Abstract
Objectives:
Preoperative chemotherapy plays a key role in management of bone sarcomas. Postoperative evaluation of histological necrosis has been the gold standard method of assessing response to preoperative chemotherapy. This study was done to evaluate the efficacy of static and dynamic magnetic resonance imaging (MRI) for assessing response preoperatively.
Materials and Methods:
Our study included 14 patients (12 osteosarcomas and 2 malignant fibrous histiocytomas) with mean age of 21.8 years, treated with preoperative chemotherapy followed by surgery. They were evaluated with static and dynamic MRI twice, before starting chemotherapy and again prior to surgery. Change in tumor volume and slope of signal intensity - time curve were calculated and correlated with percentage of histological necrosis using Pearson correlation test.
Results:
The change in dynamic MRI slope was significant (P = 0.001). Also, ≥60% reduction in slope of the curve proved to be an indicator of good histological response [positive predictive value (PPV) =80%]. Change in tumor volume failed to show significant correlation (P = 0.071). Although it showed high negative predictive value (NPV = 85.7%), PPV was too low (PPV = 57.14%).
Conclusions:
Dynamic MRI correctly predicts histological necrosis after administration of preoperative chemotherapy to bone sarcomas. Hence, it can be used as a preoperative indicator of response to neoadjuvant chemotherapy. On the other hand, volumetric assessment by static MRI is not an effective predictor of histological necrosis. This study proves the superiority of dynamic contrast-enhanced study over volumetric study by MRI.
Keywords: Dynamic MR, histological necrosis, malignant fibrous histiocytoma, MR, osteosarcoma, preoperative chemotherapy
Introduction
The approach to management of malignant bone tumors has evolved tremendously in recent years. Previously, surgery was the only treatment option. Nowadays, chemotherapy has become the first - line therapy because of its beneficial local and systemic effects.[1,2,3] With the advent of preoperative chemotherapy, long - term survival has improved from 20 to 70%.[4,5] However, response to chemotherapy regimen is not uniform.
To date, histological assessment of tumor necrosis on the resected tumor specimens has been the gold standard method of assessing response to preoperative chemotherapy.[2,6,7,8] With the effect of chemotherapy, tumor demonstrates large area of necrosis marked with sparsely fibrillar and calcified stromal cells, tumor bone with empty “turtle shells” of tumor osteoid, with few viable osteocytes in the lacunae or empty lacunae.[6] With modern therapy, 45% of the patients have shown more than 90% necrosis.[9] Percentage of tumor necrosis is also the most important prognostic factor for both disease - free survival and recurrence. Good responders to chemotherapy have better disease - free survival rates and can be treated with limb salvage surgery with low risk of recurrence. But poor responders should be treated aggressively with radical surgery and a changed postoperative chemotherapy regimen.[2,4,10]
Early information regarding tumor response to initial chemotherapy cycles will help tailoring subsequent treatment to achieve better control of the tumor.[3,5] Many imaging techniques like conventional radiography, computed tomography (CT) scan, angiography, bone scintigraphy, magnetic resonance imaging (MRI) scan, and positron emission tomography (PET) scan have been evaluated for predicting tumor response preoperatively.[11,12,13,14,15] MR imaging in various forms like static, contrast-enhanced, diffusion-weighted, dynamic contrast-enhanced with or without pharmacokinetic modeling of bone sarcomas has been applied to the study of musculoskeletal tumors with encouraging results.[3,10,16,17,18,19,20,21,22,23]
Dynamic contrast-enhanced MRI studies the kinetics of distribution of paramagnetic contrast in the micro vessels and the interstitial space of tissues.[18] It detects impaired viability by detecting disappearance of tumor vascularity using the slope value of signal intensity–time curve.[19] The role of dynamic MRI in assessing response to preoperative chemotherapy is well documented. Compared to dynamic MRI, in literature, there has not been common consensus about reliability of volumetric assessment by static MRI. Though few studies have reported its significant role in predicting histological response, many studies have given an equivocal report.[13,24] Although increase in tumor volume correlates well with the poor histological response, reduced or stable tumor volume does not guarantee a good response.[14,25,26]
Hence, we undertook this exercise to investigate the role of both volumetric and dynamic studies, by static and dynamic MRI scan, respectively, in assessment of the tumor response to preoperative chemotherapy. The results were correlated with histological necrosis.
