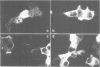
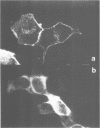
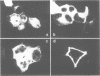
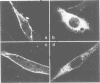
Abstract
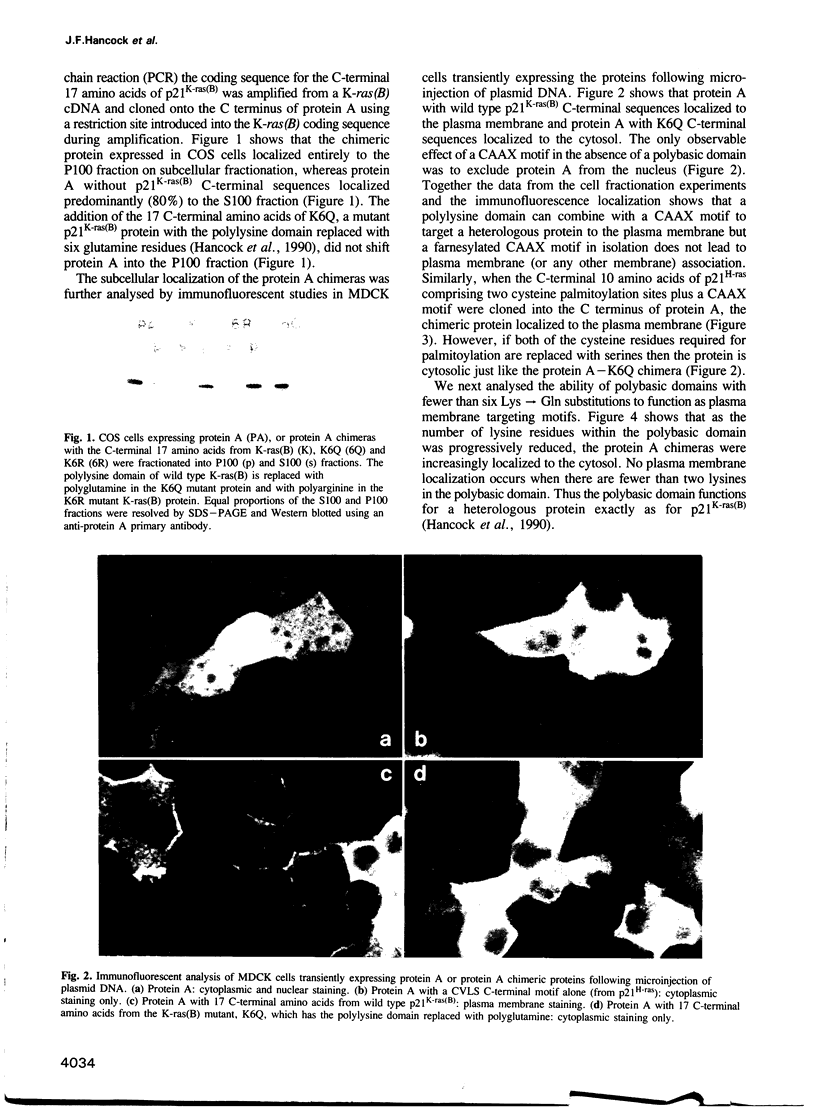
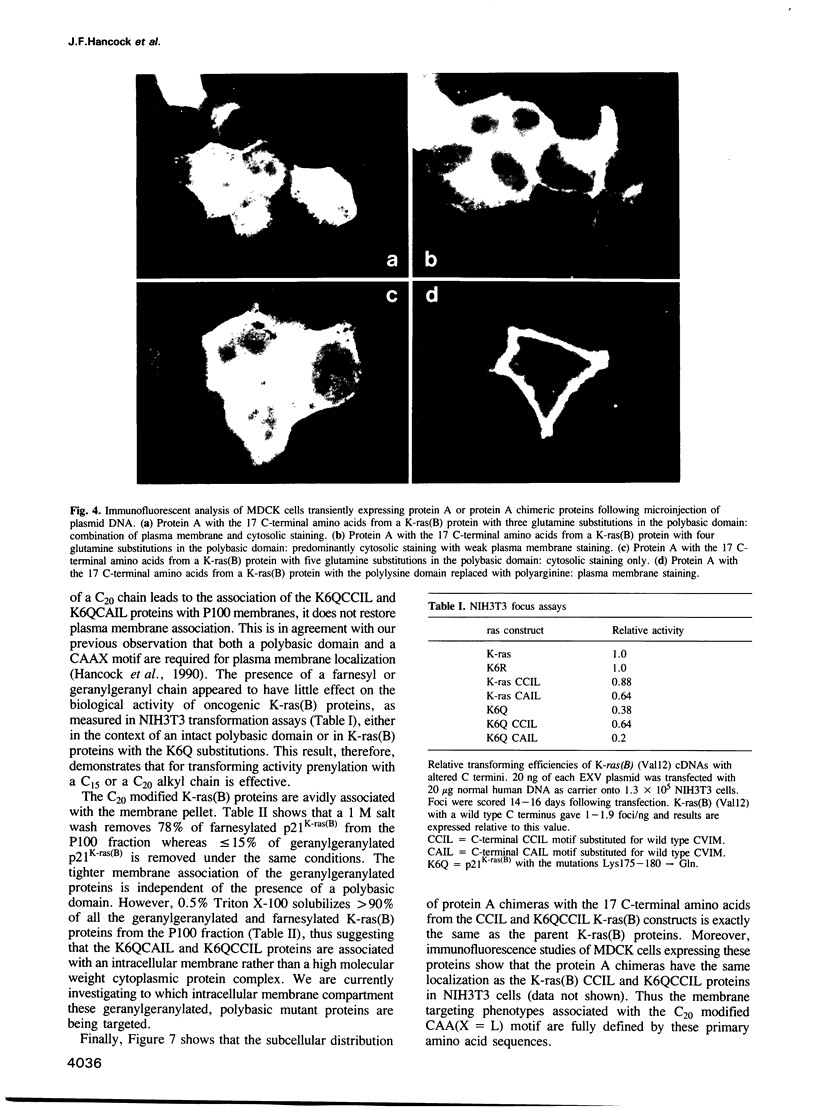
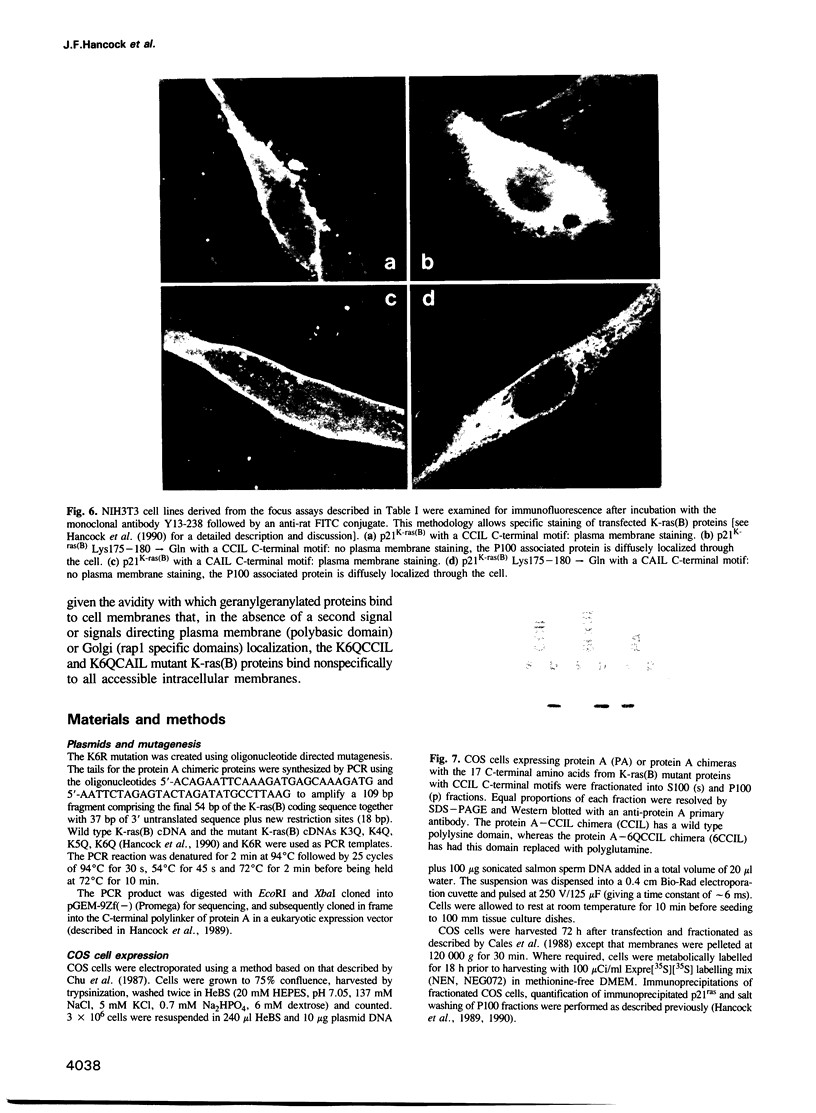
Mutational analysis of p21ras has shown that plasma membrane targeting requires the combination of a CAAX motif with a polybasic domain of six lysine residues or a nearby palmitoylation site. However, it is not known from these studies whether these signals alone target p21ras to the plasma membrane. We now show that these C-terminal sequences are sufficient to target a heterologous cytosolic protein to the plasma membrane. Interestingly, the key feature of the p21K-ras(B) polybasic domain appears to be a positive charge, since a polyarginine domain can function as a plasma membrane targeting motif in conjunction with the CAAX box and p21K-ras(B) with the polylysine domain replaced by arginines is biologically active. Since some ras-related proteins are modified by geranylgeranyl rather than farnesyl we have investigated whether modification of p21ras with geranylgeranyl affects its subcellular localization. Geranylgeranyl can substitute for farnesyl in combining with a polybasic domain to target p21K-ras(B) to the plasma membrane, but such geranylgeranylated proteins are more tightly bound to the membrane. This increased avidity of binding is presumably due to the extra length of the geranylgeranyl alkyl chain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buss J. E., Quilliam L. A., Kato K., Casey P. J., Solski P. A., Wong G., Clark R., McCormick F., Bokoch G. M., Der C. J. The COOH-terminal domain of the Rap1A (Krev-1) protein is isoprenylated and supports transformation by an H-Ras:Rap1A chimeric protein. Mol Cell Biol. 1991 Mar;11(3):1523–1530. doi: 10.1128/mcb.11.3.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béranger F., Goud B., Tavitian A., de Gunzburg J. Association of the Ras-antagonistic Rap1/Krev-1 proteins with the Golgi complex. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1606–1610. doi: 10.1073/pnas.88.