Abstract
Purpose:
Paraduodenal pancreatitis (PP) is a unique form of focal chronic pancreatitis that selectively involves the duodenum and aberrant pancreatic tissue located near the minor papilla (beyond the pancreas proper). The pseudotumoral nature of the disease often generates considerable clinical quandary and patient apprehension, and therefore merits a better understanding. The present study appraises the clinicoradiological manifestations of PP in 33 patients.
Materials and Methods:
Clinical, laboratory, and radiological manifestations of 33 patients of PP treated in gastroenterology/hepatology and hepato-pancreatico-biliary surgery units during June 2010-August 2014 were retrospectively reviewed.
Results:
All patients were young to middle-aged men (100%) with history of alcohol abuse (93.9%) and/or smoking (42.4%), who presented either with acute or gradually worsening abdominal pain (90.9%). Pancreatic enzymes and serum tumor markers remained normal or were mildly/transiently elevated. Cystic variant was detected in 57.6% (solid in 42.4%); the disease remained confined to the groove/duodenum (pure form) in 45.4%. Medial duodenal wall thickening with increased enhancement was seen in 87.87 and 81.81%, respectively, and duodenal/paraduodenal cysts were seen in 78.78%. Pancreatic calcifications and biliary stricture were seen 27.3% patients. Peripancreatic arteries were neither infiltrated nor encased.
Conclusion:
PP has a discrete predilection for middle-aged men with history of longstanding alcohol abuse and/or smoking. Distinguishing imaging findings include thickening of the pancreatic side of duodenum exhibiting increased enhancement with intramural/paraduodenal cysts. This may be accompanied by plate-like scar tissue in the groove region, which may simulate groove pancreatic carcinoma. However, as opposed to carcinoma, the peripancreatic arteries are neither infiltrated nor encased, rather are medially displaced.
Keywords: Chronic pancreatitis, computed tomography, magnetic resonance imaging, pancreatitis
Introduction
In 2004, researchers Adsay and Zamboni proposed paraduodenal pancreatitis (PP) as a superordinate term for a distinct variant of alcohol-induced focal chronic pancreatitis which had been formerly described under various titles including cystic dystrophy of heterotopic pancreas, paraduodenal wall cyst, pancreatic hamartoma of duodenum, myoadenomatosis, and groove pancreatitis.[1,2] The authors observed that all the aforementioned disorders shared common clinical and pathological features, i.e. they affected young men with a history of chronic alcohol abuse and/or smoking who presented with clinical symptoms similar to chronic pancreatitis. When reviewed from a pathological viewpoint, they observed analogous histopathologic manifestations of chronic inflammatory, cystic, and fibrotic changes involving the duodenum, adjacent pancreatico-duodenal groove, and the juxtaduodenal pancreas [Figure 1].[1,2,3,4,5,6,7,8]
Figure 1.
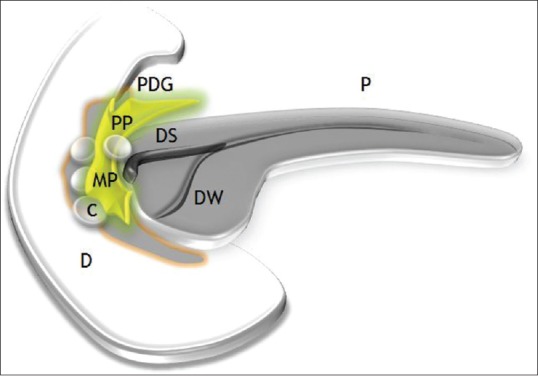
Pictorial illustration of paraduodenal pancreatitis depicting a fibroinflammatory mass (PP) in the pancreatico-duodenal groove (PDG) with concurrent duodenal and paraduodenal cysts (C) in the region of minor papilla (MP). P: Pancreas; DS: Duct of Santorini; DW: Duct of Wirsung
Pathogenetically, disturbance of the flow of pancreatic juice through the accessory duct of Santorini and the presence of heterotopic pancreatic tissue within the duodenal wall, on the background of chronic alcohol abuse, are allegedly responsible for its development [Figure 2].[1,2,3,4,5,6,7,8,9,10] The histopathologic hallmark of PP is the presence of chronic inflammatory, cystic, and fibrotic changes involving the descending duodenum, especially in the region of minor papilla, adjacent pancreatico-duodenal groove, and/or the juxtaduodenal pancreas [Figure 3].[1,2,3,4,5,6,7,8] Macroscopically, the most characteristic finding is duodenal wall thickening with pseudotumorous polypoid giant folds and dense scarring causing varying degrees of luminal compromise.[1,2,3,4,5,6,7,8,9,10] Duodenal involvement is predominantly on the pancreatic side and is most pronounced in the region of the minor papilla (supra-ampullary region).[1,2,3,4,5] Intramural cystic lesions are almost always there and can be either located submucosally or within the muscularis layer, ranging in size from less than 1 cm up to 10 cm.[1,2,3,4,5,8] If large and multiple, the cysts may barge into the adjacent groove and compress the distal bile duct. The adjoining pancreatico-duodenal groove frequently displays fibroinflammatory changes and dense firm whitish scar tissue often simulating a neoplasm.[1,2,3,4,5,6,7,8,9,10] Microscopically, the duodenal wall typically shows dense Brunner's gland hyperplasia and myoid stromal proliferation.[1,2,3,4,5,8,9,10] Frequent accompaniment is the presence of heterotopic pancreatic tissue with cystically dilated ducts often containing inspissated secretions.[1,2,3,4,5,8] The pancreatic parenchyma of the head which is normal during the early stages shows mild-to-moderate fibrosis in due course.[1,2,3,4,5] Pancreatic involvement in the form of duct ectasia with intraductal calculi, chronic parenchymal inflammation, and myofibroblastic proliferation can be encountered as the disease advances.[3,4]
Figure 2.
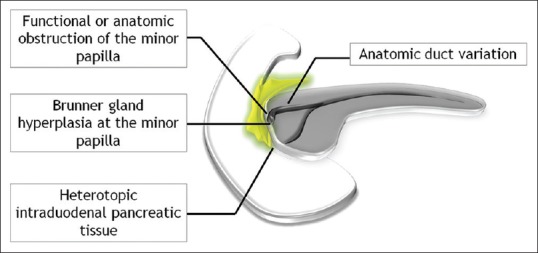
Etiopathogenesis of paraduodenal pancreatitis
Figure 3.
