Abstract
Background :
Partial liquid ventilation (PLV) and prone positioning can improve the arterial oxygenation (PaO2) in acute lung injury (ALI). We evaluated the effect of prolonged prone positioning during partial liquid ventilation (PLV) in a canine model of acute lung injury.
Methods :
Six mongrel dogs (weighing 17.4±0.7 kg each) were anesthetized, intubated and mechanically ventilated. After 1 hour of baseline stabilization, the dogs’ lungs were instilled with 40 mL/kg perfluorocarbon (PFC). PLV was first performed in the supine position for 1 hour (S1), then in the prone position for 3 hours with hourly measurements (P1, P2, P3), and finally, PLV was performed with the animal turned back to the supine position for 1 hour (S2).
Results :
After instillation of the PFC, the PaO2 significantly increased from 99.2±32.6 mmHg at baseline to 198.1±59.2 mmHg at S1 (p=0.001). When the dogs were turned to the prone position, the PaO2 further increased to 288.3±80.9 mmHg at P1 (p=0.008 vs. S1): this increase was maintained for 3 hours, but the PaO2 decreased to 129.4±62.5 mmHg at S2 (p<0.001 vs. P3). Similar changes were seen in the shunt fraction. There were no significant differences for the systemic hemodynamic parameters between the prone and supine positions.
Conclusion :
Prolonged prone positioning during PLV in an animal model of ALI appears to improve oxygenation without any hemodynamic compromise.
Keywords: Prone position; Liquid ventilation; Pulmonary gas exchange; Respiration, Artificial
INTRODUCTION
Although recent advances in mechanical ventilation have reduced mortality1, 2), the optimal ventilatory strategy for acute respiratory distress syndrome (ARDS) remains elusive. While many modalities have been shown to be effective in improving some aspects of this syndrome, whether these efforts lead to an improved outcome is not known.
One of most promising new ventilatory strategies is partial liquid ventilation (PLV)3). In experimental studies, PLV has been shown to improve gas exchange, lung mechanics and the histology of severely injured lungs4–8). The most plausible explanation for the beneficial effect of PLV is its “liquid PEEP” (positive end-expiratory pressure) effect, by which it is able to recruit collapsed lung units9). PLV has been shown to be applicable to humans and it may be beneficial in a subset of patients10–12), but not all patients respond to PLV and up to now no survival benefit has been shown12). Thus, some modification of this technique or its combined treatment with other therapeutic options may be needed to maximize PLV’s benefits.
Lying in a prone position can improve oxygenation for patients with ARDS13). This improvement has been attributed to improved ventilation and perfusion matching14, 15). The prone position also has the potential to optimise alveolar recruitment16, 17). A recent study showed that combining PLV and prone positioning can have additive effects on gas exchange in a saline-lavage model in swine18), but it is not known whether these findings are applicable to other models. Furthermore, heavy PFC may have deleterious effects on the systemic and pulmonary hemodynamics, especially if the prone position is prolonged because it might compress the heart and large blood vessels.
This study was performed to determine the effects of the prone position on oxygenation and hemodynamics during PLV in a model of acute lung injury in mongrel dogs.
MATERIALS AND METHODS
Animal preparation
The protocol of this study was approved by the Animal Use and Care Committee at the Samsung Life Science Research Institute. Six mongrel dogs (Choong-ang Laboratory Animals, Korea) with a mean body weight of 17.4±0.7 kg each were anesthetized with thiopental sodium (35 mg/kg), and they were next intubated with 6.0 mm (internal diameter) cuffed endotracheal tubes. They were then ventilated in the supine position under the volume-controlled mode using a Servo 900C ventilator (Siemens-Elema, Solna, Sweden) at a respiration rate of 20 breaths/min, a positive end expiratory pressure (PEEP) of 5 cm H2O, an inspiratory and expiratory time ratio of 1:1, an inspired O2 fraction of 1.0 and a tidal volume (VT) (120–190 mL) that was adjusted to maintain an arterial partial pressure of CO2 (PaCO2) between 35 and 45 mmHg. These initial ventilator settings were kept constant until the end of the experiment.