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calés C., Hancock J. F., Marshall C. J., Hall A. The cytoplasmic protein GAP is implicated as the target for regulation by the ras gene product. Nature. 1988 Apr 7;332(6164):548–551. doi: 10.1038/332548a0. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S., Vogel J. P., Deschenes R. J., Stock J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4643–4647. doi: 10.1073/pnas.85.13.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Finegold A. A., Johnson D. I., Farnsworth C. C., Gelb M. H., Judd S. R., Glomset J. A., Tamanoi F. Protein geranylgeranyltransferase of Saccharomyces cerevisiae is specific for Cys-Xaa-Xaa-Leu motif proteins and requires the CDC43 gene product but not the DPR1 gene product. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4448–4452. doi: 10.1073/pnas.88.10.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukada Y., Takao T., Ohguro H., Yoshizawa T., Akino T., Shimonishi Y. Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature. 1990 Aug 16;346(6285):658–660. doi: 10.1038/346658a0. [DOI] [PubMed] [Google Scholar]
- Gutierrez L., Magee A. I., Marshall C. J., Hancock J. F. Post-translational processing of p21ras is two-step and involves carboxyl-methylation and carboxy-terminal proteolysis. EMBO J. 1989 Apr;8(4):1093–1098. doi: 10.1002/j.1460-2075.1989.tb03478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Cadwallader K., Marshall C. J. Methylation and proteolysis are essential for efficient membrane binding of prenylated p21K-ras(B). EMBO J. 1991 Mar;10(3):641–646. doi: 10.1002/j.1460-2075.1991.tb07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H., Marshall C. J. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell. 1990 Oct 5;63(1):133–139. doi: 10.1016/0092-8674(90)90294-o. [DOI] [PubMed] [Google Scholar]
- Holtz D., Tanaka R. A., Hartwig J., McKeon F. The CaaX motif of lamin A functions in conjunction with the nuclear localization signal to target assembly to the nuclear envelope. Cell. 1989 Dec 22;59(6):969–977. doi: 10.1016/0092-8674(89)90753-8. [DOI] [PubMed] [Google Scholar]
