age at onset. J Neurol Neurosurg Psychiatry. 1999;66:52–6. doi: 10.1136/jnnp.66.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker FO. Huntington's disease. Semin Neurol. 2007;27:143–50. doi: 10.1055/s-2007-971176. [DOI] [PubMed] [Google Scholar]
- 5.Taylor N, Bramble D. Sleep disturbance and Huntingdon's disease. Br J Psychiatry. 1997;171:393. doi: 10.1192/bjp.171.4.393c. [DOI] [PubMed] [Google Scholar]
- 6.Goodman AO, Rogers L, Pilsworth S, et al. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington's disease. Curr Neurol Neurosci Rep. 2011;11:211–7. doi: 10.1007/s11910-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 7.Cuturic M, Abramson RK, Vallini D, Frank EM, Shamsnia M. Sleep patterns in patients with Huntington's disease and their unaffected first-degree relatives: a brief report. Behavioral Sleep Med. 2009;7:245–54. doi: 10.1080/15402000903190215. [DOI] [PubMed] [Google Scholar]
- 8.Arnulf I, Nielsen J, Lohmann E, et al. Rapid eye movement sleep disturbances in Huntington disease. Arch Neurol. 2008;65:482–8. doi: 10.1001/archneur.65.4.482. [DOI] [PubMed] [Google Scholar]
- 9.Emser W, Brenner M, Stober T, Schimrigk K. Changes in nocturnal sleep in Huntington's and Parkinson's disease. J Neurol. 1988;235:177–9. doi: 10.1007/BF00314313. [DOI] [PubMed] [Google Scholar]
- 10.Videnovic A, Leurgans S, Fan W, Jaglin J, Shannon KM. Daytime somnolence and nocturnal sleep disturbances in Huntington disease. Parkinsonism Relat Disord. 2009;15:471–4. doi: 10.1016/j.parkreldis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Wiegand M, Moller AA, Lauer CJ, et al. Nocturnal sleep in Huntington's disease. J Neurol. 1991;238:203–8. doi: 10.1007/BF00314781. [DOI] [PubMed] [Google Scholar]
- 12.Wiegand M, Moller AA, Schreiber W, Lauer C, Krieg JC. Brain morphology and sleep EEG in patients with Huntington's disease. Eur Arch Psychiatry Clin Neurosci. 1991;240:148–52. doi: 10.1007/BF02190755. [DOI] [PubMed] [Google Scholar]
- 13.Hurelbrink CB, Lewis SJ, Barker RA. The use of the Actiwatch-Neurologica system to objectively assess the involuntary movements and sleep-wake activity in patients with mild-moderate Huntington's disease. J Neurol. 2005;252:642–7. doi: 10.1007/s00415-005-0709-z. [DOI] [PubMed] [Google Scholar]
- 14.Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease. J Neurosci. 2005;25:157–63. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pallier PN, Maywood ES, Zheng Z, et al. Pharmacological imposition of sleep slows cognitive decline and reverses dysregulation of circadian gene expression in a transgenic mouse model of Huntington's disease. J Neurosci. 2007;27:7869–78. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pallier PN, Morton AJ. Management of sleep/wake cycles improves cognitive function in a transgenic mouse model of Huntington's disease. Brain Res. 2009;1279:90–8. doi: 10.1016/j.brainres.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 17.Franzen PL, Buysse DJ. Sleep disturbances and depression: risk relationships for subsequent depression and therapeutic implications. Dialogues Clin Neurosci. 2008;10:473–81. doi: 10.31887/DCNS.2008.10.4/plfranzen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogarth P, Kayson E, Kieburtz K, et al. Interrater agreement in the assessment of motor manifestations of Huntington's disease. Mov Disord. 2005;20:293–7. doi: 10.1002/mds.20332. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarino AL, Anderson K, Borowsky B, et al. An item response analysis of the motor and behavioral subscales of the unified Huntington's disease rating scale in huntington disease gene expansion carriers. Mov Disord. 2011;26:877–84. doi: 10.1002/mds.23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vignatelli L, Plazzi G, Barbato A, et al. Italian version of the Epworth sleepiness scale: external validity. Neurol Sci. 2003;23:295–300. doi: 10.1007/s100720300004. [DOI] [PubMed] [Google Scholar]
- 21.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–9. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 22.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 23.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for scoring of sleep and associated events: rules, terminology and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 25.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3:121–31. [PubMed] [Google Scholar]
- 26.Zucconi M, Ferri R, Allen R, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7:175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 28.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 29.Kang SH, Yoon IY, Lee SD, Han JW, Kim TH, Kim KW. REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics. Sleep. 2013;36:1147–52. doi: 10.5665/sleep.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoffers D, Altena E, van der Werf YD, et al. The caudate: a key node in the neuronal network imbalance of insomnia? Brain. 2014;137:610–20. doi: 10.1093/brain/awt329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villablanca JR, Marcus RJ, Olmstead CE. Effects of caudate nuclei or frontal cortex ablations in cats. II. Sleep-wakefulness, EEG, and motor activity. Exp Neurol. 1976;53:31–50. doi: 10.1016/0014-4886(76)90279-x. [DOI] [PubMed] [Google Scholar]
- 32.Wilcott RC. Skeletal and automatic inhibition from low-frequency electrical stimulation of the cat's brain. Neuropsychologia. 1974;12:487–96. doi: 10.1016/0028-3932(74)90078-5. [DOI] [PubMed] [Google Scholar]
- 33.Siegel J, Morton CR, Sandkuhler J, Xiao HM, Zimmermann M. Spinal neuronal inhibition and EEG synchrony by electrical stimulation in subcortical forebrain regions of the cat. Exp Brain Res. 1986;62:363–72. doi: 10.1007/BF00238856. [DOI] [PubMed] [Google Scholar]
- 34.Chkhenkeli SA, Sramka M, Lortkipanidze GS, et al. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–29. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]


