Abstract
Background
This study was conducted to evaluate the risk factors for infection and clinical outcomes of bacteremic spontaneous bacterial peritonitis (SBP) due to ESBL-producing E. coli and K. pneumoniae, in patients with advanced liver cirrhosis.
Methods
The ESBL production was determined by NCCLS guidelines and/or double-disk synergy tests, on stored E. coli and K. pneumoniae blood isolates collected between 1998 and 2002. Of the patients with advanced liver cirrhosis, 15 case patients, with SBP due to ESBL-producers, were compared with 30 matched controls, with SBP due to non-ESBL-producers.
Results
There were no significant differences in age, sex, Child-Pugh scores, or APACHE II scores between the two groups. Significant factors associated with infection by ESBL-producing organisms, according to univariate analysis, were: ICU care, indwelling urinary catheter, central venous catheterization, an invasive procedure within the previous 72 hours, and prior use of antibiotics within the previous 30 days. When assessing the clinical response at 72 hours after the initial antimicrobial therapy, the treatment failure rate was significantly higher in the ESBL group (73.3% vs. 16.7%, p<0.001). Also, overall 30-day mortality rates were 60% (9/15) in the ESBL groups and 23.3% (7/30) in the control group p=0.015).
Conclusion
Among patients with advanced liver cirrhosis, bacteremic SBP due to ESBL-producing E. coli and K pneumoniae was associated with adverse outcomes, and significantly higher mortality.
Keywords: Klebsiella pneumoniae, Escherichia coli, Peritonitis, Liver cirrhosis, Beta-lactamase
INTRODUCTION
Patients with liver cirrhosis often have serious infections. It is also common for cirrhosis patients to develop acute bacterial peritonitis, with no obvious primary source of infection1, 2). Cirrhotic patients with ascites are particularly susceptible to spontaneous bacterial peritonitis (SBP)3, 4). In these cases, empirical therapy with cefotaxime or ampicillin with aminoglycoside has classically been recommended, because enteric gram-negative bacilli, such as Escherichia coli and Klebsiella pneumoniae, are found to be responsible for the majority of these cases5, 6). However, in recent years, many Enterobacteriaceae have been found to harbor extended-spectrum β-lactamases (ESBLs)7). These enzymes hydrolyze broad-spectrum cephalosporins, such as cefotaxime and ceftazidime. This activity renders these antibiotics clinically ineffective7, 8). There have been several reports that the ESBL production adversely affected the clinical outcome of patients infected by Escherichia coli and Klebsiella pneumoniae9–11). Also, there has been a case report on fatal SBP due to ESBL-producing E. coli12). However, data regarding the impact of ESBL-producing organisms on the clinical outcome of SBP is very limited at this time. Thus, we conducted this study in order to evaluate the impact of ESBL-producing organisms on the clinical outcome of bacteremic SBP, due to E. coli and K. pneumoniae.
MATERIALS AND METHODS
1. Patients and bacterial strains
In order to identify the patients with advanced liver cirrhosis and bacteremic SBP due to E. coli and K. pneumoniae, the database at the Clinical Microbiology laboratory was reviewed, for the period spanning January 1998 to December 2002. The deep-frozen blood isolates of E. coli and K. pneumoniae were tested for ESBL production, which was phenotypically determined by the disk diffusion method, according to NCCLS performance standards13). The inhibition zone diameters for the bacterial strains of the cefotaxime (30 μg) and ceftazidime (30 μg) disks, both alone, and in combination with 10 μg of clavulanic acid, were determined. An increase of ≥ 5 mm in zone diameter for either of the antimicrobial agents in combination with clavulanic acid, compared to its zone diameter when tested alone, was considered to be a phenotypic confirmation of ESBL production. Bacterial strains which were resistant to cefotaxime, ceftazidime or cefpodoxime, but which revealed no synergy in combination with clavulanic acid, were subjected to double-disk diffusion tests, using cefotaxime, ceftazidime and cefepime disks14). Double-disk diffusion tests were performed as described by Thomas and Sanders15), except that the ceftazidime and amoxicillin/clavulanic acid disks were placed 15 mm apart10). Two control organisms (E. coli ATCC 25922 and K. pneumoniae ATCC 700603) were inoculated into each set of tests as a quality control.