Materials and Methods
This was a prospective case study done at our hospital between September 2008 and August 2010, after obtaining approval from institute review board.
Patients
Twenty-five patients of bone sarcoma with a mean age of 21.8 years (range 12-40 years) were included in this study after taking informed consent from them or their parents (for patients of age <18 years). Diagnoses were confirmed with core or open biopsy. Out of 25 patients, 11 patients were excluded from the study, which included six patients of Ewing's sarcoma with widespread disease, not amenable to surgical treatment. They were primarily treated with chemotherapy and radiotherapy. Among the other five patients who were excluded from the study, two patients had parosteal variety of osteosarcoma, one patient had already received one cycle of chemotherapy, one patient had metallic implant, and one patient could not complete three cycles due to the development of ulcer over the tumor mass. Finally, 14 patients formed the study group; 10 were male and 4 were female patients. Twelve patients had osteosarcoma and two patients had malignant fibrous histiocytoma (MFH). Histological subtypes were conventional osteosarcoma in 10 patients and telangiectatic osteosarcoma in 2 patients. The tumors were located in femur in nine patients, tibia in three patients, humerus in one patient, and scapula in one patient. Bone was involved over left side in nine and right side in five patients. This study included 1 intracompartmental and 13 extracompartmental tumor, as per Enneking's criteria. Six patients had chest metastasis at the time of inclusion in the study.
The treatment protocol for each patient was decided in tumor board meeting, by a team of specialists which included orthopedic surgeon, medical oncologist, radiotherapist, and pathologist. Patients were started on chemotherapy consisting of three drugs – cisplatin [100 mg/m2 body surface area (BSA) in three divided doses over 3 days], adriamycin (50 mg/m2 BSA single dose), and ifosfamide (1.5 g/m2 BSA daily for 3 days). Three to four cycles of preoperative chemotherapy were given at 3 weeks interval. We did not add methotrexate to the chemotherapy regimen due to lack of monitoring facility.
MRI evaluation
After inclusion, static MRI followed by dynamic study was performed in all 14 patients. It was done before starting chemotherapy and again repeated before surgical resection. MRI study was performed with 1.5 T magnet (Siemens, TIM technology, Avanto, Germany). Static MRI was performed first in coronal, sagittal, and axial planes. Both T1-weighted image (spin echo, TR/TE = 500 ms/12 ms, number of excitation 1) and T2-weighted images (fast spin echo, TR/TE = 3500-4000 ms/120 ms, 180° flip angle, 120 kHz band width, 15 turbo factor, number of excitations 2) were obtained with slice thickness 5 mm, interslice gap 0.5 mm, 340 mm field of view (FOV), and 512 × 256 image matrixes. Dynamic study included six sequences. First baseline sequence was taken in axial plane. Immediately after the first sequence, bolus injection of gadolinium diethylene (0.1 mmol/kg) was administered intravenously within a period of 15 s. It was followed by five fast low angle shot (FLASH) sequences without pause, for a total of 6 min (acquisition time of 72 s per sequence). Acquisition parameters for FLASH sequences included echo delay time of 1.45 ms, T1W1 (TR/TE = 4.27/1.45), 0.9 × 0.8 × 0.9 mm voxel size, 358 × 448 image matrixes, 6° flip angle, 340 mm FOV, 45-65 number of slices with 0.9 mm slice thickness, and use of surface coil and spine matrix coil.
