Abstract
Background
Hyperdynamic circulation due to reduced peripheral vascular resistance and increased cardiac output, and the development of portal hypertension are the hemodynamic changes observed in patients with liver cirrhosis. Such hemodynamic abnormalities appear in patients with late stage liver cirrhosis. Therefore, hemodynamic indices, which represent hyperdynamic circulation and portal hypertension, are significant for the prognosis of patients with liver cirrhosis. The aim of this study was to determine the hemodynamic indices associated with the prognosis of patients with liver cirrhosis.
Methods
A total of 103 patients diagnosed with liver cirrhosis between December 1999 and June 2003, with a mean follow-up period of 73 weeks, ranging from 7 to 168 weeks, were recruited. Using Child-Pugh classification, the mean arterial pressure, heart rate and hepatic venous pressure gradient (HVPG) were measured. The indices of Doppler ultrasonography, including the portal and splenic venous flows, and the resistance of the hepatic, splenic, and renal arteries were also measured using the arterial pulsatility index (PI). The prognostic values of these indices were determined by their comparison with the patient survivals.
Results
Significant hemodynamic indices for a bad prognosis were high HVPG (≥15 mmHg) and renal arterial PI (≥1.14)(p<0.05). A Child-Pugh score ≥10 was important for a poor prognosis (p<0.05).
Conclusion
Severe portal hypertension (HVPG≥15 mmHg) and high renal arterial resistance (PI≥1.14) were valuable hemodynamic indices for the prognosis of patients with liver cirrhosis. Therefore, it was concluded that the measurement of these hemodynamic indices, in addition to the Child-Pugh classification, is helpful in the prognosis of patients with liver cirrhosis.
Keywords: Liver cirrhosis, Prognosis, Portal hypertension, Hemodynamic index
INTRODUCTION
Although the Child-Pugh classification is widely used in clinical evaluation and for the prognosis of patients with liver cirrhosis, it is subjective in actual classification, lacks a discriminating ability and excludes factors other than liver function, which can pose problems for prognosis1). In particular, since liver transplantation is the most important treatment modality in patients with late stage liver cirrhosis, prognostic indices for an accurate prognosis, in addition to the Child-Pugh score, are needed for late stage liver cirrhosis patients subjected to liver transplantation. Recently, new prognostic models, such as the model for end stage liver disease (MELD), were proposed for predicting the survival of patients with liver cirrhosis1–3). However, there seems to be little reason why the MELD score should replace the well established Child-Pugh classification in the prognosis of patients with liver cirrhosis4,5). Portal hypertension and hyperdynamic circulatory changes are major complications that develop in most patients with liver cirrhosis. The development of ascites and variceal bleeding in patients with liver cirrhosis are the consequence of portal hypertension and systemic hemodynamic abnormalities, such as decreased arterial resistance and increased cardiac output6, 7). Since these hemodynamic abnormalities progress with liver disease, they are essential for making the survival prognosis8, 9). Therefore, our aim was to determine the hemodynamic prognostic factors that could represent the survival prognosis in patients with liver cirrhosis and the time and priority for the application of a liver transplantation.
MATERIALS AND METHODS
1. Subjects
Between December 1999 and June 2003, a total of 103 patients with liver cirrhosis, with a mean follow-up period of 73 weeks, ranging from 7 to 168 weeks, were included in this study. The mean age of these patients was 49.7±9.8 years (28–71), with 96 male and 7 female patients. The etiology of cirrhosis were alcohol induced, associated with hepatitis B surface antigen or hepatitic C antibody, both alcohol induced and viral and cryptogenic in 74, 10, 16 and 3 subjects, respectively. The diagnosis of cirrhosis was based on liver histology or by the combination of portal hypertension, and the clinical and ultrasonographic findings. Thirty-six. 49 and 18 of these patients belonged to Child-Pugh classes A, B and C, respectively. The MELD score was 11.8±4.3 (6–27). During the follow-up period, 30 patients died (Table 1).
Table 1.