2. Study design and definitions
Cases were defined as the patients with advanced liver cirrhosis and bacteremic SBP, due to ESBL-producing E. coli and K. pneumoniae. Among the patients with bacteremic SBP due to non-ESBL-producers, 2 controls were matched to each case patient, based on age (±5 year), sex, bacterial strain, Child-Pugh score (±2)16), and APACHE II score (±2)17). The diagnosis of SBP was based on the combination of a positive ascitic fluid culture and a polymorphonuclear leukocyte (PMNL) count of > 250 cells/L, or on the symptoms and signs of SBP, and a PMNL count of > 250 cells/μL18). Bacteremia was defined as the isolation of organism in a blood culture specimen. Bacteremic SBP was defined as SBP with concomitant bacteremia, of which the primary source was presumed to be SBP.
The medical records of the patients were retrospectively reviewed. The data collected included: age, sex, severity of illness calculated by the Acute Physiology and Chronic Health Evaluation (APACHE) II score17), duration of hospital stay prior to bacteremia, antimicrobial therapy regimen, and antimicrobial therapy within the 30 days prior to onset of bacteremia. The presence of the following comorbid conditions were also documented: neutropenia, presentation with septic shock, intensive care unit (ICU) care, and an invasive procedure within 72 hours prior to bacteremia. In addition, the presence of a central venous catheter, indwelling of a urinary catheter, or mechanical ventilation was assessed. As this was a retrospective study, the patient’s physicians, rather than the researchers, had decided the antimicrobial therapy regimens.
The main outcome criteria included the initial response to treatment, and the 7- and 30-day mortality rates. The initial responses to treatment were assessed at 72 hours after antimicrobial therapy, and classified as follows: ‘complete response’ for patients who had a resolution of fever, leukocytosis, a PMNL count in ascitic fluid and all other signs of infection; ‘partial response’ for patients who had an abatement of the abnormalities associated with the above parameters, but without complete resolution; ‘failure’ for patients who had an absence of abatement, or a deterioration in any of the clinical parameters, and ‘death’ for those patients who had died10).
3. Statistical analysis
The Student’s t-test was used to compare the continuous variables, and χ2 or Fisher’s exact tests were utilized, when required, to compare the categorical variables. All P values were two-tailed, with p<0.05 being considered statistically significant. The SPSS (version 10.0) software package was used for these analyses.
RESULTS
Fifteen consecutive patients were included in the cases group, with 30 matched patients selected for the control group. Demographic characteristics of the two groups were similar (Table 1).
Table 1.
Demographic Characteristics of Study Population
| Characteristic | ESBL (n=15) | Non-ESBL (n=30) | p |
|---|---|---|---|
| Age, mean years±standard deviation (median, range) | 58±11 (57, 43–78) | 55±11 (56, 38–74) | 0.301 |
| Male | 13 (86.7%) | 25 (83.3%) | 1.000 |
| Klebsiella pneumoniae | 10 (66.7%) | 20 (66.7%) | 1.000 |
| Nosocomial infection | 14 (93.3%) | 28 (93.3%) | 1.000 |
| Child-Pugh score | 12.40±0.74 | 12.13±1.17 | 0.425 |
| Bilirubin, mg/dL | 8.85±4.13 | 7.24±2.20 | 0.094 |
| Albumin, g/dL | 2.49±0.13 | 2.54±0.13 | 0.176 |
| Prothrombin time, INR | 1.89±0.21 | 1.98±0.27 | 0.188 |
| APACHE II score (range) | 12.73±4.01 (8–21) | 12.37±1.59 (10–17) | 0.662 |
INR, International Normalized Ratio; APACHE, Acute Physiologic and Chronic Health Evaluation
The factors associated with bacteremic SBP due to ESBL-producing organisms are shown in table 2. According to univariate analysis, the significantly associated factors for infection due to ESBL producer were: ICU care, an invasive procedure within the previous 72 hours, an indwelling urinary catheter, central venous catheterization, and prior use of antibiotics within the previous 30 days (All p<0.05) (Table 2). Of the 15 case patients (ESBL group), 14 had received ‘cefotaxime±aminoglycoside’, with one patient having received ‘imipenem’, as the initial empirical antibiotics. In terms of definitive antimicrobial therapy, 8 patients had received imipenem, 5 ‘cefotaxime±aminoglycoside’ and 2 ‘ciprofloxacin’. All the control patients (non-ESBL group) had received ‘cefotaxime±aminoglycoside’.
