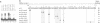Abstract
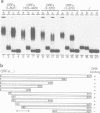
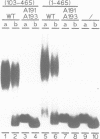
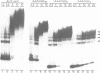
Ac encodes the 807 amino acid ORFa protein which binds specifically to multiple AAACGG motifs that are subterminally located in both ends of Ac. The wild-type ORFa protein and a number of deletion and amino acid exchange mutants were expressed in Escherichia coli, renatured and used for mobility shift assays. At least 136 amino acids from the N-terminus and 537 C-terminal amino acids may be removed from the ORFa protein without destroying the DNA binding domain, whereas a protein starting at amino acid 189 is DNA binding deficient. Certain basic amino acids between positions 190 and 200 are essential for DNA binding, as their substitution with uncharged amino acids leads to the loss of this function. The DNA binding domain of ORFa protein has an overall basic character, but no obvious sequence homology to any other known DNA binding protein. The homologies to the major open reading frames of transposable elements Tam3 from Antirrhinum majus and Hobo from Drosophila are found between the C-terminal two thirds of the three proteins. The ORFa protein forms discrete complexes with target DNA that appear, depending on the protein concentration, as a 'ladder' of bands on gels, indicating the occupation of target DNA by multiple ORFa protein molecules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B., Coupland G., Fedoroff N., Starlinger P., Schell J. Phenotypic assay for excision of the maize controlling element Ac in tobacco. EMBO J. 1987 Jun;6(6):1547–1554. doi: 10.1002/j.1460-2075.1987.tb02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The product of the Drosophila zeste gene binds to specific DNA sequences in white and Ubx. EMBO J. 1987 May;6(5):1387–1392. doi: 10.1002/j.1460-2075.1987.tb02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel S., Pirrotta V. Self-association of the Drosophila zeste protein is responsible for transvection effects. EMBO J. 1990 Sep;9(9):2959–2967. doi: 10.1002/j.1460-2075.1990.tb07488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi B. R., Hong T. J., Findley S. D., Gelbart W. M. Evidence for a common evolutionary origin of inverted repeat transposons in Drosophila and plants: hobo, Activator, and Tam3. Cell. 1991 Aug 9;66(3):465–471. doi: 10.1016/0092-8674(81)90010-6. [DOI] [PubMed] [Google Scholar]
- Coen E. S., Carpenter R. A semi-dominant allele, niv-525, acts in trans to inhibit expression of its wild-type homologue in Antirrhinum majus. EMBO J. 1988 Apr;7(4):877–883. doi: 10.1002/j.1460-2075.1988.tb02891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland G., Baker B., Schell J., Starlinger P. Characterization of the maize transposable element Ac by internal deletions. EMBO J. 1988 Dec 1;7(12):3653–3659. doi: 10.1002/j.1460-2075.1988.tb03246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigie R., Mizuuchi K. Transposition of Mu DNA: joining of Mu to target DNA can be uncoupled from cleavage at the ends of Mu. Cell. 1987 Nov 6;51(3):493–501. doi: 10.1016/0092-8674(87)90645-3. [DOI] [PubMed] [Google Scholar]
- Frey M., Reinecke J., Grant S., Saedler H., Gierl A. Excision of the En/Spm transposable element of Zea mays requires two element-encoded proteins. EMBO J. 1990 Dec;9(12):4037–4044. doi: 10.1002/j.1460-2075.1990.tb07625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusswinkel H., Schein S., Courage U., Starlinger P., Kunze R. Detection and abundance of mRNA and protein encoded by transposable element activator (Ac) in maize. Mol Gen Genet. 1991 Feb;225(2):186–192. doi: 10.1007/BF00269846. [DOI] [PubMed] [Google Scholar]
- Greenblatt I M, Brink R A. Twin Mutations in Medium Variegated Pericarp Maize. Genetics. 1962 Apr;47(4):489–501. doi: 10.1093/genetics/47.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C., Fusswinkel H., Li J., Oellig C., Kunze R., Müller-Neumann M., Heinlein M., Starlinger P., Doerfler W. Overproduction of the protein encoded by the maize transposable element Ac in insect cells by a baculovirus vector. Mol Gen Genet. 1988 Nov;214(3):373–378. doi: 10.1007/BF00330469. [DOI] [PubMed] [Google Scholar]
- Hehl R., Nacken W. K., Krause A., Saedler H., Sommer H. Structural analysis of Tam3, a transposable element from Antirrhinum majus, reveals homologies to the Ac element from maize. Plant Mol Biol. 1991 Feb;16(2):369–371. doi: 10.1007/BF00020572. [DOI] [PubMed] [Google Scholar]