Clinical Characteristics of the Study Subjects
| Characteristics | Patients (n=103) |
|---|---|
| Age (year) | 49.7±9.8 |
| Sex (male:female) | 96:7 |
| Etiology of cirrhosis | |
| Alcohol | 74 (71.8%) |
| HBV or HCV | 10 (9.7%) |
| Combined | 16 (15.5%) |
| Cryptogenic | 3 (2.9%) |
| Child-Pugh class | |
| A | 36 (35.0%) |
| B | 49 (47.6%) |
| C | 18 (17.5%) |
| Child-Pugh score | 7.7±1.9 |
| MELD score | 11.8±4.3 |
| Survival | 72 (69.9%) |
| Death | 30 (29.1%) |
HBV, hepatitis B virus; HCV, hepatitis C virus; MELD, model for end stage liver disease.
2. Methods
For the hemodynamic studies, the mean arterial pressure, heart rate, hepatic venous pressure gradient (HVPG), flow rates of the portal and splenic veins, and pulsatility index (PI) of the hepatic, splenic and renal arteries were measured. The HVPG and PI are representative of the portal pressure and arterial resistance, respectively. The HVPG was measured by hepatic vein catheterization. The portal and splenic venous flows and the PI of the liver, spleen and kidney were evaluated by Doppler ultrasonography using a 3.5MHz convex probe (SSD-1700, Aloka, Tokyo, Japan). The mean arterial pressure was noninvasively measured with an automatic sphygmomanometer (Hewlett-Packard M1205A; Hewlett-Packard, Palo Alto, CA, USA). The heart rate was derived from continuous electrocardiogram monitoring.
(1) Measurement of hepatic venous pressure gradient
The right hepatic vein was percutaneously catheterized through the femoral vein, and the pressure in both the wedged and free positions was recorded using a 7 French balloon-tipped catheter. The HVPG was determined by subtracting the free hepatic venous pressure from the wedged hepatic venous pressure (Figure 1)10–12).
Figure 1.
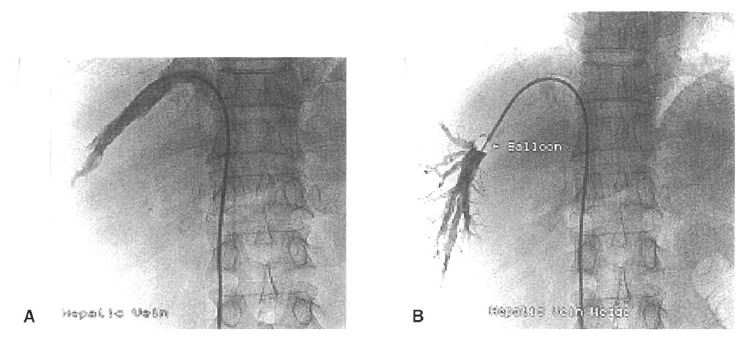
Measurement of the hepatic venous pressure gradient by right hepatic vein catheterization. Seven French balloon catheters were placed in the right hepatic vein to measure the free hepatic venous pressure (A), and the wedged hepatic venous pressure was measured by inflating the balloon catheter at the right hepatic vein (B).
(2) Measurement of Doppler indices of venous flow and arterial resistance in the liver, spleen, and kidney
The portal venous velocity and cross-sectional area of the portal vein were estimated from a subcostal scan at its crossing point with the hepatic artery. When the sample point was adjusted to the center of the portal vein, the portal venous velocity was recorded during quiet suspended expiration, which was averaged over a few seconds. The portal venous flow was determined by the following formula: cross-sectional area × mean velocity × 6013). The mean velocity of the splenic vein was also measured at the splenic hilum, and the blood flow of the splenic vein was determined using the same method as for the portal vein. Measurement of the PI by Doppler ultrasonography allowed assessment of the arterial resistance14–16). After the interlobar artery of the kidney was identified, using color flow, the time velocity wave of the Doppler signal from the interlobar artery was recorded by applying the Doppler sample volume. Tchrough the time velocity wave, the peak systolic velocity, end diastolic velocity, and mean velocity were measured and the renal arterial PI [(peak systolic velocity - end diastolic velocity)/mean velocity] determined (Figure 2)11, 14–16).
Figure 2.