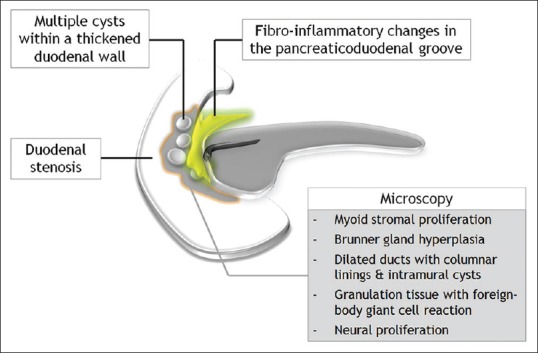
Schematic illustration of the pathological manifestations of paraduodenal pancreatitis
Characteristic imaging findings include focal wall thickening of the pancreatic side of duodenum with varying degrees of luminal compromise and intramural and/or paraduodenal cysts.[7,9,10] Often, this is accompanied by plate-like scar tissue in the groove region, which may cause smooth distal bile duct narrowing and may encroach into the adjacent pancreatic parenchyma. However, the imaging diagnosis may not always be straightforward and, therefore, a thorough knowledge of the imaging appearances of PP across an array of imaging modalities is necessary for the radiologist to make an apposite diagnosis. Most important is the differentiation from groove pancreatic carcinoma, keeping in view optimal therapeutic planning.[7] The present study appraises the clinicoradiological manifestations of PP in 33 patients treated at our institution.
Materials and Methods
This is a retrospective analysis of 33 patients of PP treated in gastroenterology/hepatology and hepato-pancreatico-biliary surgery units at our institute, during June 2010-August 2014. In lieu of the retrospective nature of the study, institutional review board approval was not required as per our institution's policy. A detailed clinical, laboratory, and radiological workup led to the diagnosis of PP. The clinical details were collated along with available laboratory parameters including serum amylase, lipase, liver function tests (LFT), and tumor markers such as carbohydrate antigen (CA 19-9) and carcinoembryonic antigen (CEA). Histopathologic confirmation by fine needle aspiration (FNA) or post-surgical biopsy for exclusion of neoplasia, in relevant cases, was also reviewed. All patients underwent either a contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) evaluation. In addition, results of transabdominal ultrasonography (USG) and endoscopic ultrasound (EUS) examination, wherever available, were reviewed.
All CT studies were performed on a Discovery 750HD 64-row spectral CT scanner (General Electric, USA). The helical scan parameters included: 120 kV with automated mA, 0.6 s rotation time, speed 55 mm/rotation, pitch of 1.375:1, detector coverage 40 mm, and matrix size of 512 × 512. A low osmolarity non-ionic contrast medium (Iomeron; 1.5-2.0 ml/kg body weight, 400 mg/ml) was administered intravenously at a rate of 3.0-3.5 ml/s (approximately 70-100 ml) and scans obtained in the arterial phase, pancreatic phase, and portal phase with a delay of 30 s, 50 s, and 70 s, respectively, with a slice of 2.5 mm thickness. MR imaging was performed on a 3 T Signa HDXT MR scanner (General Electric, USA) using a phased-array TORSOPA coil to enhance signal reception. Unenhanced axial sequences were acquired with a slice thickness of 5 mm at 1 mm intervals, including T1W and T2W single-shot fast spin echo (SSFSE) sequences with and without fat suppression, followed by a fat-saturated dynamic T1W gadolinium-enhanced study (with breath hold). Two-dimensional fast imaging employing steady-state acquisition (2D FIESTA) sequences were also obtained in axial and coronal planes with a slice thickness of 3 mm and at 0.5 mm intervals. Three-dimensional magnetic resonance cholangiopancreatography (3D MRCP) sequences were obtained (in axial and coronal planes) by respiration-triggered heavily weighted T2 sequence FRFSE-XL) with contiguous thin sections (1.4 mm/0.7 overlap). In addition, T2 SSFSE sequences were also obtained (with breath hold) in thick slabs of 40 mm in coronal oblique planes at 20° increments, keeping the common bile duct as the center of rotation. Imaging parameters for SSFSE sequences included: Repetition time (TR) 2100 ms, time to echo (TE) 80.1 ms, slab thickness 0.5 mm, field of view (FOV) 38 cm, and matrix 288 × 192. Imaging parameters for FIESTA sequences were: TR 4.7 ms, TE 2.1 ms, slab thickness 3 mm, FOV 35 cm, flip angle 70°, and matrix 224 × 352. USG was performed by four radiologists (R.S.), (Y.P.), (A.A.) and (S.T.) with more than 6, 9, 10 and 12 years experience on Toshiba Xario™ ultrasound system (Toshiba Medical Systems Corporation, Japan) with a 3.5 MHz convex probe or on iU22 (Philips Medical Systems, Bothell, WA, USA) using a convex array transducer (3.5 MHz).
The imaging manifestations were broadly categorized into four: duodenal involvement, involvement of the pancreatico-duodenal groove, involvement of pancreas proper, and biliary manifestations. Descending duodenum was evaluated for the presence of wall thickening, mural hyperenhancement, intra/paraduodenal cystic lesions, and/or luminal compromise. Based upon the presence of predominantly solid scar tissue or cystic lesions in the pancreatico-duodenal groove, the disease was categorized as either solid or cystic variant. When solid scar tissue was present, its enhancement characteristics were evaluated along with the presence of displacement/encasement of peripancreatic vessels (to differentiate it from groove pancreatic carcinoma). Pancreatic involvement was evaluated for concurrent involvement of the head in the form of a hypoenhancing focal area. Also, intrapancreatic cystic lesions in the head were documented. The pancreas was also evaluated for the presence of main duct dilatation, calcifications (localized to the head/diffuse involvement), or pancreatic parenchymal atrophy. Based upon whether the changes remained confined only to the duodenum and/or groove region or, in addition, also involved the head of the pancreas, the disease was classified as “pure” or “segmental” forms, respectively. Also, the biliary tree was evaluated for the presence of biliary stricture and/or an elongated ectatic gallbladder (popularly termed as banana gallbladder). All USG, CT, and MR examinations were read by experienced radiologists (A.A.), (A.M.) and (S.T.) with more than 10, 11 and 12 years experience, respectively; and EUS findings by an experienced gastroenterologist (V.B.) with more than 15 years experience, and the necessary information was tabulated as per the aforementioned checklist.
Results
We had 33 patients of PP with a mean age 46.27 years (range 28-66 years), whose demographic and clinical details are given in Table 1. We had no female patient and the entire study group consisted of men, of which 31 (93.93%) gave a history of long-term alcohol abuse (>8-10 years) while 14 (42.42%) also had a history of cigarette smoking. Thirty of the 33 (90.9%) patients presented with gradually worsening or acute episodes of abdominal pain and 16 (48.8%) had concurrent complaints of nausea and/or vomiting. Five of the 33 (15.15%) patients gave a history of weight loss, while only 2 (6%) presented with features of gastric outlet obstruction. Ten of the 33 (30.3%) patients also complained of darkening of urine and/or yellowing of eyes compatible with jaundice; 3 of these 10 patients also had underlying alcohol-related liver cirrhosis and elevated LFT could partly be explained by this.
Table 1.
Demographic data, clinical history, and laboratory parameters

The laboratory parameters including serum amylase, lipase, LFT, and tumor markers (CA 19-9, CEA) of the study population are presented in Table 1. In almost all patients, the pancreatic enzymes (serum amylase and lipase) were either within the normal range or marginally elevated. In 28 of the 33 (84.84%) patients, tumor markers were within the normal range. CA19-9 was marginally elevated in 2 (6.06%) patients and significantly elevated in 3 (9.09%) patients, albeit the CEA levels remained normal in all these patients.