Table 2.
Factors associated with Infection by ESBL-Producers in Bacteremic Spontaneous Bacterial Peritonitis due to Escherichia coli and Klebsiella pneumoniae
| ESBL (n=15) | Non-ESBL (n=30) | p | |
|---|---|---|---|
| Hospital stay≥2 weeks | 8 (53.3%) | 16 (53.3%) | 1.000 |
| ICU care | 3 (20%) | 0 (0%) | 0.032 |
| Indwelling urinary catheter | 5 (33.3%) | 0 (0%) | 0.002 |
| Central venous catheterization | 4 (26.7%) | 0 (0%) | 0.009 |
| Invasive procedure within previous 72 hours | 3 (20%) | 0 (0%) | 0.032 |
| Prior use of antibiotics within previous 30 days | 12 (80%) | 9 (30%) | 0.002 |
| 3rd cephalosporins | 11 (73.3%) | 9 (30%) | 0.006 |
| Aminoglycosides | 6 (40%) | 3 (10%) | 0.042 |
| Metronidazoles | 8 (53.3%) | 2 (6.7%) | 0.001 |
| Ciprofloxacin | 1 (6.7%) | 1 (3.0%) | 1.000 |
| The number of kinds of antibiotics administered within previous 30 days, mean ± standard deviation (median, range) | 2.13±1.60 (2, 0–6) | 0.50±0.90 (0, 0–3) | <0.001 |
Data are numbers (%) of patients, unless otherwise indicated.
When assessing clinical response 72 hours after the initial antimicrobial therapy, the treatment failure rate was considerably higher in the ESBL group than that in the control group (73.3% vs. 16.7%, p<0.001). The 30-day mortality rate was also significantly higher in the ESBL group than in the control group (60% vs. 23.3%, p=0.015) (Table 3). Of the 10 patients with bacteremic SBP due to ESBL-producing K. pneumoniae, 6 died within 30 days, and of the 5 patients with bacteremic SBP due to ESBL-producing E. coli, 3 died within 30 days (Figure 1).
Table 3.
Clinical Outcome of ESBL-Producers and non-ESBL-Producers in Bacteremic Spontaneous Bacterial Peritonitis due to Escherichia coli and Klebsiella pneumoniae
| ESBL (n=15) | Non-ESBL (n=30) | p | |
|---|---|---|---|
| Complete response at 72 hours after antimicrobial therapy | 0 (0%) | 16 (53.3%) | <0.001 |
| Treatment failure at 72 hours after antimicrobial therapy* | 11 (73.3%) | 5 (16.7%) | <0.001 |
| 7-day mortality | 5 (33.3%) | 4 (13.3%) | 0.135 |
| 30-day mortality | 9 (60%) | 7 (23.3%) | 0.015 |
Data are numbers (%) of patients.
For patients with an absence of abatement or a deterioration in any of the clinical parameters associated with infection, such as fever and leukocytosis
Figure 1.