Tumor height, width, and depth were measured from the axial, sagittal, and coronal images in the static MRI. Then, tumor volume was calculated using the formula for ellipsoid mass “V = π/6 × height × width × depth.” Tumor volume ratio (tumor volume after treatment divided by tumor volume before treatment) was calculated. Changes in tumor volume were classified as increased (ratio > 1.05), stable (ratio between 0.95 and 1.05), or decreased (ratio < 0.95). Decreased or stable tumor volume indicated good response to chemotherapy.[24]
In a software program for dynamic analysis, all dynamic sequences were loaded. Imaging plane was selected representing largest area of the tumor. Due to heterogeneity in enhancement, three circular regions of interest of around 1 cm diameter were selected in the areas of maximum enhancement. Signal intensity was measured and plotted against time in a graph. In all three regions of interest, slope of the curve (percentage increase in signal intensity per minute over the baseline value) was calculated using the equation “slope (%/minute) = (SImax – SIprior) × 100/(SIprior × Tmax).” SIprior is the baseline signal intensity before contrast, SImax is signal intensity at Tmax, and Tmax is the time point at which SI/SIprior increased by at least 3% from image to image.[19] Average of three slope values was considered as the final pre-chemotherapy dynamic MRI slope value. Again, in post-chemotherapy dynamic MR image, three regions of similar size were selected in exactly the same areas as visualized in pre-chemotherapy image and the slope was calculated. Slope values obtained before chemotherapy were compared with the values after chemotherapy and the difference was calculated. More than 60% reduction in slope value after chemotherapy was considered as good response.[19]
Histological evaluation
After completion of preoperative chemotherapy, patients were taken for surgery with a mean time interval of 25 days between chemotherapy and surgery. Twelve patients were operated with limb salvage surgery [nine tumor resection, extracorporeal irradiation and reconstruction (ECIR); two tumor resection and reconstruction with custom-made megaprosthesis (CMP); and one resection of scapula]. Amputation was performed in two patients (one transfemoral amputation and one forequarter amputation). After surgical resection of the tumor, the specimens were sent to pathology laboratory and were evaluated histologically. Each specimen was placed in formalin and then sliced coronally or axially at its largest cross section. Five-millimeter-thick slice was prepared which was cut into multiple tissue bits of 5-10 mm length and 5-10 mm breadth. The tissue bits were decalcified and processed further using xylene, alcohol, and formalin. Subsequently, the bits were embedded in paraffin. After embedding, 3.5-4 mm thin sections were cut, mounted, stained with hematoxylin and eosin stain, and then viewed under microscope for necrosis and viable tumor cells.
Percentage area of necrosis was calculated in each slide and summed up to give percentage necrosis of the whole tumor. Tumor necrosis was scored according to Huvos grading (grade I, less than 50% necrosis; grade II, 50-90% necrosis; grade III, more than 90% necrosis with some foci of viable tumor cells; and grade IV, 100% necrosis with no viable tumor cells). At least 90% necrosis (grades III and IV) was considered as good response to preoperative chemotherapy.[6] Based on this histological response of the tumor, patients were divided into two groups, i.e. good responders and poor responders. Those with >90% necrosis (grades III and IV) were considered as good responders and those with <90% necrosis were considered as poor responders.
Statistical analysis
The overall degree of relationship between the change in radiological parameters and histological necrosis was assessed by means of Pearson's correlation analysis using correlation coefficient, Rp. The criterion for statistical significance with a two-tailed test was chosen at a P value of less than 0.05. Both the parameters were individually correlated with the histological response. Using the cut-off criteria for positive response, positive and negative predictive values were calculated. Statistical analysis was done using SPSS software for windows (version 16.0, SPSS Inc).
Results
Histological evaluation of tumor necrosis
Histological examination of the surgically resected specimens showed mean necrosis of 56%. The necrotic foci showed cellular degeneration with sparsely fibrillar, calcified stromal cells and empty tumor osteoid. Areas of fibrosis and hyalinization with inflammatory cell infiltrate were also seen. Considering necrosis of the tumor, according to Huvos grading, nine patients were poor responders (six grade I and three grade II) and five were good responders (three grade III and two grade IV). In two cases of poor response, there was no necrosis at all.
Tumor volume change
Pre-chemotherapy tumor volume of all patients ranged from 70.73 to 4244.42 cm3 (mean 852.8 cm3). Following chemotherapy, increase in tumor volume (tumor volume ratio >1.05) was seen in six poor responders (five Huvos grade I and one grade II necrosis) and one good responder (grade III necrosis). Reduction of volume (tumor volume ratio <0.95) was seen in four good responders (two grade III and two grade IV necrosis) and three poor responders (one grade I and two grade II necrosis). None of the patients showed stable tumor volume. Among the five good responders, four patients showed 9.39-39.20% (59.8-761.85 cm3) decrease in volume and one patient showed 32.88% (207.15 cm3) increase in volume. Among the nine poor responders, the tumor volume increased by 6.47-76.62% (12.66-774.35 cm3) in six patients, while three patients experienced reduction by 9.91-36.13% (25.92-112.76 cm3).