- Houba-Hérin N., Becker D., Post A., Larondelle Y., Starlinger P. Excision of a Ds-like maize transposable element (Ac delta) in a transient assay in Petunia is enhanced by a truncated coding region of the transposable element Ac. Mol Gen Genet. 1990 Oct;224(1):17–23. doi: 10.1007/BF00259446. [DOI] [PubMed] [Google Scholar]
- Kammann M., Laufs J., Schell J., Gronenborn B. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR). Nucleic Acids Res. 1989 Jul 11;17(13):5404–5404. doi: 10.1093/nar/17.13.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P. D., Doll R. F., Rio D. C. Drosophila P element transposase recognizes internal P element DNA sequences. Cell. 1989 Oct 20;59(2):359–371. doi: 10.1016/0092-8674(89)90297-3. [DOI] [PubMed] [Google Scholar]
- Knight J. D., Li R., Botchan M. The activation domain of the bovine papillomavirus E2 protein mediates association of DNA-bound dimers to form DNA loops. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3204–3208. doi: 10.1073/pnas.88.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Starlinger P. The putative transposase of transposable element Ac from Zea mays L. interacts with subterminal sequences of Ac. EMBO J. 1989 Nov;8(11):3177–3185. doi: 10.1002/j.1460-2075.1989.tb08476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze R., Stochaj U., Laufs J., Starlinger P. Transcription of transposable element Activator (Ac) of Zea mays L. EMBO J. 1987 Jun;6(6):1555–1563. doi: 10.1002/j.1460-2075.1987.tb02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. F., Zou A. H., Jayaram M., Getzoff E., Harshey R. DNA-protein complexes during attachment-site synapsis in Mu DNA transposition. EMBO J. 1991 Jun;10(6):1585–1591. doi: 10.1002/j.1460-2075.1991.tb07679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung P. C., Teplow D. B., Harshey R. M. Interaction of distinct domains in Mu transposase with Mu DNA ends and an internal transpositional enhancer. Nature. 1989 Apr 20;338(6217):656–658. doi: 10.1038/338656a0. [DOI] [PubMed] [Google Scholar]
- Li M. G., Starlinger P. Mutational analysis of the N terminus of the protein of maize transposable element Ac. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6044–6048. doi: 10.1073/pnas.87.16.6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S., Rio D. C. Cytotype control of Drosophila P element transposition: the 66 kd protein is a repressor of transposase activity. Cell. 1990 Jul 27;62(2):269–284. doi: 10.1016/0092-8674(90)90365-l. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Ollo R., Maniatis T. Drosophila Krüppel gene product produced in a baculovirus expression system is a nuclear phosphoprotein that binds to DNA. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5700–5704. doi: 10.1073/pnas.84.16.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman R. F., Fedoroff N. V., Messing J. The nucleotide sequence of the maize controlling element Activator. Cell. 1984 Jun;37(2):635–643. doi: 10.1016/0092-8674(84)90395-7. [DOI] [PubMed] [Google Scholar]
- Postle K., Good R. F. DNA sequence of the Escherichia coli tonB gene. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5235–5239. doi: 10.1073/pnas.80.17.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. M., Engels W. R. Modified P elements that mimic the P cytotype in Drosophila melanogaster. Genetics. 1989 Dec;123(4):815–824. doi: 10.1093/genetics/123.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Saedler H., Nevers P. Transposition in plants: a molecular model. EMBO J. 1985 Mar;4(3):585–590. doi: 10.1002/j.1460-2075.1985.tb03670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P. K., Pelham H. R. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988 Sep 9;54(6):855–864. doi: 10.1016/s0092-8674(88)91219-6. [DOI] [PubMed] [Google Scholar]
- Streck R. D., Macgaffey J. E., Beckendorf S. K. The structure of hobo transposable elements and their insertion sites. EMBO J. 1986 Dec 20;5(13):3615–3623. doi: 10.1002/j.1460-2075.1986.tb04690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Su W., Jackson S., Tjian R., Echols H. DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 1991 May;5(5):820–826. doi: 10.1101/gad.5.5.820. [DOI] [PubMed] [Google Scholar]
- Surette M. G., Buch S. J., Chaconas G. Transpososomes: stable protein-DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell. 1987 Apr 24;49(2):253–262. doi: 10.1016/0092-8674(87)90566-6. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]