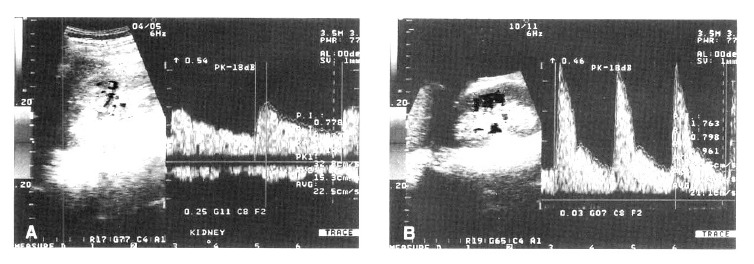
In a Child-Pugh class A cirrhotic patient, Doppler ultrasonography shows a pulsatility index of 0.778 (A). In a Child-Pugh class C cirrhotic patient, Doppler ultrasonography shows a pulsatility index of 1.763 (B).
Using right intercostal scanning of the liver, the branch of the hepatic artery around the portal hilus was identified using color Doppler. After the Doppler sample volume was located in the branch of the hepatic artery, the time velocity wave of the Doppler signal was recorded11, 17). Also, color Doppler allowed for the identification of the main branches of the splenic artery at the splenic hilus. The time velocity wave was recorded after the Doppler sample volume was placed inside these vessels11). The PI of the hepatic and splenic arteries were determined using the same method as for the renal artery. The Doppler indices were estimated from the average of three repeated measurements.
3. Statistical analysis
All data are expressed as the mean ± SD. The Kaplan-Meier method and Cox proportional hazard regression analysis were used to investigate the survival analysis of patients with liver cirrhosis according to the hemodynamic parameters. A p value <0.05 was considered significant. All statistics were analyzed using the SPSS version 10.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
The survival rate was lower in patients with Child-Pugh scores of 10 points or more compared to those of less than 10 points (p=0.001) (Figure 3A). Patients with MELD scores of 11 points or more showed bad prognosis compared with those below 11 points (p=0.007) (Figure 3B). The prognosis was bad in patients with an HVPG of 15 mmHg or more compared with those of less than 15 mmHg (p=0.025) (Figure 4A). The survival rate was lower in patients with a renal arterial PI of 1.14 or more compared with those of less than 1.14 (p=0.021) (Figure 4B) (Table 2). The following hemodynamic variables did not correlate with shorter survival: mean arterial pressure (<85 mmHg), heart rate (≥75), portal venous flow (≥700 mL/min), splenic venous flow (≥600 mL/min), hepatic arterial PI (≥1.30) and splenic arterial PI (≥1.10) (p>0.05) (Table 2). In the Cox’s proportional regression analysis, the Child-Pugh and Meld scores and the renal PI were related to the mortality of patients with liver cirrhosis (Table 3). The Child-Pugh and Meld scores were included separately in the multivariate analysis to avoid interaction due to their duplicate variables.
Figure 3.
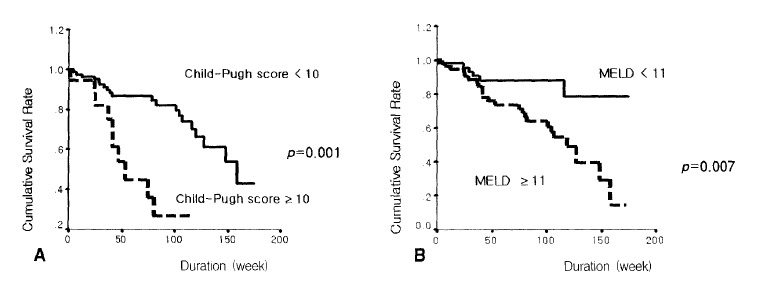
Comparison of the cumulative survival rates of a Child-Pugh (A) with a score of 10 and a model for end stage liver disease (MELD) with a score of 11 (B).
Figure 4.
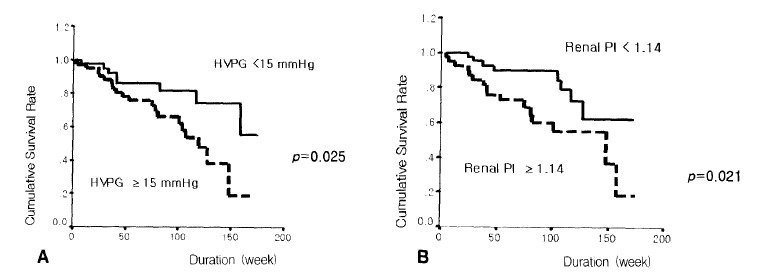
Comparison of the cumulative survival rates of a hepatic venous pressure gradient (HVPG) of 15 mmHg (A) and a renal arterial pulsatility index (PI) of 1.14 (B).