No statistically significant correlation was found between change in tumor volume and histological response (Rp = 0.496, P = 0.071) as depicted in Figure 1. Predictive value of decreased tumor volume for a good response was 57.14% (four of seven patients), and predictive value of increased tumor volume for a poor response was 85.7% (six of seven patients). Sensitivity was 80% and specificity was 66.67%. We observed changes in all three dimensions of the tumor mass including longitudinal dimension (height of the tumor) in post-chemotherapy static MR imaging.
Figure 1.
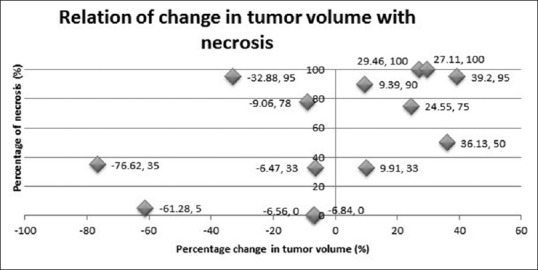
Correlation of tumor volume change with histological necrosis
Dynamic MRI slope
The mean dynamic MRI slope value for all patients before chemotherapy was 41.53% per minute (range 15.33-67.09% per minute). Following preoperative chemotherapy, there was fall in slope values in 12 patients and increase in 2 patients. Post-chemotherapy mean slope value for five good responders was 15.52% per minute (range 11.27-22.7%) and for nine poor responders, it was 26.66% per minute (range 10.41-40.29%). There was reduction in slope value of an average of 67.55% of the pre-chemotherapy value in good responders and of 20.21% in poor responders. One responding patient showed less than 60% reduction in slope (55.97%) and one poor responder showed more than 60% reduction in slope (61.46%). These two patients were classified incorrectly, using criteria of 60% reduction in slope following chemotherapy. Figures 2A, B and 3A, B show dynamic MR images and signal intensity–time curves in a case with 50% necrosis, respectively, and Figures 4A, B, and 5A, B show the same in a case with no necrosis.
Figure 2.
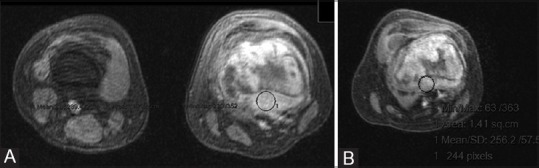
(A and B): (A) Pre-chemotherapy dynamic MR image of osteosarcoma of distal femur in a 15-year-old girl showing greater enhancement (B) Post-chemotherapy dynamic MR image showing reduced enhancement following chemotherapy
Figure 3 (A and B).
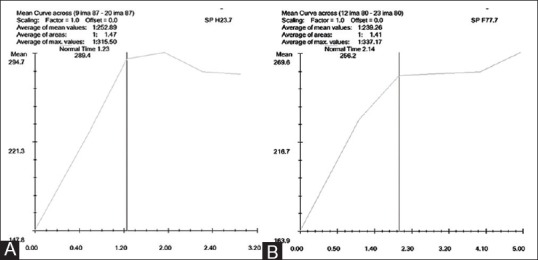
(A) Pre-chemotherapy signal intensity–time curve [signal intensity on the x-axis and time (minute) on the y-axis] showing dynamic MRI slope value of 67.09% (B) Post-chemotherapy signal intensity–time curve [signal intensity on the x-axis and time (minute) on the y-axis] showing dynamic MRI slope value of 34.13% (49.13% reduction in slope value following chemotherapy)
Figure 4 (A and B).

(A) Pre-chemotherapy dynamic MR image of telangiectatic osteosarcoma of proximal tibia in a 15-year-old boy showing greater enhancement (B) Post-chemotherapy dynamic MR image showing reduced enhancement following chemotherapy
Figure 5 (A and B).