All 33 patients underwent either a contrast-enhanced CT or MRI of the abdomen. In 24 patients, the study was conducted according to a dedicated pancreatic protocol. Twenty-two patients also had a transabdominal USG, although the pancreas could not be evaluated in 10 due to overlying bowel shadows. Sixteen patients also had a EUS examination in addition to CT or MR imaging. The spectrum of imaging manifestations (as per the checklist) is enlisted in Table 2.
Table 2.
Imaging characteristics
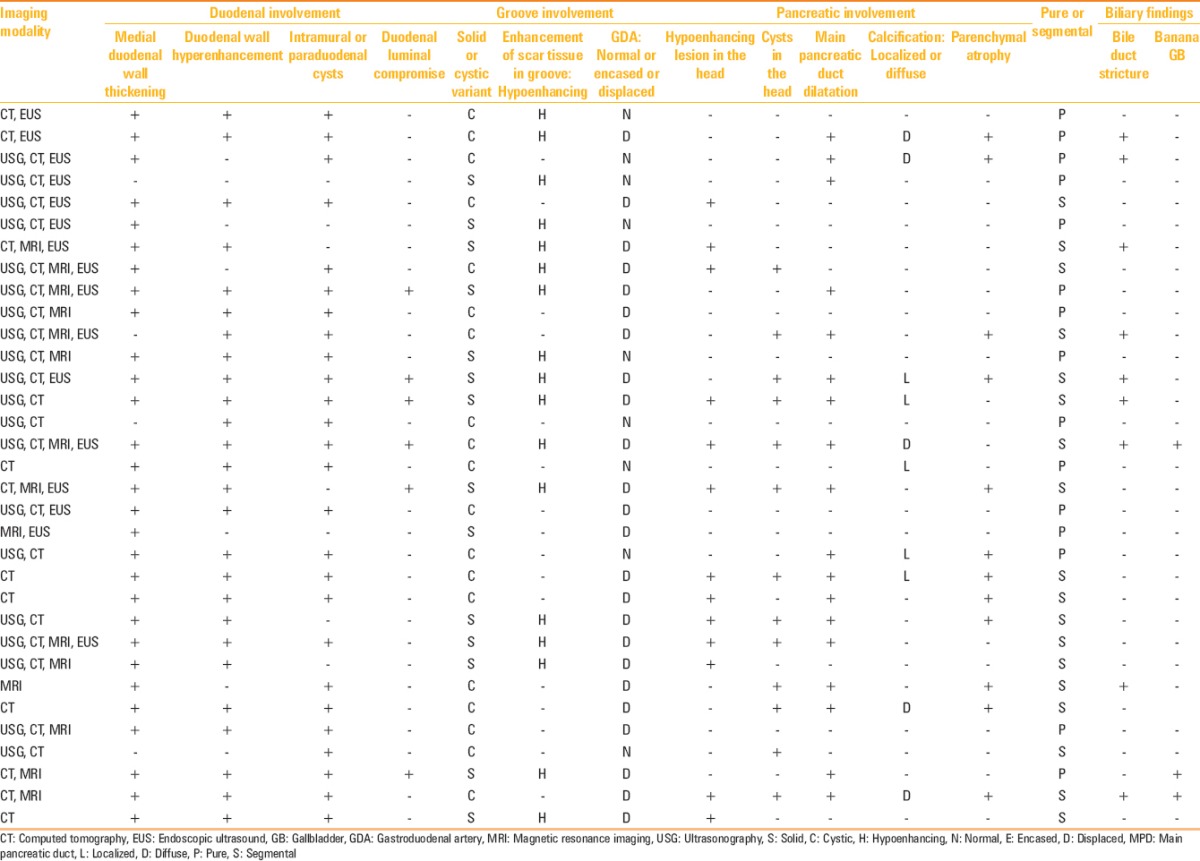
Medial duodenal wall thickening with increased enhancement was the commonest imaging manifestation seen in 87.87% and 81.81% patients, respectively. Twenty-six (78.78%) patients displayed duodenal or paraduodenal cysts [Figure 4]. The disease was predominantly cystic in 19 (57.6%) patients, while 14 exhibited a predominantly solid form (42.4%) [Figure 5]. Gastroduodenal artery remained normal in course and caliber in 9 (27.3%) patients, while it was medially displaced in the remaining 24 (72.7%) patients. In none of the patients, the peripancreatic vessel was either encased or attenuated. Solid scar tissue when present (17 patients) was hypoenhancing relative to the adjoining pancreas. Only 2 of the 17 (11.76%) patients with a hypoenhancing solid scar tissue showed delayed enhancement of the scar. The so-called pure form involving only the duodenum and/or the groove region was observed in 15 (45.45%) patients, while 18 (54.54%) patients exhibited segmental form characterized by concurrent involvement of the pancreatic head [Figure 6]. Pancreas proper displayed atrophic changes in 12 (36.3%) patients, while 10 (30.3%) patients showed pancreatic calcifications, confined to the head in 5 patients and was diffuse in the remaining 5 patients [Figure 7]. As many as 18 (54.54%) patients showed main pancreatic duct dilatation with smooth caliber transition if at all in the head region. The distal bile duct showed smooth stenosis at its distal end in 9 (27.3%) patients. None had an abrupt bile duct cut-off to suggest malignancy. An elongated and distended (banana-shaped) gallbladder was encountered in 3 (9.09%) patients [Figure 8].
Figure 4.
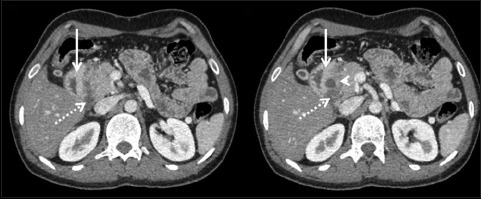
Axial contrast-enhanced CT displaying medial duodenal wall thickening with mural hyperenhancement (arrow). In addition, paraduodenal cyst is noted (arrowhead) along with a hypoenhancing soft tissue (dotted arrow) sandwiched between the pancreatic head and the descending duodenum
Figure 5(A-D).
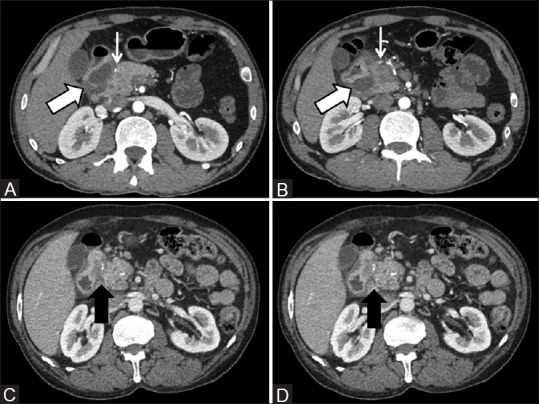
(A and B) Cystic variant of paraduodenal pancreatitis displaying extensive cystic formations in the groove (thick arrow) causing medial displacement of the gastroduodenal artery which otherwise is normal in caliber (thin arrow) (C and D) Solid variant of paraduodenal pancreatitis wherein a hypoattenuating sheet-like fibrous tissue is seen within the pancreatico-duodenal groove (arrow)
Figure 6(A and B).
