Abstract
Background
Sinus node dysfunction (SND) is caused not only by intrinsic sinus node disease, but also by the extrinsic factors. Among the extrinsic factors, autonomic imbalance is most common. Symptomatic SND usually requires permanent pacemaker therapy. However, the clinical characteristics and patient response to medical therapy for hypervagotonic SND have not been properly clarified.
Materials and Methods
Thirty two patients (14 men, 18 women, 51 ± 14 years) with hypervagotonic SND were included in this study, but those patients who had taken calcium antagonists, beta-blockers or other antiarrhythmic drugs were excluded. Hypervagotonic SND was diagnosed if the abnormal electrophysiologic properties of the sinus node were normalized after the administration of atropine (0.04 mg/kg).
Results
The presenting arrhythmias were 16 cases of sinus bradycardia (50.0%), 12 of sinus pause (37.5%), 3 of sinoatrial block (9.4%) and 1 of tachy-bradycardia (3.1%). Nine (28.1%) patients had hypertension, 7 (21.9%) smoked, 2 (6.3%) had diabetes mellitus, and 1 (3.1%) had hypercholesterolemia. Among the patients, 3 had no remarkable symptoms, 13 had dizziness, 7 had syncope, 3 had weakness and 6 had shortness of breath. Twenty five (78.1%) patients were treated with theophylline, 1 patient with tachy-bradycardia syndrome was treated with digoxin and propafenone, and 6 (18.8%) were treated with no medication. During the 43±28 month follow-up, 25 patients remained asymptomatic, but 6 who took no medication developed mild dizziness. One patient needed permanent pacemaker implantation owing to recurrent syncope despite of theophylline treatment.
Conclusion
These results show that hypervagotonic SND has a benign course and most of the patients can be managed safely without implanting a pacemaker. (Ed note: I like the abstract. It is short and direct, as it should be.)
Keywords: Sick sinus syndrome, Vagus, Autonomic hyperactivity, Electrophysiologic study
INTRODUCTION
Sinus node dysfunction (SND) is one of major indications for the implantation of a permanent pacemaker1). SND is often caused by extrinsic factors such as drugs, infection, and autonomic imbalance, however, its clinical characteristics and response to drugs have not been clarified if the SND is caused by enhanced vagal tone (hypervagotonia). In unpaced patients with SND, symptoms such as dizziness and syncope do not necessarily indicate a poor prognosis, and the total mortality and the sudden death rate of such patients do not not seem to be higher than those in the general population2).
We conducted this study in order to observe the demographic features, associated medical diseases and clinical courses of patients with hypervagotonic SND.
MATERIALS AND METHODS
The subjects of this study were 32 patients who underwent electrophysiologic study on the suspicion of SND, and they were followed up for more than 6 months. The resting electrocardiography (ECG) of the 32 patients showed sinus bradycardia, sinus pause, sinoatrial exit block or tachycardia-bradycardia. Hypervagotonic SND was defined if the sinus cycle length, sinus node recovery time (SNRT) and/or sinoatrial conduction time (SACT) returned to normal after the administration of atropine (0.04 mg/kg)3). Those patients were excluded that had taken beta blockers, calcium channel blockers, antiarrhythmic drugs, or if they had a positive head-up tilt test. Other criteria for exclusion were if the patient had significant hepatic, renal, or thyroid disease. A written informed consent was obtained from all subjects.
An electrophysiologic study was performed after the patients had fasted for more than 6 hours and all kinds of cardiovascular active drugs were with drawn for more than 48 hours before the test. We placed 6–7 Fr electrode catheters at the high right atrium, the His-bundle area and the right ventricular apex. Programmed electrical stimulation was performed at a level two times higher than the diastolic threshold. SNRT was measured as an interval between the last beat of rapid atrial pacing for 45 seconds and the first spontaneous sinus beat. SACT was measured using the Strauss or Narula method. We defined the SND if the SNRT (over 550 msec of corrected SNRT) was over 1550 msec or the SACT was over 125 msec. Patients were then followed up and asked about the presence of recurrent symptoms. Information on clinical status, symptoms, drug treatment, and side effects were recorded at each visit.
RESULTS
Among the subject patients, 14 (43.8%) were male and 18 were female. The mean age was 51 ± 14 years. Of them, one patient had myocardial infarction and two had angina pectoris. As for associated cardiovascular risk factors, hypertension was found in 9, smoking in 7, diabetes mellitus in 2, and hyperlipidemia in 1 (Table 1).
Table 1.