Table 2.
Univariate analysis of the prognostic values
| Variables | Log-Rank test | p-value |
|---|---|---|
| HVPG ≥ 15 | 5.06 | 0.025 |
| MAP < 85 mmHg | 2.27 | 0.132 |
| HR ≥ 75 | 0.00 | 0.948 |
| PVF ≥ 700 | 0.40 | 0.526 |
| SVF ≥ 600 | 0.71 | 0.399 |
| Hepatic arterial PI ≥ 1.30 | 0.05 | 0.830 |
| Splenic arterial PI ≥ 1.10 | 0.61 | 0.436 |
| Renal arterial PI ≥ 1.14 | 5.36 | 0.021 |
| Child-Pugh score ≥ 10 | 14.76 | 0.001 |
| MELD score ≥ 11 | 7.23 | 0.007 |
HVPG, hepatic venous pressure gradient; MAP, mean arterial pressure; HR, heart rate; PVF, portal venous flow; SVF, splenic venous flow; PI, pulsatility index; MELD, model for end stage liver disease.
Table 3.
Multivariate analysis of the prognostic values
| Variables | B | S.E. | p-value |
|---|---|---|---|
| Renal arterial PI | 3.766 | 1.595 | 0.018 |
| HVPG | 0.069 | 0.049 | 0.159 |
| MAP | 0.001 | 0.022 | 0.955 |
| HR | 0.028 | 0.016 | 0.079 |
| PVF | 0.000 | 0.001 | 0.706 |
| SVF | 0.000 | 0.001 | 0.745 |
| Age | −0.035 | 0.027 | 0.188 |
| Child-Pugh score | 0.436 | 0.145 | 0.003 |
PI, pulsatility index; HVPG, hepatic venous pressure gradient; MAP, mean arterial pressure; HR, heart rate; PVF, portal venous flow; SVF, splenic venous flow.
DISCUSSION
Portal and systemic hemodynamic changes develop in most patients with liver cirrhosis6, 7). In liver cirrhosis, increased intrahepatic vascular resistance causes an increase in the portal pressure and portosystemic shunt11, 18). These changes induce hyperdynamic systemic circulation, such as increased cardiac output and decreased systemic vascular resistance. The hyperdynamic systemic circulation and an increased splanchnic flow lead to an increase in the portal inflow and maintenance of portal hypertension6, 7, 11). Therefore, portal hypertension in patients with liver cirrhosis is initiated by an increased intrahepatic vascular resistance and maintained by an increased portal inflow due to a hyperdynamic circulatory abnormality11, 18). In other words, changes in the portal and systemic hemodynamics are a causal sequence. Portal hypertension induces serious complications; in particular, variceal bleeding is a major cause of death with liver cirrhosis. These hemodynamic abnormalities can be profound in the end stage of liver cirrhosis. Hence, portal hypertension and its related hyperdynamic circulation are important for the survival prognosis of patients with liver cirrhosis8, 9, 12).
The Child-Pugh classification has been used for the prognosis of patients with liver cirrhosis19), but it has various defects, including a limited discriminatory ability and subjective interpretation in estimating ascites and encephalopathy1). Above all, factors for hemodynamic abnormality are excluded in the Child-Pugh classification. The MELD score, recently proposed for the prognosis of liver cirrhosis, uses the serum builirubin and creatinine levels, international normalized ratio for prothrombin time, and the etiology of liver disease1–3). The MELD score was designed to overcome the limitations of the Child-Pugh score in patients at the end stage of liver disease. However, even though the serum creatinine level is included in the MELD score, the effect of creatinine on hemodynamic abnormality is weak. Furthermore, despite an actual decrease in the glomerular filtration rate in patients with liver cirrhosis, most of these patients showed normal serum creatinine levels due to muscular atrophy and a poor nutritional status. Systemic and renal hemodynamic alternations are already present in the early phase of liver cirrhosis, when common renal function tests are normal14, 20). Hence, it is our opinion that additional factors are required to reflect hemodynamic abnormalities for an accurate prognosis.