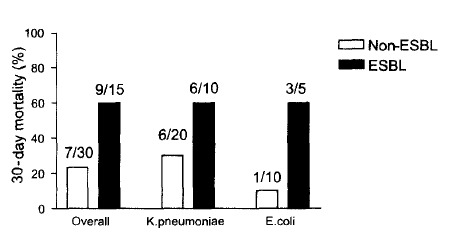
30-day mortality rate according to strain type and ESBL production in bacteremic spontaneous bacterial peritonitis due to Escherichia coli and Klebsiella pneumoniae.
DISCUSSION
In this study, the critical impact of ESBL-producing organisms on clinical outcome has been demonstrated for cases of bacteremic SBP due to E. coli and K. pneumoniae. Of the patients with advanced liver cirrhosis, whose demographic characteristics (including residual liver function) were similar, those with bacteremic SBP due to ESBL-producers had significantly higher occurrences of treatment failure and mortality rates than did those with SBP due to non-ESBL-producers.
At present, carbapenems are recommended for the treatment of infections caused by ESBL-producing organisms, primarily based on the in vitro effect, the results of animal experiments, and the limited clinical data19). Broad-spectrum cephalosporins, such as cefotaxime and ceftazidime, were ineffective in the treatment of infections caused by ESBL-producing organisms, even when the organisms were apparently susceptible to cephalosporin in vitro8). In our study, most of the patients had advanced liver cirrhosis and had received cefotaxime as the empirical antibiotic, which was easily hydrolyzed by the ESBLs. The possibility exists that patients infected by ESBL-producers could not tolerate ineffective therapies, or delayed effective antimicrobial therapies against ESBL-producing organisms, due to their severely impaired liver functions. Patients with advanced liver cirrhosis have multifactorial impairments of the immune system, which include changes in humoral immunity with decreased synthesis in the complement system, which is part of the defense mechanism against infections caused by gram-negative organisms20). Patients with liver cirrhosis also have a lower number of Kupffer’s cells, and alterations in cellular immunity lead to a decrease in the number of neutrophils, hence an impairment of their function21). Cefotaxime is widely regarded as the drug of choice for treating SBP5, 6). However, the development of ESBLs in the organisms most commonly associated with SBP has serious implications for the antibiotic management of this condition12).
The main limitation of the present investigation is that it was observational and not randomized, and, thus, an unknown risk factor for mortality might have been unequally distributed between the 2 groups. We can also not exclude the possibility of unmeasured confounding factors. In addition, our study population was composed of patients with bacteremic SBP, who might have had more severe infections than those who had SBP without bacteremia. Therefore, further study is warranted on the impact of ESBL-producing strains on outcome in SBP without concomitant bacteremia.
Our data suggest that ESBL production adversely affects the clinical outcome, and is associated with significantly higher mortality rates in bacteremic SBP due to E. coli and K. pneumoniae. Extended-spectrum cephalosporins might not constitute the optimal therapeutic option for the treatment of SBP if ESBL-producing organisms predominate, especially in patients with risk factors for infection due to ESBL-producers.