(A) Pre-chemotherapy signal intensity–time curve [signal intensity on the x-axis and time (minute) on the y-axis] showing dynamic MRI slope value of 36.7% (B) Post-chemotherapy signal intensity–time curve [signal intensity on the x-axis and time (minute) on the y-axis] showing dynamic MRI slope value of 21.9% (40.3% reduction in slope value following chemotherapy)
Significant correlation was found between percentage change in slope and tumor necrosis (Rp = 0.894, P = 0.001) as depicted in Figure 6. More than 60% reduction in slope was predictive of good response [positive predictive value (PPV) =80%], while less than 60% reduction was predictive of poor response [negative predictive value (NPV) =88.89%]. While pre-chemotherapy slope value did not correlate well with necrosis (Rp = 0.384, P = 0.175), post-chemotherapy slope value showed significant negative correlation with tumor necrosis (Rp = −0.706, P = 0.004).
Figure 6.
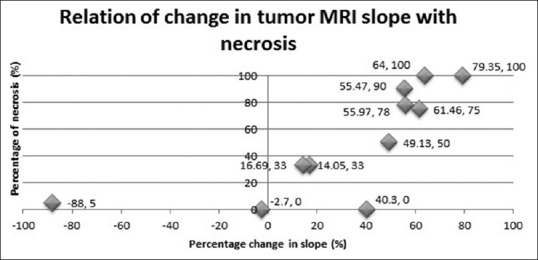
Correlation of change in dynamic MRI slope with histological necrosis
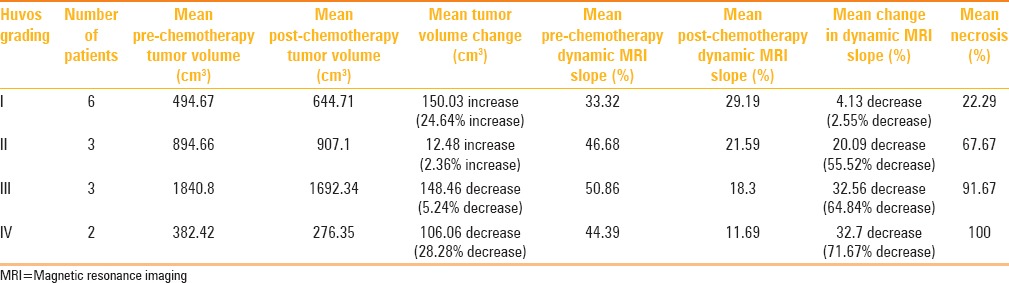
Table 1 shows the changes in tumor volume and dynamic MRI slope, and tumor necrosis in response to preoperative chemotherapy in patients in different Huvos grades.
Table 1.
Changes in static and dynamic MRI parameters (tumor volume and dynamic MRI slope) and histological necrosis in response to preoperative chemotherapy
Discussion
Reduction in the tumor volume occurs due to necrosis, decrease in the volume of normal supporting stroma, and resolution of inflammatory edema.[25] Many studies have been done in the past to find out the usefulness of tumor volume measured by CT or MRI to predict histological response. As CT does not provide good contrast to show the distinction between extraosseous tumor and adjacent normal tissue, volume measurement may be inaccurate with CT.[10] Few studies have found significant correlation between change in tumor volume assessed by MRI and histological necrosis.[14,21,22,26] Although, in our study, change in tumor volume showed trend toward correlation with necrosis (Rp = 0.497), this correlation was not found to be significant (P = 0.071). These findings are supported by other studies which also failed to find a significant correlation.[13,24,25]
Although changes in volume do not accurately reflect the extent of necrosis,[19] increase in volume is associated with poor histological response and decrease in volume indicates good response.[14,25,26] We also found increased tumor volume as a better predictor of poor response (NPV = 85.7%) but decreased tumor volume as a poor predictor of good response to chemotherapy (PPV = 57.14%).