The animals were paralyzed by a bolus injection of 1 mg vecuronium bromide and then with hourly 0.1 mg/kg injections of the same drug, and they were anesthetized by a continuous infusion of sodium pentobarbital at a rate of 6 mg/kg/hour. Physiological saline was infused at a rate of 4 mL/kg/hour as a maintenance fluid. The right femoral artery was catheterized for blood gas sampling and monitoring the arterial pressure, and a Swan-Ganz catheter (5 Fr, Baxter, Irvine, CA, USA) was inserted into the pulmonary artery via the left external jugular vein.
The heart rate, mean arterial pressure (MAP), central venous pressure (CVP), pulmonary artery pressure (PAP), and pulmonary capillary wedge pressure (PCWP) were monitored using a Hewlett-Packard Monitoring System Model 78354C (Hewlett-Packard Gmbh, Boeblingen, Germany). A separate catheter was inserted via the external jugular vein into the right atrium to infuse oleic acid. The core body temperature was monitored with a pulmonary artery catheter, and it was maintained between 36 and 38°C with the use of heat lamps and a heating pad.
Measurements
Arterial and venous blood gas tensions, oxygen saturation and arterial hemoglobin concentration were measured from the blood drawn from the femoral artery and pulmonary artery by using a model 288 Blood Gas Analyzer (CIBA-Corning Diagnostic Corp., Medfield, MA, USA). The oxygen content (CxO2) was calculated as 1.35×% Sat×Hb+PaO2×0.003, where % Sat is the percentage oxygen saturation of the blood and Hb is hemoglobin. The shunt fraction was calculated as (CcO2-CaO2) / (CcO2-CvO2)×100, where subscripts c, a, and v denote the pulmonary capillary, arterial and mixed venous blood, respectively. The exhaled VT, the mean peak airway pressure and peak airway pressures were recorded from the ventilator display. The effective tidal volume (VTeff) was calculated by subtracting the compressible volume of the ventilator circuit from the VT. The airway pressures were monitored using a P23XL pressure transducer (Ohmeda, Inc., Madison, WI, USA), and they were recorded with a TA11 recording system (Gould Instruments, Valley View, OH, USA). The plateau pressure (Pplat) was measured by occluding the expiratory valve for 3 s while observing the pressure display to confirm a stable pressure. The static compliance of the respiratory system was calculated by dividing the VTeff by (Pplat PEEP) and then it was normalized for the individual body weight.
The cardiac output (CO) was measured by a COM-2TM CO monitor (Edwards Life Sciences Cooperation, Irvine, CA, USA), and by using at least three 5 mL injections of cold normal saline at the end-expiration. The cardiac index (CI) was calculated by dividing the measured CO by the body surface area (BSA), where BSA=0.12×(body weight)2/3. The systemic vascular resistance index (SVRI) and the pulmonary vascular resistance index (PVRI) were calculated by the following formulas and expressed as dyne×second×cm−5/m2 of BSA [SVRI=80×(mean AP/CI), PVRI=80×(mean PAP-PCWP)/CI].
Experimental protocol
The animals were hydrated to maintain a PCWP≥6 mmHg. After the surgical procedure and a stabilization period of 1 hour, the baseline (Base) parameters were collected. Lung injury was induced by an oleic acid infusion to the right atrium for 5 min (0.08 mL/kg, Sigma-Aldrich, St. Louis, MO, USA). Sixty minutes after the oleic acid infusion, bilateral lung lavage was performed using warmed isotonic saline (60 mL/kg body weight, 37°C), and this was repeated until the PaO2 after the last lavage was less than 150 mmHg.
After 1 hour period to allow stabilization of the lung injury, (Injury), the animals were instilled with PFC (FC-5080, 3M Ltd., St. Paul, MN, USA) through the side port of a closed suction catheter (Trachcare®, Ballard Medical Products, Draper, Utah, USA) during the inspiration phase. 40 mL/kg of PFC was initially instilled over 15 min; one-third was instilled in the supine position, one-third was instilled in the right lateral decubitus position and the remainder was instilled in the left lateral decubitus position, respectively. Replacement of vaporized PFC was done hourly after the measurements by filling the PFC until meniscus in the endotracheal tube was observed at the level corresponding to two thirds of the chest cage’s height. PLV was performed in the supine position for the first hour (S1), in the prone position for 3 hours (P1, P2, P3, respectively), and the animals were turned back to the supine position for the last hour (S2). After the final measurement, the animals were euthanized with a bolus injection of KCl.