Baseline characteristics of patients
| Baseline characteristics | Number of patients (%) |
|---|---|
| Male | 14 (43.7) |
| Age (years) | 51±14 |
| Underlying heart disease | |
| No organic heart disease | 29 (90.6) |
| Angina pectoris | 2 (6.3) |
| Old myocardial infarction | 1 (3.1) |
| Cardiovascular risk factors | |
| None | 13 (40.6) |
| Hypertension | 9 (28.1) |
| Smoking | 7 (21.9) |
| Diabetes Mellitus | 2 (6.3) |
| Hyperlipidemia | 1 (3.1) |
Three patients had no remarkable symptoms while 13 others had dizziness, 7 had syncope, 3 had weakness and 6 had shortness of breath. Sinus bradycardia was the most common ECG finding, which was found in 16 patients, and sinus pause was found in 12 patients, sinoatrial block in 3 and tachy-bradycardia in 1 (Table 2). For the patients with sinus bradycardia, the heart rate was 27–50 beats per minute and sinus pause was also found in 3 patients. One patient had additional asymptomatic Mobitz type II second-degree atrioventricular block which was an atrioventricular (AV) nodal block. The prolonged SNRT and SACT became normal after the administration of 0.04 mg/kg atropine (Figure 1), and the sinoatrial and AV block also disappeared. One patient with tachycardia-bradycardia syndrome was found to have paroxysmal atrial fibrillation, which was followed by sinus pause for up to 2.5 second.
Table 2.
Clinical presentations of patients
| Clinical presentations | Number of patients (%) |
|---|---|
| Symptoms | |
| None | 3 (9.4) |
| Dizziness | 13 (40.6) |
| Syncope | 7 (21.9) |
| Fatigue or weakness | 3 (9.4) |
| Dyspnea | 6 (18.7) |
| Electrocardiographic findings | |
| Sinus bradycardia | 16 (50) |
| Sinus pause | 12 (37.5) |
| Sinoatrial block | 3 (9.4) |
| Tachycardia-bradycardia | 1 (3.1) |
Figure 1.
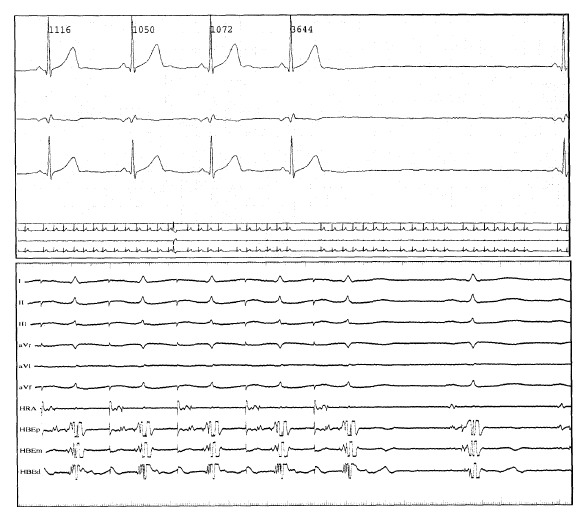
Hotter electrocardiogram (upper panel) and the record of the electrophysiologic test showing hypervagotonic sinus node dysfunction in a patient with frequent episodes of dizziness. The Hotter electrocardiogram shows sinus pause of 3.6 seconds (upper panel). After administration of atropine (lower panel) sinus node recovery time (SNRT) is normalized from 2,967 msec at the baseline state to 704 msec (cSNRT from 1,955 msec to 184 msec).
Theophylline (200–300 mg/day) was administered to 24 patients (75%), digoxin and propafenone to 1 (3.1%) with tachycardia-bradycardia, and no medications were administered to 6 (18.8%). All the patients were followed up and asked to visit the outpatient clinic every 1–2months for 43±28months. During the follow-up, 6 patients had occasional dizziness and 1 had recurrent sinus pause on the Holter electrocardiographic record, and this was associated with syncope despite of theophylline treatment, which forced the patient to have permanent pacemaker implantation (Table 3).
Table 3.
Treatment modalities
| Treatment | Number of patients (%) |
|---|---|
| Drugs | |
| Theophylline | 24(75.0) |
| Digoxin and Propafenone | 1(3.1) |
| No medication | 6 (18.8) |
| Permanent pacemaker | 1 (3.1) |
DISCUSSION
SND results in syncope, dizziness and lightheadedness, and it may be caused by sinus node disease and various extrinsic conditions such as drug, decreased blood flow to the sinus node and autonomic imbalance3, 4).
The sinus node characteristically responds to various environmental stimulis. In this process the autonomic nervous system is known to play an important role5).
The sinus node is richly innervated with postganglionic adrenergic and cholinergic nerve terminals6). By releasing acetylcholine, vagal stimulation slows down the sinus nodal discharge rate and prolongs intranodal and even internodal conduction time. Acetylcholine increases and norepinephrine decreases refractoriness of the sinus node. The negative chronotropic effects of acetylcholine are due to the inhibition of the hyperpolarization-activated pacemaker current If7).