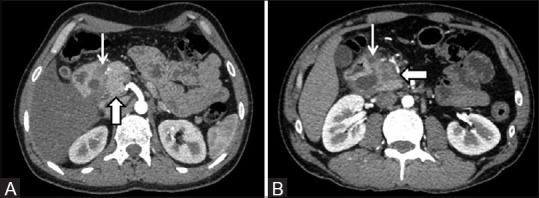
(A) “Pure” form of disease characterized by a normal-appearing pancreatic head (thick arrow) and the inflammatory changes remaining confined to the groove region (thin arrow) (B) “Segmental” form of disease wherein the head of the pancreas is concurrently involved and shows a hypoenhancing lesion (thick arrow). Predominantly cystic form of the disease can be seen within the groove (thin arrow)
Figure 7(A and B).
(A) A 50-year-old patient with paraduodenal cystic lesions (arrowheads) displaying calcifications localized to the pancreatic head (arrow). Also, note the changes of alcohol-related liver disease (dotted arrows) and ascites (asterisk) (B) A 52-year-old patient with paraduodenal pancreatitis and a large paraduodenal cyst (arrowhead) showing an atrophic pancreas with diffuse calcifications (arrows)
Figure 8.

Thick slab 2D MRCP in a 33-year-old patient of paraduodenal pancreatitis showing excessive widening of the space between the descending duodenum (D) and the bile duct (thin arrow). An ectatic and elongated banana-shaped gallbladder can also be seen (thick arrow)
Only, one of our patients required a pancreaticoduodenectomy to relieve his symptoms of unrelenting pain and weight loss owing to progressive gastric outlet obstruction. Rest of the patients were managed conservatively with or without endotherapy. All were followed up either in gastroenterology or hepatology and hepato-pancreatico-biliary surgery units. The follow-up duration ranged from 1 to 42 months (mean 10 months). Those patients who presented with elevated tumor marker levels were subjected to EUS- guided FNA to exclude malignancy. In spite of the imaging manifestations being not suspicious of carcinoma, all these patients were subjected to EUS-guided FNA to exclude malignancy. None of these patients displayed evidence of malignancy on FNA (four patients) or post-surgical histopathologic evaluation (one patient). Follow-up evaluation demonstrated declining trend of serum tumor marker levels following the acute episode.
Discussion
In 2004, Adsay and Zamboni suggested that all the entities including pancreatic duodenal hamartoma, paraduodenal wall cyst, cystic dystrophy of the pancreas, myoadenomatosis, and groove pancreatitis represented the same lesional spectrum or different stages of the same disease, which primarily affected the duodenum in the region of minor papilla.[2] Historically, the term “groove pancreatitis” first appeared in 1982 in the reports of Stolte et al., although the condition was first described in 1973 in the German literature by Becker and Bauchspeichel as “segmentare pancreatitis”.[11,12] They observed an intriguing variant of segmental chronic pancreatitis wherein a sheet-like fibroinflammatory mass remained principally localized within the pancreatico-duodenal groove. Subsequently, in 1991, Becker and Mischke observed two subtypes wherein the disease either remained confined to the groove region (pure form) or, in addition, contiguously permeated the dorsocranial pancreas (segmental form).[13] But even before the description of groove pancreatitis, a similar description of the disease can be obtained in the French literature in which Potet and Duclert described the entity as cystic dystrophy of the pancreas in the year 1970.[14] They observed duodenal wall thickening with intraparietal cystic lesions (lined by pancreatic duct-like epithelium), which were believed to represent cystic formations within the heterotopic intraduodenal pancreatic tissue. Dense accompanying inflammatory granulation tissue was observed in the duodenal wall, while the histological examination of the pancreas proper was normal.[14] Later, in the 1996 World Health Organization classification of tumors and pseudotumoral lesions of the pancreas, the entity was referred to as para-ampullary duodenal wall cyst.[15] Besides, the entity has also been referred to as pancreatic hamartoma of the duodenum which symbolizes its pseudotumoral nature owing to a complex amalgamation of intraduodenal pancreatic tissue with distorted ducts and acini, Brunner gland, and smooth muscle hyperplasia on a background of dense fibrosis.[1,2,3,4,5] Dense proliferation and trabeculation of duodenal musculature seen in conjunction with intramural macrocystic or microcystic changes has also earned it the name of myoadenomatosis.[1,2,3,4,5,8] Based upon their shared pathological and clinical manifestations, all the aforementioned entities/terminologies are now collectively termed as “paraduodenal pancreatitis”.[1,2,3,4,5,6,7,8]
PP usually occurs in men in their 40s or 50s and is associated with a history of chronic alcohol abuse and/or smoking.[1,2,3,4,5,6,7,8,9,10] Chronic alcohol abuse is the key factor in the development of PP and the disease typically affects young to middle-aged male patients.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15] According to one of the propositions, chronic alcohol intake increases the viscosity of the pancreatic juice, ensuing the formation of intraductal protein plugs which obstruct the flow of pancreatic secretions, thereby elevating the pancreatic duct pressures.[16,17] That the process is primarily located in the region of Santorini duct suggests the possibility of anatomical or functional obstruction of the minor papilla, which has been attributed to alcohol-induced periampullary Brunner gland hyperplasia causing occlusion or dysfunction of the minor papilla [Figure 2].[1,2,3,4,5,18] Another likely explanation is the presence of heterotopic pancreatic elements within the duodenal wall which get trapped during embryogenesis.[1,2,3,4,8,9,10] Alcohol is probably also injurious to the acinar cells in the heterotopic pancreatic elements, which causes their fatty degeneration, inflammation, and necrosis. Also, the presence of heterotopic pancreatic tissue may contribute to outflow obstruction of an anatomically aberrant minor papilla.[6] The fibroinflammatory process primarily being centered in the region of the minor papilla also raises the possibility of an underlying anatomic variation in the ductal anatomy, such as congenitally absent or narrow Santorini duct or the presence of pancreas divisum, making it particularly susceptible to the effects of alcohol-related injury.[19,20] Besides, smoking is also thought to be an important risk factor for PP, with the combination of alcohol abuse and smoking aggravating and hastening the evolution of the disease.[5,19,20] In addition to causing outflow resistance at the minor papilla, alcohol and smoking are also believed to cause ischemic injury of the juxtapapillary duodenum and the adjacent groove.[5,19,20]
All 33 patients in our study were men (100%) in the age range of 28-66 years (mean age 46.27 years). Thirty-one of the 33 (93.93%) patients had a history of long-term alcohol abuse, while 14 (42.42%) patients also gave a history of smoking for more than 10 years. Initial clinical symptoms include recurrent episodes of acute or gradually worsening abdominal pain, which may be localized to the upper abdomen or may radiate to the back.[1,5,9,10] This may be accompanied with nausea and vomiting which worsens following food consumption. Although most patients usually present within 3-6 months of experiencing the symptoms, the duration is highly variable ranging from a few weeks to more than a year.[17] Often, as the disease progresses, the pain episodes become more frequent and severe, and the patients start manifesting symptoms of gastric outlet obstruction owing to progressive inflammatory narrowing and/or scarring of the duodenum.[5,9,10,21] This may lead to progressive loss of body weight with attendant tiredness and fatigue, thus raising concerns for a potential malignancy.[5,9,10] However, as opposed to pancreatic or gastroabdominal malignancies, serum tumor markers such as CA 19-9 and CEA usually remain within the normal range or may show marginal or transient elevation.[9,22] The pancreatic enzymes (serum amylase and lipase) are mildly elevated as opposed to acute pancreatitis. Only rarely does the patient manifest clinically with painless obstructive jaundice secondary to distal bile duct involvement.[1,2,3,4,5,9,10,21] Moreover, unlike chronic pancreatitis due to other causes, PP is usually not associated with symptoms of pancreatic insufficiency, such as diabetes and steatorrhea, as the major portion of the gland is by and large spared, though some patients may manifest pancreatic insufficiency secondary to alcohol-induced chronic calcific pancreatitis.[3,4] In our series, 7 of the 33 (21.2%) patients were found to have elevated serum glucose levels presumably due to their relatively late presentation during the course of the disease by which time chronic alcohol-induced pancreatic injury and insufficiency had already set in.