Statistics
The results are expressed as means±standard deviations (SD). The data from the serial measurements were analysed using analysis of variance (ANOVA) for repeated measurements on SPSS version 10.0 software (SPSS, Chicago, Il, USA). Simple contrast analysis was used to compare the measurements with Injury, and repeated contrast analysis was used to compare individual data with the previous measurement. A p value of less than 0.05 was considered as statistically significant.
RESULTS
Parameters of gas exchange
After lung injury, the PaO2 decreased from 484.6±34.0 mmHg at the baseline measurement (Base) to 99.2±32.6 mmHg at Injury. After initiation of PLV, the PaO2 significantly increased to 198.1±59.2 mmHg at S1 from Injury (p=0.001). After turning the animals to the prone position, the PaO2 further increased to 288.3±80.9 mmHg at P1 (p=0.008), and this remained stable for the 3 hours the animals stayed in the prone position. When the animals were turned back to the supine position, the PaO2 significantly decreased from 302.2±73.0 mmHg at P3 to 129.4±62.5 mmHg at S2 (p<0.001) (Figure 1). There were no significant changes for the PaCO2 or pH throughout the duration of the study.
Figure 1.
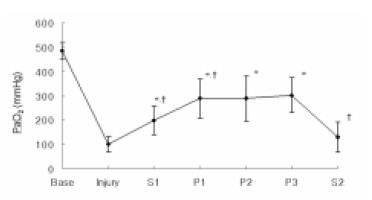
Sequential changes of PaO2 according to positioning during partial liquid ventilation (PLV). Base, baseline; Injury, 1hr after induction of injury; S1, PLV in the supine position; P1, 1hr after PLV in the prone position; P2, 2hrs after PLV in the prone position; P3, 3hrs after PLV in the prone position; S2, PLV in the supine position after the prone position.*p<0.05 compared with Injury, †p<0.05 compared with previous value.
The shunt fraction increased, after the induction of lung injury, from 16.8±2.7% at Base to 51.8±18.8% at Injury. After initiation of PLV, the shunt fraction significantly decreased to 27.6±3.2% (p=0.033) at S1. It further decreased to 21.5±3.7% at P1 (p=0.041) and this remained stable for the 3 hours the animals stayed in the prone position. The shunt fraction significantly increased again when the animals were turned back to the supine position, to 42.9±13.4% at S2 (p=0.003) (Figure 2).
Figure 2.
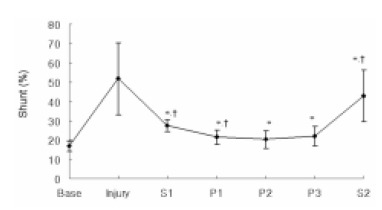
Sequential changes of the shunt fraction according to positioning during partial liquid ventilation (PLV). Base, baseline; Injury, 1hr after induction of injury; S1, PLV in the supine position; P1, 1hr after PLV in the prone position; P2, 2hrs after PLV in the prone position; P3, 3hrs after PLV in the prone position; S2, PLV in the supine position after the prone position. *p<0.05 compared with Injury, †p<0.05 compared with previous value.
Parameters of respiratory system mechanics
The Pplat showed no significant change after the instillation of the lungs with PFC, but after the dogs were placed in the prone position, this significantly decreased to 17.8±5.5 cm H2O at P1 compared with 20.7±6.2 cmH2O at Injury (p=0.012) and 20.3±4.9 cm H2O at S1 (p=0.002). The Pplat significantly increased when the animals were turned back to the supine position (p=0.012) (Figure 3).
Figure 3.
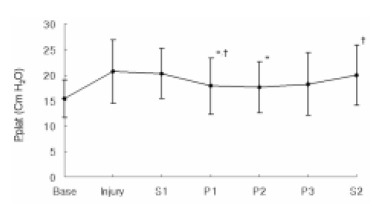
Sequential changes in plateau pressure (Pplat) according to positioning during partial liquid ventilation (PLV). Base, baseline; Injury, 1hr after induction of injury; S1, PLV in the supine position; P1, 1hr after PLV in the prone position; P2, 2hrs after PLV in the prone position; P3, 3hrs after PLV in the prone position; S2, PLV in the supine position after the prone position. *p<0.05 compared with Injury, †p<0.05 compared with previous value.
The change of Cst paralleled the change of Pplat by showing a significant increase when the animals were turned to the prone position; the Cst was 1.3±0.6 mL/cm H2O/kg at P1 compared with 1.1±0.4 mL/cm H2O/kg at Injury (p=0.026). After turning the animals back to the supine position, the value of Cst significantly decreased (p=0.040) (Figure 4).