All our patients, except one, had extracompartmental tumor, i.e., tumor spreading beyond well delineated surgical compartment of its origin.[27] Review of literature shows that most of the changes occur in vascularity or in size of the extraosseous component of the tumor.[19,25] Fletcher et al.[18] did not find any change in the intramedullary extent of the tumor, i.e. the longitudinal dimension. In contrast, in our study, we found changes occurring in all three dimensions of the tumor, including the intraosseous length.
In dynamic MRI, the pharmacokinetics of the intravenously injected gadolinium is studied. In this, the slope of signal intensity–time curve indirectly reflects the change in vascularity and, hence, the viability of the tumor. Slope value reflects the increase in signal intensity per unit time in a specific area of tumor, i.e. rate of uptake in the vascularized portion of the tumor. It shows steeper slope in the vascularized portion and gradual slope in the area of necrosis.[19] Dynamic study by contrast-enhanced MRI detects impaired viability by detecting the disappearance of tumor vascularity with the effect of chemotherapy,[19] indirectly by measuring reduction in slope values over consecutive MRI. It helps differentiating regions of necrosis, viable tumor, muscle, and blood vessels, as they display distinct signal intensity–time curves in dynamic images.[17] It also helps differentiating extraosseous tumor, muscle infiltrated by the tumor, edematous muscle, and normal muscle.[28] Recently, in a study by Guo et al.,[29] they found significant correlation between histological response and the pharmacokinetic parameters of dynamic contrast -enhanced MRI. They also found its efficacy in prediction of event -free and overall survival of osteosarcoma patients.
Tumor viability is also indicated by rapid upward slope (greater than 30% per minute), early enhancement of areas within 6 s after arrival of the bolus in a feeding artery, and early wash in and wash out of the contrast agent. More gradual slopes are observed in areas of necrosis, cystic areas, edema, peritumoral inflammation, and paucicellular cartilaginous and myxoid regions.[19,30] The degree of devitalization varies in different parts of the tumor. Dynamic MRI has the advantage of correctly determining the area of viable tumor by showing different enhancement pattern and change in slope values.[6,19]
The prediction of histological response by dynamic MR imaging has proved to be very significant in our study. This is in accordance with the findings of other studies which establish its role in assessing necrosis in malignant bone tumor.[18,19] The cut-off criteria of >60% reduction in the slope of Erlemann et al.[19] could predict good histological response in 80% of our patients, while correctly predicting poor response in 88.89% of patients. Fletcher et al.[18] had used post-chemotherapy slope value of 40% to classify responders. Slope value of less than 40% per minute after full course of neoadjuvant chemotherapy indicated good response to it. But in our study, all the patients showed less than 40% post-chemotherapy slope value, except one poor responder who showed more than 40% slope value.
The prime limitation of our study was its small sample size. Procedural errors in performing contrast MRI were minimized by consistency of the method of contrast injection, using the portal at peripheral site. Others factors like chemotherapy-induced cardiomyopathy and infection that could lead to relative change in uptake of contrast by tumor did not develop in any patient.
Conclusion
The present study proves the efficacy of dynamic MRI as an accurate, quantitative, and noninvasive method of assessing response of malignant bone tumors to preoperative chemotherapy. It has the advantage of accurately localizing the sites of viable residual tumor following therapy. This study also indicates that although tumor volume change provides some information about the responding nature of the tumor, the volumetric assessment should not be trusted as an accurate method of evaluating tumor response to preoperative chemotherapy.