Sinus bradycardia frequently occurs in healthy, young, well-trained athletes8). While sleeping, the normal heart rate can slow down to 35–40 beats/min, particularly in adolescents and young adults, with marked sinus arrhythmia occasionally producing pauses of 2 seconds or longer. Sinus bradycardia also occurs during vomiting or vasovagal syncope, after carotid sinus stimulation, and by the administration of parasympathomimetic drugs, lithium, amiodarone, beta blockers, clonidine, propafenone or calcium antagonists9, 10).
Because sinus node function is influenced by autonomic innervations, it is important to discriminate intrinsic SND from the hypervagotonic condition. Alboni11) and Park12) have reported that atropine decreases sinus cycle length, SNRT, and SACT by 30%1) and these reports verified the negative chronotropic effect of the vagus nerve on the sinus node.
Enhanced parasympathetic tone itself may be physiologic, as during sleep, or it may be pathologic13, 14). The latter may be triggered by gastrointestinal, genitourinary, pharyngeal or other disorders involving tissues that are richly innervated by the vagus, or it may represent enhanced sensitivity to, a disproportionate increase in, or a reaction to vagal traffic triggered by normal reflexes such as baroreceptor stimulation.
Vagally induced SND may respond to atropine, but it needs to be treated only if the patient is symptomatic. Hypervagotonic SND should be distinguished from the pathologic condition of the sinus node because of differences in treatment and prognosis. Hypervagotonia as a cause of SND is often suspected by its transient nature and with the symptoms associated with the physiological increase of vagal tone, such as micturition, nausea and vomiting. However, the role of hypervagotonia in SND should be confirmed by the response to a vagolytic drug. Electrophysiologic abnormalities in hypervagotonic SND should be corrected with atropine because the efferent mechanism involves vagotonia.
There have been a number of reports substantiating the positive chronotropic effect of theophylline. The most probable mechanism by which the drug exerts this action is the antagonism of the cardiac effect of adenosine, which has been found to depress sinus node automaticity15, 16). Several electrophysiology studies reported that theophylline improves sinus node function in subjects with sinus bradycardia and enhances nodal conduction17, 18). In uncontrolled studies performed in patients with symptomatic SND, oral theophylline increased the resting and exercise heart rate, improved symptoms, and reduced sinus pauses during the follow-up period19).
In the present study, theophylline was the most common drug used, and it eliminated most of the symptoms associated with SND. In 70.4% of patients who received theophylline, there was no recurrence of symptoms even after discontinuation of the drug. The increase of the heart rate and a slight positive inotropic action may account for the effect of this drug on patients with SND20, 21).
The dosage appropriate for eliminating the symptom was 200–300 mg/day, although some studies suggested administering a much higher dosage. Drug discontinuation was required for 11% of the patients in other studies22). However, in this study, no one had any serious side effects associated with theophylline administration. A higher dosage may account for the development of side effects and the discontinuation of the drug.
There was no relation between associated medical illness and hypervagotonic SND in terms of severity of sinus node dysfunction and clinical presentation. These results suggest that it is important to identify hypervagotonia when the attending physician is evaluating sinus node dysfunction and determining whether medical treatments are adequate and safe for patients with hypervagotonic SND.
REFERENCES
- 1.Sutton R, Kenny RA. The natural history of sick sinus syndrome. Pacing Clin Electrophysiology. 1986;9:1110–1114. doi: 10.1111/j.1540-8159.1986.tb06678.x. [DOI] [PubMed] [Google Scholar]
- 2.Shaw DB, Holman RR, Gowers JI. Survival in sino-atrial disorder (sick sinus syndrome) Br Med J. 1980;280:139–141. doi: 10.1136/bmj.280.6208.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai JM, Scheinman MM, Strauss HC, Massie B, O’Young J. Electrophysiologic effects of combined autonomic blockade in patients with sinus node disease. Circulation. 1981;63:953–960. doi: 10.1161/01.cir.63.4.953. [DOI] [PubMed] [Google Scholar]