- Kawata M., Farnsworth C. C., Yoshida Y., Gelb M. H., Glomset J. A., Takai Y. Posttranslationally processed structure of the human platelet protein smg p21B: evidence for geranylgeranylation and carboxyl methylation of the C-terminal cysteine. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8960–8964. doi: 10.1073/pnas.87.22.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella B. T., Erdman R. A., Maltese W. A. Carboxyl-terminal isoprenylation of ras-related GTP-binding proteins encoded by rac1, rac2, and ralA. J Biol Chem. 1991 May 25;266(15):9786–9794. [PubMed] [Google Scholar]
- Lai R. K., Perez-Sala D., Cañada F. J., Rando R. R. The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7673–7677. doi: 10.1073/pnas.87.19.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Isoprenoid modification of G25K (Gp), a low molecular mass GTP-binding protein distinct from p21ras. J Biol Chem. 1990 Oct 15;265(29):17883–17890. [PubMed] [Google Scholar]
- Marshall C. J., Hall A., Weiss R. A. A transforming gene present in human sarcoma cell lines. Nature. 1982 Sep 9;299(5879):171–173. doi: 10.1038/299171a0. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Casey P. J., Gilman A. G., Gutowski S., Sternweis P. C. G protein gamma subunits contain a 20-carbon isoprenoid. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5873–5877. doi: 10.1073/pnas.87.15.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz T. F., Vogetseder W., Mitterer M., Böck G., Johnson J. P., Dierich M. P. Individual epitopes of an 85,000 MW membrane adherence molecule are variably expressed on cells of different lineage. Immunology. 1988 Aug;64(4):581–586. [PMC free article] [PubMed] [Google Scholar]
- Seabra M. C., Reiss Y., Casey P. J., Brown M. S., Goldstein J. L. Protein farnesyltransferase and geranylgeranyltransferase share a common alpha subunit. Cell. 1991 May 3;65(3):429–434. doi: 10.1016/0092-8674(91)90460-g. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]
- Yamane H. K., Farnsworth C. C., Xie H. Y., Howald W., Fung B. K., Clarke S., Gelb M. H., Glomset J. A. Brain G protein gamma subunits contain an all-trans-geranylgeranylcysteine methyl ester at their carboxyl termini. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5868–5872. doi: 10.1073/pnas.87.15.5868. [DOI] [PMC free article] [PubMed] [Google Scholar]