USG is often the first diagnostic tool used in patients with suspected pancreatitis. Although widely accessible and readily available, the sonographic appearances of PP have sparingly been reported.[17,23,24] USG findings vary as the disease progresses from the initial inflammatory stage to an advanced fibro-cicatricial phase, and often the differentiation from malignant processes is tricky based upon USG findings alone.[17,22,23,24] During the initial stages, mild-to-moderate inflammatory thickening of the second portion of the duodenum can be appreciated displaying prominent hyperechoic duodenal folds amid the presence of intramural or paraduodenal cystic lesions [Figure 9]. Unlike periampullary tumors which display focal hypoechoic mural thickening, the duodenal wall thickening in PP is more general, but nevertheless remains more pronounced along the medial wall. In addition, a band-like hypoechoic area may be seen widening the groove with or without associated heterogeneity of the adjacent pancreatic head.[17] Duodenal peristaltic activity is often preserved and appreciable till late. Progressive scarring of the duodenum and dorsocranial pancreatic head renders the groove area heterogeneously hyperechoic intermixed with anechoic ductal structures. Irregular and heteroechoic texture of the pancreatic head and attendant narrowing of the distal bile duct and the proximal main pancreatic duct can easily simulate a neoplasm.[17]
Figure 9(A and B).

Transabdominal ultrasonography (A) depicting thickened (arrows) descending duodenal wall (D2) accompanying a relatively bulky heteroechoic (arrowheads) head of pancreas (HOP) in an alcoholic patient with paraduodenal pancreatitis (B) A different patient of paraduodenal pancreatitis with duodenal thickening (D2) and accompanying cystic formation (arrowhead)
Characteristic imaging findings on CT include reactive parietal thickening along the medial aspect of the descending duodenum. The thickened duodenal wall often shows prominent enhancement and, depending upon the degree of duodenal thickening/scarring and consequential luminal compromise, there may be attendant findings of upstream gastric dilatation.[7,9,10,23,24,25] Frequently, duodenal thickening is associated with intramural or paraduodenal cystic lesions, the presence of which is a useful indicator toward the diagnosis [Figure 6]. These cysts greatly vary in number and size, ranging from subcentimetric up to 8-10 cm, and are best discernible following contrast administration. Often, this is accompanied by a poorly enhancing mass within the C-loop of the duodenum and the head of the pancreas (pancreatico-duodenal groove).[1,5,9,10,25,26,27,28,29] The soft tissue within the groove may be difficult to discern on the unenhanced scan; however, it stands out as a hypoattenuating tissue following intravenous contrast administration. On delayed imaging, it may show mild enhancement reflecting its fibrous nature.[25] Based upon the predominance of the solid scar tissue or cystic lesions, two variants of PP have been described: (1) The “cystic” type showing multiple paraduodenal and/or duodenal wall cysts which may protrude into the duodenal lumen and (2) the “solid” type characterized by a marked medial duodenal wall thickening with a sheet-like solid mass in the groove [Figure 7].[5] Pretis et al., in their series of 112 patients, reported cystic variant in 79 (70%) patients and solid type in 33 (30%) patients,[30] whereas of the 33 patients in the present study, the disease was predominantly cystic in 19 (57.6%) and the remaining 14 patients exhibited a predominantly solid variant (42.4%).
The sheet-like fibrotic scarring can remain confined to the groove region (pure form) or may extend to involve the adjoining portion of the head of the pancreas (segmental), thus simulating a scirrhous groove (pancreatic) carcinoma.[24,28] The pure form of the disease is relatively uncommon; in a surgical series of 600 patients, the pure form was detected in only 2% cases, although the changes in the pancreatic head may not always identifiable on imaging alone.[9,31]
The importance of differentiating segmental variant from groove pancreatic carcinoma remains challenging. However, unlike in carcinoma, the peripancreatic vessels in PP are neither constricted, attenuated nor encased.[32] Graziani et al., suggested that identification of the gastroduodenal artery is a useful diagnostic sign that can help differentiate groove carcinoma from PP. A normal-sized artery, if displaced leftward, favors paraduodenal (groove) pancreatitis, while an encased or constricted artery seen encased within the soft tissue favors a groove carcinoma.[32] Pure form involving only the duodenum and/or the groove region was observed in 15 (45.45%) patients and segmental form (with concurrent pancreatic head involvement) was seen in 18 of our patients (54.54%). In none of our cases, the vessels were either encased or attenuated. They remained normal in course and caliber in 9 (27.3%) patients, while they were medially displaced in the remaining 24 (72.7%) patients, but were encased or attenuated in none.
The pancreas proper typically remains normal in bulk and displays preserved parenchymal enhancement, especially during the early stages of the disease. However, in advanced disease, patients may show changes of chronic calcific pancreatitis and/or duct dilatation. Pretis et al., in their study population of 112 patients reported “diffuse” form of the disease, i.e. chronic pancreatitis changes involving the entire gland, in 90 (80%) patients. Pancreatic calcifications were observed in 68 (61%) patients.[30] More recently, Zaheer et al., reported pancreatic duct dilatation in 8 of their 12 patients (66.66%), calcifications localized to the head in 4 (33%), diffuse calcification in 1 (8.33%), and pancreatic glandular atrophy in 3 (25%) patients.[31] Twelve of our 33 (36.3%) patients showed pancreatic parenchymal atrophy, while 10 (30.3%) patients showed pancreatic calcifications and as many as 18 (54.54%) patients showed main pancreatic duct dilatation.