REFERENCES
- 1.Wyke RJ. Problems of bacterial infection in patients with liver disease. Gut. 1987;28:623–641. doi: 10.1136/gut.28.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caly WR, Strauss E. A prospective study of bacterial infections in patients with cirrhosis. J Hepatol. 1993;18:353–358. doi: 10.1016/s0168-8278(05)80280-6. [DOI] [PubMed] [Google Scholar]
- 3.Andreu M, Sola R, Sitges-Serra A, Alia C, Gallen M, Vila MC, Coll S, Oliver MI. Risk factors for spontaneous bacterial peritonitis in cirrhotic patients with ascites. Gastroenterology. 1993;104:1133–1138. doi: 10.1016/0016-5085(93)90284-j. [DOI] [PubMed] [Google Scholar]
- 4.Campillo B, Richardet JP, Kheo T, Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clin Infect Dis. 2002;35:1–10. doi: 10.1086/340617. [DOI] [PubMed] [Google Scholar]
- 5.Rimola A, Navasa M, Arroyo V. Experience with cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis. Diagn Microbiol Infect Dis. 1995;22:141–145. doi: 10.1016/0732-8893(95)00089-s. [DOI] [PubMed] [Google Scholar]
- 6.Rimola A, Salmeron JM, Clemente G, Rodrigo L, Obrador A, Miranda ML, Guarner C, Planas R, Sola R, Vargas V. Two different dosages of cefotaxime in the treatment of spontaneous bacterial peritonitis in cirrhosis: results of a prospective, randomized, multicenter study. Hepatology. 1995;21:674–649. [PubMed] [Google Scholar]
- 7.Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;14:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterson DL, Ko WC, von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, Bonomo RA, Rice LB, McCormack JG, Yu VL. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamase: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39:2206–2212. doi: 10.1128/JCM.39.6.2206-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiappa DA, Hayden MK, Matushek MG, Hashemi FN, Sullivan J, Smith KY, Miyashiro D, Quinn JP, Weinstein RA, Trenholme GM. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli bloodstream infection: a case-control and molecular epidemiologic investigation. J Infect Dis. 1996;174:529–536. doi: 10.1093/infdis/174.3.529. [DOI] [PubMed] [Google Scholar]
- 10.Lautenbach E, Patel JB, Bilker WB, Edelstein PH, Fishman NO. Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for infection and impact of resistance on outcomes. Clin Infect Dis. 2001;32:1162–1171. doi: 10.1086/319757. [DOI] [PubMed] [Google Scholar]
- 11.Kim YK, Pai H, Lee HJ, Park SE, Choi EH, Kim J, Kim JH, Kim EC. Bloodstream infections by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae in children: epidemiology and clinical outcome. Antimicrob Agents Chemother. 2002;46:1481–1491. doi: 10.1128/AAC.46.5.1481-1491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson DL, Singh N, Gayowski T, Marino IR. Fatal infection due to extended-spectrum β-lactamaseproducing Escherichia coli: implications for antibiotic choice for spontaneous bacterial peritonitis. Clin Infect Dis. 1999;28:683–684. doi: 10.1086/517217. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards . Performance standards for antimicrobial disk susceptibility tests. Approved standard. NCCLS document M100-S12. Wayne, PA: National Committee for Clinical Laboratory Standards; 2002. [Google Scholar]
- 14.Tzelepi E, Giakkoupi P, Sofianou D, Loukova V, Kemeroplou A, Tsakris A. Detection of extended spectrum β-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J Clin Microbiol. 2000;38:542–546. doi: 10.1128/jcm.38.2.542-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomason KS, Sanders CC. Detection of extended-spectrum β-lactamases in members of the family Enterobacteriaceae: comparison of the double-disk and three-dimensional tests. Antimicrob Agents Chemother. 1992;36:1877–1882. doi: 10.1128/aac.36.9.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660–664. doi: 10.1002/hep.1840070408. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Drapier EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 18.Rimola A, Garcia-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 19.Paterson DL. Recommendation for treatment of severe infections caused by Enterobacteriaceae producing extended-spectrum beta-lactamases (ESBLs) Clin Microbiol Infect. 2000;6:460–463. doi: 10.1046/j.1469-0691.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- 20.Thulstrup AM, Sorensen HT, Schonheyder HC, Moller JK, Tage-Jensen U. Population-based study of the risk and short-term prognosis for bacteremia in patients with liver cirrhosis. Clin Infect Dis. 2000;31:1357–1361. doi: 10.1086/317494. [DOI] [PubMed] [Google Scholar]
- 21.Rimola A, Soto R, Bory F, Arroyo V, Piera C, Rodes J. Reticuloendothelial system phagocytic activity in cirrhosis and its relation to bacterial infections and prognosis. Hepatology. 1984;4:53–58. doi: 10.1002/hep.1840040109. [DOI] [PubMed] [Google Scholar]