Acknowledgments
Dr. Prakash Muthusamy and Mr. Rajaram are acknowledged for their help.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The memorial hospital experience. Cancer. 1992;69:698–708. doi: 10.1002/1097-0142(19920201)69:3<698::aid-cncr2820690317>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Wunder JS, Paulian G, Huvos AG, Heller G, Meyers PA, Healey JH. The histological response to chemotherapy as a predictor of the oncological outcome of operative treatment of Ewing sarcoma. J Bone Joint Surg Am. 1998;80:1020–33. doi: 10.2106/00004623-199807000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Kawai A, Sugihara S, Kunisada T, Uchida Y, Inoue H. Imaging assessment of the response of bone tumors to preoperative chemotherapy. Clin Orthop Relat Res. 1997;337:216–25. doi: 10.1097/00003086-199704000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Raymond AK, Chawla SP, Carrasco CH, Ayala AG, Fanning CV, Grice B, et al. Osteosarcoma chemotherapy effect: A prognostic factor. Semin Diagn Pathol. 1987;4:212–36. [PubMed] [Google Scholar]
- 5.Rosen G, Caparros B, Huvos AG, Kosloff C, Nirenberg A, Cacavio A, et al. Preoperative chemotherapy for osteogenic sarcoma: Selection of postoperative adjuvant chemotherapy based on the response of the primary tumor to preoperative chemotherapy. Cancer. 1982;49:1221–30. doi: 10.1002/1097-0142(19820315)49:6<1221::aid-cncr2820490625>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: Pathologic aspect in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–8. [PubMed] [Google Scholar]
- 7.Gherlinzoni F, Picci P, Bacci G, Campanacci D. Limb sparing versus amputation in osteosarcoma. Correlation between local control, surgical margins and tumor necrosis: Istituto Rizzoli experience. Ann Oncol. 1992;3(Suppl 2):S23–7. doi: 10.1093/annonc/3.suppl_2.s23. [DOI] [PubMed] [Google Scholar]
- 8.Picci P, Rougraff BT, Bacci G, Neff JR, Sangiorgi L, Cazzola A, et al. Prognostic significance of histopathologic response to chemotherapy in nonmetastatic Ewing's sarcoma of the extremities. J Clin Oncol. 1993;11:1763–9. doi: 10.1200/JCO.1993.11.9.1763. [DOI] [PubMed] [Google Scholar]
- 9.Hendershot E, Pappo A, Malkin D, Sung L. Tumor necrosis in pediatric osteosarcoma: Impact of modern therapies. J Pediatr Oncol Nurs. 2006;23:176–81. doi: 10.1177/1043454206289786. [DOI] [PubMed] [Google Scholar]
- 10.Shin KH, Moon SH, Suh JS, Yang WI. Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res. 2000;376:200–8. doi: 10.1097/00003086-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 11.Smith J, Heelan RT, Huvos AG, Caparros B, Rosen G, Urmacher C, et al. Radiographic changes in primary osteogenic sarcoma following intensive chemotherapy. Radiology. 1982;143:355–60. doi: 10.1148/radiology.143.2.6978499. [DOI] [PubMed] [Google Scholar]
- 12.Carrasco CH, Charnsangavej C, Raymond AK, Richli WR, Wallace S, Chawla SP, et al. Osteosarcoma: Angiographic assessment of response to preoperative chemotherapy. Radiology. 1989;170:839–42. doi: 10.1148/radiology.170.3.2916040. [DOI] [PubMed] [Google Scholar]
- 13.Welling RM, Davies AM, Pynsent PB, Carter SR, Grimer RJ. The value of computed tomographic measurements in osteosarcoma as a predictor of response to adjuvant chemotherapy. Clin Radiol. 1994;49:19–23. doi: 10.1016/s0009-9260(05)82908-3. [DOI] [PubMed] [Google Scholar]
- 14.Holscher HC, Bloem JL, Nooy MA, Taminieu AH, Eulderink F, Hermans J. The value of MR imaging in monitoring the effect of chemotherapy on bone sarcomas. AJR Am J Roentgenol. 1990;154:763–9. doi: 10.2214/ajr.154.4.2107673. [DOI] [PubMed] [Google Scholar]
- 15.Cheon GJ, Kim MS, Lee JA, Lee SY, Cho WH, Song WS, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009;50:1435–40. doi: 10.2967/jnumed.109.063602. [DOI] [PubMed] [Google Scholar]