Characteristic imaging manifestations on MRI include the presence of a sheet-like fibrous scar within the pancreato-duodenal groove that appears hypointense relative to the normally hyperintense pancreatic parenchyma on T1W.[9,10,21,23,28] On T2W, it shows variable signal ranging from hypointense, isointense, or slightly hyperintense relative to the pancreas [Figure 10]. The T2W signal intensity changes are considered demonstrative of the pathologic characteristics of the disease – the bright signal signifies active edema and inflammation, while hypointense signal represents fibrosis.[1,21,23,28] Following intravenous contrast administration, the scar tissue exhibits a delayed and inhomogeneous progressive enhancement reflecting its fibrous nature. Ishigami et al., reported that patchy focal enhancement in the portal venous phase is a helpful imaging manifestation favoring paraduodenal (groove) pancreatitis over groove carcinoma, which was seen in 14 of 15 (93%) patients studied by them. Patchy focal enhancement putatively reflects the enhancement of pancreatic tissue within the inflammatory mass.[24] The dorsocranial portion of the head of the pancreas may display hypointense signal (on T1W MRI) reflecting varying degrees of focal parenchymal fibrosis. However, this can progress to involve the entire gland reflecting disease chronicity and ensuing loss of glandular cells, protein, and lipid contents of the gland.[28,29]
Figure 10(A and B).
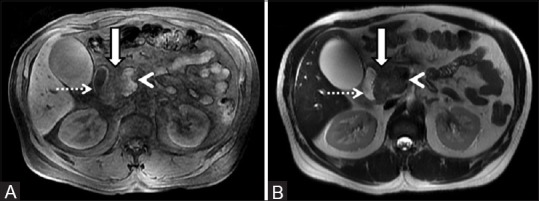
Unenhanced MRI of abdomen in a 48-year-old chronic alcoholic male showing a mass (arrow) in the groove region sandwiched between the pancreatic head (arrowhead) and descending duodenum (dotted arrow). The mass depicts hypointense signal on T1W (A) and isointense signal on the corresponding T2-weighted image (B)
Most cases also manifest features of duodenal inflammation as evidenced by medial duodenal wall thickening and/or cystic formations within the duodenal wall. These cysts are best visualized on T2W imaging and corresponding MRCP [Figure 11].[27,28,29] Also, a characteristic tubulocystic pattern corresponding to the path of the duct of Santorini/minor papilla (groove region) may be seen.[6,7]
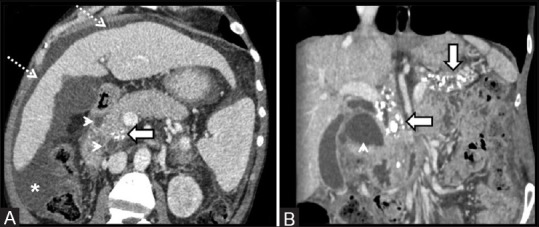
Figure 11(A and B).
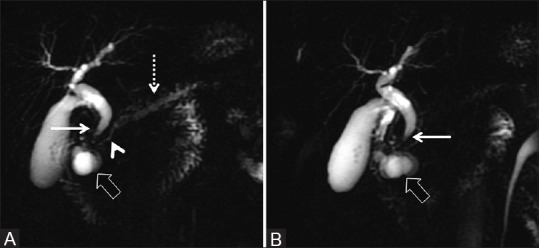
(A and B) Thick slab 2D MRCP depicting characteristic features of paraduodenal pancreatitis as evidenced by paraduodenal cystic formations (thick arrow) with associated widening of the space between the descending duodenum, bile duct, and the pancreatic duct. There is associated smooth tapering of the distal bile duct (thin arrow). The pancreatic duct in the head region shows smooth decrease in diameter (arrowhead) with modest dilatation of the upstream duct (dotted arrow)
On MRCP, the main pancreatic duct usually is normal in appearance; however, it may smoothly decrease in its diameter near the ampulla. In addition, at times, the main duct appears ectatic and irregular with changes of chronic pancreatitis. Of our 33 patients, 18 (54.5%) manifested pancreatic duct dilatation at presentation with or without side duct ectasias. The distal bile duct frequently shows mild smooth concentric long segmental stenosis as opposed to irregular narrowing or shouldering caused by pancreatic carcinoma. Also, 9 (27.3%) patients in our study demonstrated smooth distal bile duct narrowing, while none had an abrupt bile duct cut-off to suggest malignancy. Another finding commonly seen is the widening of the space between distal pancreatic and common bile ducts and duodenal lumen on MRCP.[28] Blasbalg et al., reported this to be a sensitive finding seen in almost all patients in their study group. Another intriguing finding is the elongated and distended gallbladder (banana-shaped gallbladder) which has been attributed to long-standing biliary dilatation.[28] We encountered this in only three of our patients (9%).
Differentiation of PP from pancreatic carcinoma is particularly difficult in cases where there is pseudotumoral inflammatory enlargement of the head of the pancreas. An important feature is the mild smooth and progressive narrowing of the main pancreatic duct in the head of the pancreas. The pancreatic duct narrowing is relatively longer and smoother (penetrating duct sign) and the upstream duct dilation is only modest when compared to that seen in cases of pancreatic carcinoma. More recently, Kalb et al., studied the performance of MRI in distinguishing PP from groove carcinoma. They concluded that when three strict diagnostic criteria were used to define PP (including duodenal wall thickening, abnormal increased enhancement of the descending duodenum, and cystic change in the region of the Santorini duct), a high diagnostic accuracy of 87.2% (41 of 47 patients) could be achieved and carcinoma could be rightly excluded with a negative predictive value of 92.9% (26 of 28 cases).[7]
Owing to its ability to differentiate the histologic layers of the gastrointestinal (GI) tract walls, EUS allows excellent and much more detailed evaluation of the duodenum, the paraduodenal structures including the groove region, and the adjoining pancreas. EUS examination delineates with high precision the inflammatory changes of PP seen in the form of parietal thickening of the second portion of the duodenum with prominent intramural and paraduodenal cystic changes [Figure 12]. The groove region frequently shows hypoechoic scar tissue with a relatively bulky heteroechoic head of the pancreas. Smooth biliary narrowing can be demonstrated with greater clarity on EUS examination. Some patients may also exhibit pancreatic inflammation, calcifications, pseudocysts, and duct dilatation.[9,28,29] At times, the hypoechoic mass imperceptibly merges with the head of the pancreas, simulating a malignancy. But the smooth progressive narrowing of the main pancreatic duct as it courses through the head of the pancreas can help differentiation from a pancreatic malignancy which causes abrupt irregular duct narrowing with pronounced dilatation of the upstream duct. EUS not only provides supplementary information to that acquired with abdominal CT and MRI, but EUS-guided biopsy and histopathologic examination of the soft tissue within the groove can also help exclude malignancy in confounding cases.[33] However, needle tissue samples are not always easy to interpret and the relatively small sample tissue may not be able to confidently eliminate the possibility of carcinoma.[33,34] Nevertheless, the presence of spindled stromal cells, foamy cells, disproportionate Brunner glands, and granular debris in appropriate clinical settings can be an important clue to the diagnosis.[8,33,34] The drawbacks of EUS examination are its limited availability, operator dependence, and deterrence of examination in patients with duodenal stenosis.[9,22]
Figure 12(A and B).