- 4.Alboni P, Baggioni GF, Scarfo S, Cappato S, Cappato R, Percoco GF, Paparella N, Antonioli GE. Rote of sinus node artery disease in sick sinus syndrome in inferior wall acute myocardial infarction. Am J Cardiol. 1991;67:1180–1184. doi: 10.1016/0002-9149(91)90923-9. [DOI] [PubMed] [Google Scholar]
- 5.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27:1053–1060. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 6.Zipes DP. Autonomic modulation of cardiac arrhythmias. In: Zipes DP, Jalife J, editors. Cardiac electrophysiology: from cell to bedside. 3rd ed. Philadelphia: WB Saunders; 2000. pp. 300–314. [Google Scholar]
- 7.DiFrancesco D. The pacemaker current (If) plays an important rote in regulating S4 node pacemaker activity. Cardiovasc Res. 1995;30:307–308. [PubMed] [Google Scholar]
- 8.Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J. 1982;47:213–220. doi: 10.1136/hrt.47.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leier CV, Johnson TM, Hashimoto H, Schaal SF. Effects of commonly used cardioactive drugs on atrial and sinoatrial conductionn in man. J Cardiovasc Pharmacol. 1980;2:553–566. doi: 10.1097/00005344-198009000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Alpert MA, Flaker GC. Arrhythmias associated with sinus node dysfunction: pathogenesis, recognition, and management. JAMA. 1983;250:2160–2166. [PubMed] [Google Scholar]
- 11.Alboni P, Malcame C, Pedroni P, Masoni A, Narula OS. Electrophysiology of normal sinus node with and without autonomic blockade. Circulation. 1982;65:1236–1242. doi: 10.1161/01.cir.65.6.1236. [DOI] [PubMed] [Google Scholar]
- 12.Park HW, Kim JW, Kim SH, Cho JH, Ahn YK, Park JH, Jeong MH, Cho JG, Park JC, Kang JC. Electrophysiologic properties of the AV conduction system in patients with siuns node dysfunction. Korean J Med. 1998;55:342–348. [Google Scholar]
- 13.Brodsky M, Wu D, Denes P, Kanakis C, Rosen KM. Arrhythmias documented by 24 hour continuous electrocardiographic monitoring in 50 mate medical students without apparent heart disease. Am J Cardiol. 1977;39:390–395. doi: 10.1016/s0002-9149(77)80094-5. [DOI] [PubMed] [Google Scholar]
- 14.Romano M, Clarizia M, Onofrio E, Caiazzo MR, Adinolfi L, Cutillo S, Chiariello M, Condorelli M. Heart rate, PR, and QT intervals in normal children: a 24 hour Hotter monitoring study. Clin Cardiol. 1988;11:839–842. doi: 10.1002/clc.4960111208. [DOI] [PubMed] [Google Scholar]
- 15.DiMarco JP, Sellers TD, Berne RM, West GA, Belardinelli L. Adenosine: electrophysiologic effects and therapeutic use for terminating paroxysmal supraventricular tachycardia. Circulation. 1983;68:1254–1263. doi: 10.1161/01.cir.68.6.1254. [DOI] [PubMed] [Google Scholar]
- 16.Szentimiklosi AJ, Nemeth N, Szegi J, Papp J, Szekeres L. Effect of adenosine on sinoatrial and ventricular automaticity on the guinea pig. Naunyn Schmiedebergs. Arch Pharmacol. 1980;311:147–149. doi: 10.1007/BF00510253. [DOI] [PubMed] [Google Scholar]
- 17.Benditt DG, Benson DW, Jr, Kreitt J, Dunningan A, Pritzker MR, Crouse L, Scheinman MM. Electrophysiologic effects of theophylline in young patients with recurrent symptomatic bradyarrhythmias. Am J Cardiol. 1983;52:1223–1229. doi: 10.1016/0002-9149(83)90578-7. [DOI] [PubMed] [Google Scholar]
- 18.Alboni P, Rossi P, Ratto B, Pedroni P, Gatto E, Antonioli GE. Electrophysiologic effects of oral theophylline in sinus bradycardia. Am J Cardiol. 1990;65:1037–1039. doi: 10.1016/0002-9149(90)91012-u. [DOI] [PubMed] [Google Scholar]
- 19.Saito D, Matsubara K, Yamanari H, Obayashi N, Uchida S, Maekawa K, Sato T, Mizuo K, Kobayashi H, Haraokaj S. Effects of oral theophylline on sick sinus syndrome. J Am Coll Cardiol. 1993;21:1199–1204. doi: 10.1016/0735-1097(93)90246-w. [DOI] [PubMed] [Google Scholar]
- 20.Marcus ML, Skelton CL, Grauer LE, Epstein SE. Effects of theophylline on myocardial mechanism. Am J Physiol. 1972;222:1361–1365. doi: 10.1152/ajplegacy.1972.222.6.1361. [DOI] [PubMed] [Google Scholar]
- 21.Matthay RA, Berger HJ, Loke J, Gottschalk A, Zaret BL. Effects of aminophylline upon right and left ventricular performance in chronic obstructive pulmonary disease. Am J Med. 1978;65:903–910. doi: 10.1016/0002-9343(78)90741-6. [DOI] [PubMed] [Google Scholar]
- 22.Alboni P, Menozzi C, Brignole M, Paparelle N, Gaggioli G, Lolli G, Cappato R. Effects of permanent pacemaker and oral theophylline in sick sinus syndrome. Circulation. 1997;96:260–266. doi: 10.1161/01.cir.96.1.260. [DOI] [PubMed] [Google Scholar]