The HVPG has been accepted as the gold-standard method for assessing the severity of portal hypertension11, 21). In our study, severe portal hypertension (HVPG≥15 mmHg) was of bad prognostic significance. Therefore, the HVPG measurement appears to be helpful in the prognosis of patients with liver cirrhosis. Besides the prognostic significance in liver cirrhosis, portal pressure measurements are necessary to determine the effects of pharmacological therapies on portal hypertension. It has been proved that a 20% reduction in the baseline HVPG following the administration of propranolol is associated with a low risk of variceal bleeding22). Therefore, it is our opinion that the HVPG measurement is reasonable for the prognostic value and treatment of patients with clinically significant sequelae of portal hypertension.
The methods used to investigate the portal and systemic hemodynamics through Doppler ultrasonography include: measuring the portal and splenic venous flows and the resistive index and PI of the hepatic, splenic and renal arteries7, 9, 11–17). Measurements of the resistive index and PI by Doppler ultrasonography can represent the arterial resistance. In the present study, the PI was used to estimate the arterial resistance as this is superior to the resistive index in terms of the accuracy of calculations14–16). Of the Doppler hemodynamic indices, a high renal arterial PI (≥1.14) was of bad prognostic significance in the present study. Renal dysfunction in patients with cirrhosis is considered a consequence of renal arterial vasoconstriction secondary to a decrease in the effective circulating volume9, 14). The degree of renal arterial constriction is directly proportional to that of the renal arterial resistance using Doppler ultrasonography14, 16). A decrease in the effective circulating volume can become profound in the end stage of liver cirrhosis9,14,16). Hence, it was natural that the parameter of a high renal arterial resistance was of bad prognostic significance in the present study. Our study suggests that the renal arterial PI, as measured by non-invasive Doppler ultrasonography, represent an important hemodynamic abnormality in liver cirrhosis, and can be used as a prognostic factor in these patients.
In conclusion, severe portal hypertension (HVPG≥15 mmHg) and high renal arterial resistance (renal arterial PI≥1.14) are hemodynamic factors of bad prognostic significance in patients with liver cirrhosis. It is concluded that the measurement of these hemodynamic indices may be helpful in the prognosis of liver cirrhosis and in prioritizing the allocation of liver transplantation in patients with liver cirrhosis.
REFERENCES
- 1.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, Kremers WK, Krom RA, Kim WR. MELD and PELD: application of survival models to liver allocation. Liver Transpl. 2001;7:567–580. doi: 10.1053/jlts.2001.25879. [DOI] [PubMed] [Google Scholar]
- 3.Jeong EM, Hwang SG, Park HH, Park JH, Kim HT, Oh SW, Kho KH, Hong SP, Park PW, Rim GS, Kim SH. The anayisis of mortality rate according to CTP sand MELD score in patients with liver cirrhosis. Korean J Hepatol. 2003;9:98–106. [PubMed] [Google Scholar]
- 4.Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlicb J, Mendel H, Pichler L, Wichlas M, Kreil A, Schmid M, Ferlitsch A, Lipinski E, Brunner H, Lammer J, Ferenci P, Gangl A, Peck-Radosavljevic M. Child-Pugh versus MELD score in predicting survival in patients undergoing transjuguiar intrahepatic portosystemic shunt. Gut. 2003;52:879–885. doi: 10.1136/gut.52.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee DH, Son JH, Kim TW. New scoring systems for severity outcome of liver cirrhosis and hepatocellular carcinoma: current issues concerning the Child-Turcotte-Pugh score and the Model of End-Stage Liver Disease (MELD) score. Korean J Hepatol. 2003;9:167–179. [PubMed] [Google Scholar]
- 6.Fernandez-Seara J, Prieto J, Quiroga J, Zozaya JM, Cobos MA, Rodriguez-Eire JL, Garcia-Plaza A, Leal J. Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology. 1989;97:1304–1312. doi: 10.1016/0016-5085(89)91704-6. [DOI] [PubMed] [Google Scholar]