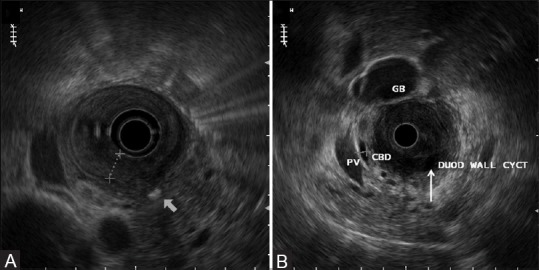
EUS showing medial duodenal wall thickening (A) with accompanying intraparietal cysts (thin arrow) within the duodenum (B) Also, note focal pancreatic calcification (thick arrow) (A)
Therapeutic options for treating PP can be broadly categorized into conservative therapy and surgical intervention.[1,2,3,4,5,9,10,35,36,37,38,39,40] Conservative therapy is primarily useful during the early stages or acute phase of the disease and includes abstinence from alcohol and smoking, use of analgesics, proton pump inhibitors and a pancreatic enzyme supplement, nutritional support, and/or endoscopic cyst drainage or stenting.[36,37,38] Isayama et al., demonstrated minor papillotomy (followed by stenting and drainage of the Santorini duct) as an innovative and feasible minimally invasive endoscopic therapy for managing symptomatic patients; however, the procedure's long-term success rates are uncertain.[38] In one of the recently published series, using endotherapy as the first-line intervention in PP,[36] Arvanitakis et al., reported complete clinical success in 70.7% of patients, with an overall survival rate of 94.1% after a median follow-up of 54 months. Out of 51 patients, a total of 39 patients underwent initial endoscopic treatment: Cystenterostomy (n = 20), pancreatic and/or biliary duct drainage (n = 19), and/or duodenal dilation (n = 6), while only 9 patients required surgery (25%). In accordance with this study, our patients also could be managed with a combination of conservative management as well as endotherapy in the form of pancreatic or biliary stenting, sphincterotomy, and/or duodenal dilatation.
Surgical intervention is generally reserved for those with unrelenting obstructive GI symptoms or confounding cases where exclusion of malignancy is difficult.[39] Preferred surgical intervention includes a radical pancreato-duodenectomy (using the Whipple procedure) which not only relieves the patient from GI obstruction but also from chronic abdominal pain. Complete remission of abdominal pain following pancreato-duodenectomy was reported in 76% of subjects by Casetti et al.[5] On the other hand, all the patients reported pain relief and increase in body weight in the surgical series of Rahman et al.[40]
To conclude, PP is a unique variant of chronic pancreatitis seen in men in their fourth or fifth decade with a history of chronic alcohol abuse and/or smoking, who present with recurrent episodes of upper abdominal pain often accompanied by obstructive GI symptoms. Laboratory examination reveals marginal elevation of pancreatic enzymes while the serum tumor marker levels, except in a few patients, generally remain normal. Improved diagnostic techniques such as MDCT, MRI/MRCP, and EUS can superiorly delineate the duodenal and juxtaduodenal abnormalities encountered in PP, which include medial duodenal wall thickening exhibiting increased enhancement, intramural and/or paraduodenal cysts, with or without a plate-like scar tissue in the groove region, which may at times contiguously involve the pancreatic head. As opposed to groove pancreatic carcinoma, the peripancreatic arteries are neither infiltrated nor attenuated/encased; rather they get medially displaced. A correct preoperative imaging diagnosis can curtail further diagnostic workup including an invasive biopsy and, therefore, limit the overall patient risk, cost, delayed diagnosis, and attendant patient apprehension. Also, it can aid in optimal therapeutic planning including the decision of whether or not to use preoperative chemotherapy (in patients with pancreatic carcinoma) or subject the patient to radical surgery such as pancreaticoduodenectomy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Arora A, Dev A, Mukund A, Patidar Y, Bhatia V, Sarin SK. Paraduodenal pancreatitis. Clin Radiol. 2014;69:299–306. doi: 10.1016/j.crad.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Adsay NV, Zamboni G. Paraduodenal pancreatitis: A clinico-pathologically distinct entity unifying “cystic dystrophy of heterotopic pancreas”, “para-duodenal wall cyst”, and “groove pancreatitis”. Semin Diagn Pathol. 2004;21:247–54. doi: 10.1053/j.semdp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Zamboni G, Capelli P, Scarpa A, Bogina G, Pesci A, Brunello E, et al. Nonneoplastic mimickers of pancreatic neoplasms. Arch Pathol Lab Med. 2009;133:439–53. doi: 10.5858/133.3.439. [DOI] [PubMed] [Google Scholar]
- 4.Klöppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20(Suppl 1):S113–31. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 5.Casetti L, Bassi C, Salvia R, Butturini G, Graziani R, Falconi M, et al. “Paraduodenal” pancreatitis: Results of surgery on 58 consecutives patients from a single institution. World J Surg. 2009;33:2664–9. doi: 10.1007/s00268-009-0238-5. [DOI] [PubMed] [Google Scholar]
- 6.Nankoe SR, Wilcox R, Roggin KK. Paraduodenal pancreatitis (groove pancreatitis) mimicking pancreatic adenocarcinoma. Clin Gastroenterol Hepatol. 2012;10:A31–2. doi: 10.1016/j.cgh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, et al. Paraduodenal pancreatitis: Clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475–81. doi: 10.1148/radiology.13112056. [DOI] [PubMed] [Google Scholar]
- 8.Fléjou JF. Paraduodenal pancreatitis: A new unifying term and its morphological characteristics. Diagn Histopathol. 2012;18:31–6. [Google Scholar]
- 9.Manzelli A, Petrou A, Lazzaro A, Brennan N, Soonawalla Z, Friend P. Groove pancreatitis. A mini-series report and review of the literature. JOP. 2011;12:230–3. [PubMed] [Google Scholar]
- 10.Levenick JM, Gordon SR, Sutton JE, Suriawinata A, Gardner TB. A comprehensive, case-based review of groove pancreatitis. Pancreas. 2009;38:e169–75. doi: 10.1097/MPA.0b013e3181ac73f1. [DOI] [PubMed] [Google Scholar]
- 11.Stolte M, Weiss W, Volkholz H, Rösch W. A special form of segmental pancreatitis: “Groove pancreatitis”. Hepatogastroenterology. 1982;29:198–208. [PubMed] [Google Scholar]
- 12.Becker V, Bauchspeichel D. Spezielle Pathologische Anatomic Bd. VI. In: Doerr W, Seifert G, Uhlinger E, editors. Berlin, Heidelberg, New York: Springer; 1973. pp. 252–445. [Google Scholar]