- 16.de Baere T, Vanel D, Shapeero LG, Charpentier A, Terrier P, di Paola M. Osteosarcoma after chemotherapy: Evaluation with contrast material-enhanced subtraction MR imaging. Radiology. 1992;185:587–92. doi: 10.1148/radiology.185.2.1410378. [DOI] [PubMed] [Google Scholar]
- 17.Dyke JP, Panicek DM, Healey JH, Meyers PA, Huvos AG, Schwartz LH, et al. Osteogenic and Ewing sarcoma: Estimation of necrotic fraction during induction chemotherapy with dynamic contrast-enhanced MR imaging. Radiology. 2003;228:271–8. doi: 10.1148/radiol.2281011651. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher BD, Hanna SL, Fairclough DL, Gronemeyer SA. Pediatric musculoskeletal tumors: Use of dynamic, contrast-enhanced MR imaging to monitor response to chemotherapy. Radiology. 1992;184:243–8. doi: 10.1148/radiology.184.1.1319075. [DOI] [PubMed] [Google Scholar]
- 19.Erlemann R, Sciuk J, Bosse A, Ritter J, Kusnierz-Glaz CR, Peters PE, et al. Response of osteosarcoma and Ewing sarcoma to preoperative chemotherapy: Assessment with dynamic and static MR imaging and skeletal scintigraphy. Radiology. 1990;175:791–6. doi: 10.1148/radiology.175.3.2188300. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida Y, Yakushiji T, Awai K, Katahira K, Nakayama Y, Shimomura O, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: Initial results. Eur Radiol. 2006;16:2637–43. doi: 10.1007/s00330-006-0342-y. [DOI] [PubMed] [Google Scholar]
- 21.Abudu A, Davies AM, Pynsent PB, Mangham DC, Tilman RM, Carter SR, et al. Tumor volume as a predictor of necrosis after chemotherapy in Ewing's sarcoma. J Bone Joint Surg Br. 1999;81:317–22. doi: 10.1302/0301-620x.81b2.8979. [DOI] [PubMed] [Google Scholar]
- 22.Moon SH, Shin KH, Suh JS, Yang WI, Noh JK, Hahn SB, et al. Tumor volume change after chemotherapy as a predictive factor of disease free survival for osteosarcoma. Yonsei Med J. 2005;46:119–24. doi: 10.3349/ymj.2005.46.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toms AP, White LM, Kandel R, Bleakney RR, Noseworthy M, Lee S, et al. Limitation of single slice dynamic contrast enhanced MR in pharmacokinetic modeling of bone sarcomas. Act Radiol. 2009;50:512–20. doi: 10.1080/02841850902922761. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Chin SY, Kim KH, Jeon DG, Cho KJ. Osteosarcoma after preoperative chemotherapy: Tissue characterization with specimen MR and the role of enhanced MR imaging. J Korean Radiol Soc. 1999;40:965–73. [Google Scholar]
- 25.Holscher HC, Bloem JL, Vanel D, Hermans J, Nooy MA, Taminiau AH, et al. Osteosarcoma: Chemotherapy-induced changes at MR imaging. Radiology. 1992;182:839–44. doi: 10.1148/radiology.182.3.1535905. [DOI] [PubMed] [Google Scholar]
- 26.Holscher HC, Bloem JL, van der Woude HJ, Hermans J, Nooy MA, Taminiau AH, et al. Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin Radiol. 1995;50:384–90. doi: 10.1016/s0009-9260(05)83135-6. [DOI] [PubMed] [Google Scholar]
- 27.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;153:106–20. [PubMed] [Google Scholar]
- 28.Lang P, Honda G, Robert T, Vahlensieck M, Jonston JO, Rosenau W, et al. Musculoskeletal neoplasm: Perineoplastic edema versus tumor on dynamic postcontrast MR images with spatial mapping of instantaneous enhancement rates. Radiology. 1995;197:831–9. doi: 10.1148/radiology.197.3.7480764. [DOI] [PubMed] [Google Scholar]
- 29.Guo J, Reddick WE, Glass JO, Ji Q, Billups CA, Wu J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a prognostic factor in predicting event-free and overall survival in pediatric patients with osteosarcoma. Cancer. 2012;118:3776–85. doi: 10.1002/cncr.26701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Woude HJ, Bloem JL, Verstraete KL, Taminiau AH, Nooy MA, Hogendoorn PC. Osteosarcoma and Ewing's sarcoma after neoadjuvant chemotherapy: Value of dynamic MR imaging in detecting viable tumor before surgery. AJR Am J Roentgenol. 1995;165:593–8. doi: 10.2214/ajr.165.3.7645476. [DOI] [PubMed] [Google Scholar]