- 7.Baik SK, Choi YJ, Kwon SO, Kim HS, Lee DK, Uh Y, Chang SJ. Splanchnic and extrasplanchnic vascular hemodynamics in liver cirrhosis. Korean J Gastroenterol. 2000;35:466–474. [Google Scholar]
- 8.Llach J, Gines P, Arroyo V, Rimola A, Tito L, Badalamenti S, Jimenez W, Gaya J, Rivera F, Rodes J. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482–487. doi: 10.1016/0016-5085(88)90441-6. [DOI] [PubMed] [Google Scholar]
- 9.Seo Jl, Baik SK, Kim JW, Rhim DW, Park YS, Kim HS, Lee DK, Kwon SO, Chang SJ. Renal function indices predicting the prognosis of patients with liver cirrhosis. Korean J Hepatol. 2001;7:140–146. [Google Scholar]
- 10.Escorsell A, Bandi JC, Andreu V, Moitinho E, Garcia-Pagan JC, Bosch J, Rodes J. Desensitization to the effects of intravenous octreotide in cirrhotic patients with portal hypertension. Gastroenterology. 2001;120:161–169. doi: 10.1053/gast.2001.20892. [DOI] [PubMed] [Google Scholar]
- 11.Choi YJ, Baik SK, Park DH, Kim MY, Kim HS, Lee DK, Kwon SO, Kim YJ, Park JW. Comparison of Doppler ultrasonography and the hepatic venous pressure gradient in assessing portal hypertension in liver cirrhosis. J Gastroenterol Hepatol. 2003;18:424–429. doi: 10.1046/j.1440-1746.2003.02992.x. [DOI] [PubMed] [Google Scholar]
- 12.Baik SK, Park DH, Kim MY, Choi YJ, Kim HS, Lee DK, Kwon SO, Kim YJ, Park JW, Chang SJ. Captopril reduces portal pressure effectively in portal hypertensive patients with low portal venous velocity. J Gastroenterol. 2003;38:1150–1154. doi: 10.1007/s00535-003-1222-8. [DOI] [PubMed] [Google Scholar]
- 13.Schepke M, Raab P, Hoppe A, Brensing K, Paar D, Potyka U, Sauerbruch T. Propranolol stereoisomer plasma concentrations and portal haemodynamic response in patients with liver cirrhosis. Aliment Pharmacol Ther. 1999;13:1451–1458. doi: 10.1046/j.1365-2036.1999.00622.x. [DOI] [PubMed] [Google Scholar]
- 14.Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology. 1993;17:219–224. [PubMed] [Google Scholar]
- 15.Lin GS, Spratt RS. Hemodynamic imaging with pulsatility-index and resistive-index color Doppler US. Radiology. 1997;204:870–873. doi: 10.1148/radiology.204.3.9280274. [DOI] [PubMed] [Google Scholar]
- 16.Baik SK, Kim KH, Jeong YS, Kim HS, Lee DK, Kwon SO. Pulsatility index of renal artery in patients with liver cirrhosis. J Korean Soc Med Ultrasound. 2000;19:71–76. [Google Scholar]
- 17.Schneider AW, Kalk JF, Klein CP. Hepatic arterial pulsatility index in cirrhosis: correlation with portal pressure. J Hepatol. 1999;30:876–881. doi: 10.1016/s0168-8278(99)80142-1. [DOI] [PubMed] [Google Scholar]
- 18.Mahl TC, Groszmann RJ. Pathophysiology of portal hypertension and variceal bleeding. Surg Clin North Am. 1990;70:251–266. doi: 10.1016/s0039-6109(16)45080-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim WR, Poterucha JJ, Wiesner RH, LaRusso NF, Lindor KD, Petz J, Therneau TM, Malinchoc M, Dickson ER. The relative role of the Child-Pugh classification and the Mayo natural history model in the assessment of survival in patients with primary sclerosing cholangitis. Hepatology. 1999;29:1643–1648. doi: 10.1002/hep.510290607. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo V, Planas R, Gaya J, Deulofeu R, Rimola A, Perez-Ayuso RM, Rivera F, Rodes J. Sympathetic nervous activity, renin-angiotensin system and renal excretion of prostaglandin E2 in cirrhosis: relationship to functional renal failure and sodium and water excretion. Eur J Clin Invest. 1983;13:271–278. doi: 10.1111/j.1365-2362.1983.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 21.Armonis A, Patch D, Burroughs A. Hepatic venous pressure measurement: an old test as a new prognostic marker in cirrhosis? Hepatology. 1997;25:245–248. doi: 10.1053/jhep.1997.v25.ajhep0250245. [DOI] [PubMed] [Google Scholar]
- 22.Feu F, Garcia-Pagan JC, Bosch J, Luca A, Teres J, Escorsell A, Rodes J. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346:1056–1059. doi: 10.1016/s0140-6736(95)91740-3. [DOI] [PubMed] [Google Scholar]