- 13.Becker V, Mischke U. Groove pancreatitis. Int J Pancreatol. 1991;10:173–82. doi: 10.1007/BF02924155. [DOI] [PubMed] [Google Scholar]
- 14.Potet F, Duclert N. Cystic dystrophy on aberrant pancreas of the duodenal wall. Arch Fr Mal App Dig. 1970;59:223–38. [PubMed] [Google Scholar]
- 15.Klöppel G, Solcia E, Longnecker D, Capella C, Sobin L. 2nd ed. Berlin, Germany: Springer-Verlag; 1996. World Health Organization International Histological Classification of Tumours. Histological Typing of Tumours of the Exocrine Pancreas; p. 23. [Google Scholar]
- 16.Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: An overview. World J Gastroenterol. 2006;12:7421–7. doi: 10.3748/wjg.v12.i46.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wronski M, Karkocha D, Slodkowski M, Cebulski W, Krasnodebski IW. Sonographic findings in groove pancreatitis. J Ultrasound Med. 2011;30:111–5. doi: 10.7863/jum.2011.30.1.111. [DOI] [PubMed] [Google Scholar]
- 18.Cavallini G, Talamini G, Vaona B, Bovo P, Filippini M, Rigo L, et al. Effect of alcohol and smoking on pancreatic lithogenesis in the course of chronic pancreatitis. Pancreas. 1994;9:42–6. doi: 10.1097/00006676-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Shudo R, Obara T, Tanno S, Fujii T, Nishino N, Sagawa M, et al. Segmental groove pancreatitis accompanied by protein plugs in Santorini's duct. J Gastroenterol. 1998;33:289–94. doi: 10.1007/s005350050086. [DOI] [PubMed] [Google Scholar]
- 20.Hartwig W, Werner J, Ryschich E, Mayer H, Schmidt J, Gebhard MM, et al. Cigarette smoke enhances ethanol-induced pancreatic injury. Pancreas. 2000;21:272–8. doi: 10.1097/00006676-200010000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Sunnapwar A, Prasad SR, Menias CO, Shanbhogue AK, Katre R, Raut A. Nonalcoholic, nonbiliary pancreatitis: Cross-sectional imaging spectrum. AJR Am J Roentgenol. 2010;195:67–75. doi: 10.2214/AJR.09.4048. [DOI] [PubMed] [Google Scholar]
- 22.Balakrishnan V, Chatni S, Radhakrishnan L, Narayanan VA, Nair P. Groove pancreatitis: A case report and review of literature. JOP. 2007;8:592–7. [PubMed] [Google Scholar]
- 23.Triantopoulou C, Dervenis C, Giannakou N, Papailiou J, Prassopoulos P. Groove pancreatitis: A diagnostic challenge. Eur Radiol. 2009;19:1736–43. doi: 10.1007/s00330-009-1332-7. [DOI] [PubMed] [Google Scholar]
- 24.Ishigami K, Tajima T, Nishie A, Kakihara D, Fujita N, Asayama Y, et al. Differential diagnosis of groove pancreatic carcinomas vs.groove pancreatitis: Usefulness of the portal venous phase. Eur J Radiol. 2010;74:e95–100. doi: 10.1016/j.ejrad.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Itoh S, Yamakawa K, Shimamoto K, Endo T, Ishigaki T. CT findings in groove pancreatitis: Correlation with histopathological findings. J Comput Assist Tomogr. 1994;18:911–5. doi: 10.1097/00004728-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Castell-Monsalve FJ, Sousa-Martin JM, Carranza-Carranza A. Groove pancreatitis: MRI and pathologic findings. Abdom Imaging. 2008;33:342–8. doi: 10.1007/s00261-007-9245-x. [DOI] [PubMed] [Google Scholar]
- 27.Shanbhogue AK, Fasih N, Surabhi VR, Doherty GP, Shanbhogue DK, Sethi SK. A clinical and radiologic review of uncommon types and causes of pancreatitis. Radiographics. 2009;29:1003–26. doi: 10.1148/rg.294085748. [DOI] [PubMed] [Google Scholar]
- 28.Blasbalg R, Baroni RH, Costa DN, Machado MC. MRI features of groove pancreatitis. AJR Am J Roentgenol. 2007;189:73–80. doi: 10.2214/AJR.06.1244. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Johnston R, Sainani NI, Sahani DV. Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis) Radiol Clin North Am. 2012;50:447–66. doi: 10.1016/j.rcl.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Pretis ND, Amodio A, Casetti L, Granato A, Marchi GD, Gabbrielli A, et al. Paraduodenal pancreatitis: An Italian experience on 112 patients. JOP. 2013;14:539. [Google Scholar]
- 31.Zaheer A, Haider M, Kawamoto S, Hruban RH, Fishman EK. Dual-phase CT findings of groove pancreatitis. Eur J Radiol. 2014;83:1337–43. doi: 10.1016/j.ejrad.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graziani R, Tapparelli M, Malagò R, Girardi V, Frulloni L, Cavallini G, et al. The various imaging aspects of chronic pancreatitis. JOP. 2005;6(Suppl):73–88. [PubMed] [Google Scholar]
- 33.Kwon MJ, Nam ES, Cho SJ, Shin HS, Kim JS, Kim DJ. A case of paraduodenal pancreatitis and immunohistochemical analysis. Korean J Pathol. 2010;44:199–203. [Google Scholar]
- 34.Chute DJ, Stelow EB. Fine-needle aspiration features of paraduodenal pancreatitis (groove pancreatitis): A report of three cases. Diagn Cytopathol. 2012;40:1116–21. doi: 10.1002/dc.21722. [DOI] [PubMed] [Google Scholar]
- 35.Ito R, Shiba H, Okamoto T, Fujioka S, Gocho T, Yanaga K. Groove pancreatitis with several cystic lesions around the pancreatic head treated conservatively: Report of a case. Case Rep Gastroenterol. 2008;2:405–9. doi: 10.1159/000164310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arvanitakis M, Rigaux J, Toussaint E, Eisendrath P, Bali MA, Matos C, et al. Endotherapy for paraduodenal pancreatitis: A large retrospective case series. Endoscopy. 2014;46:580–7. doi: 10.1055/s-0034-1365719. [DOI] [PubMed] [Google Scholar]
- 37.Laugier R, Grandval P. Does paraduodenal pancreatitis systematically need surgery? Endoscopy. 2014;46:588–90. doi: 10.1055/s-0034-1377268. [DOI] [PubMed] [Google Scholar]
- 38.Isayama H, Kawabe T, Komatsu Y, Sasahira N, Toda N, Tada M, et al. Successful treatment for groove pancreatitis by endoscopic drainage via the minor papilla. Gastrointest Endosc. 2005;61:175–8. doi: 10.1016/s0016-5107(04)02460-5. [DOI] [PubMed] [Google Scholar]
- 39.Egorov VI, Vankovich AN, Petrov RV, Starostina NS, Butkevich ATs, Sazhin AV, et al. Pancreas-preserving approach to “paraduodenal pancreatitis” treatment: Why, when, and how? Experience of treatment of 62 patients with duodenal dystrophy. Biomed Res Int 2014. 2014 doi: 10.1155/2014/185265. 185265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman SH, Verbeke CS, Gomez D, McMahon MJ, Menon KV. Pancreatico-duodenectomy for complicated groove pancreatitis. HPB (Oxford) 2007;9:229–34. doi: 10.1080/13651820701216430. [DOI] [PMC free article] [PubMed] [Google Scholar]