Figure 4.
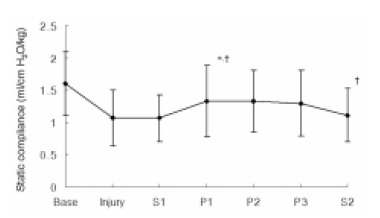
Sequential changes in static compliance (Cst) according to position during partial liquid ventilation (PLV). Base, baseline; Injury, 1hr after induction of injury; S1, PLV in supine position; P1, 1hr after PLV in prone position; P2, 2hrs after PLV in prone position; P3, 3hrs after PLV in prone position; S2, PLV in supine position after prone position. *p<0.05 compared with Injury, †p<0.05 compared with previous value.
Systemic and pulmonary hemodynamic parameters
There were no significant differences in CI or SVRI for the duration of the study (Table 1). The MAP increased significantly at P2 (p=0.026) and P3 (p=0.001) compared with Injury, but there were no significant differences according to the position of the animals (Table 1). The heart rate also increased significantly after the instillation of PFC, and it remained elevated compared with Injury for the duration of the study.
Table 1.
Sequential changes in hemodynamic parameters.
| Base | Injury | S1 | P1 | P2 | P3 | S2 | |
|---|---|---|---|---|---|---|---|
| CI (L/M2/min) | 3.4±0.9 | 3.0±0.7 | 2.9±0.8 | 3.0±0.7 | 3.5±1.2 | 3.3±0.8 | 3.4±0.9 |
| SVRI (dyne-sec) | 3460.1±1365.8 | 2673.2±764.9 | 2967.2±1316.3 | 2411.6±1027.4 | 2990.9±750.9 | 3290.1±97.3 | 2808.3±692.7 |
| MAP (mmHg) | 137.5±9.8 | 99.7±13.7 | 98.8±12.3 | 96.5±41.6 | 122.8±7.8* | 129.5±9.6* | 116.3±12.2 |
| PAP (mmHg) | 14.3±2.1 | 14.5±3.0 | 17.2±4.0 | 16.7±2.0 | 19.0±4.0 | 20.2±3.6* | 23.7±4.8*,† |
| PVRI (dyne-sec) | 237.1±122.0 | 327.5±138.8 | 418.3±161.7 | 341.4±64.2 | 362.5±79.7 | 427.4±119.9† | 484.6±149.8 |
Data are shown as means ±SD.
Base, baseline; Injury, 1hr after induction of injury; S1, PLV in the supine position; P1, 1hr after PLV in the prone position; P2, 2hrs after PLV in the prone position; P3, 3hrs after PLV in the prone position; S2, PLV in the supine position after the prone position; MAP, mean arterial pressure; CI, cardiac index; SVRI, systemic vascular resistance index; PAP, mean pulmonary arterial pressure; PVRI, pulmonary vascular resistance index.
p < 0.05 compared with Injury,
p < 0.05 compared with previous value.
The mean PAP showed a tendency to increase throughout the duration of this study, and it was significantly increased at P3 (p=0.024) and S2 (p=0.014) compared with Injury (Table 1). The most significant change was noted from P3 to S2, when the PAP increased from 20.2±3.6 mmHg to 23.7±4.8 mmHg (p=0.017). The PVRI also showed a tendency to increase after the lung injury, but the difference did not reach a level of significance compared with Injury (p>0.05).
DISCUSSION
The major findings of this study were that prone position enhanced oxygenation during PLV compared with supine position in a combined oleic acid and saline lavage model. This improved oxygenation lasted up to 3 hours, and there were no discernable adverse systemic hemodynamic effects attributable to lying in the prone position for 3 hours during PLV.
This study confirmed the findings of a previous study done on swine that showed the prone position improved the oxygenation during PLV, but that study used a different model of lung injury18). This increase in oxygenation may be due to better lung recruitment in the prone position during PLV. PLV has been shown to reopen collapsed lung units by its “iquid PEEP” effect9), thus increasing the end-expiratory lung volume19). Recruitment of collapsed lung regions is easier in the prone position16, 17) because there is a more uniform gravitational gradient of pleural pressures than in the supine position20–22). This decreased vertical gradient of pleural pressure that is noted with the prone position may be more advantageous during PLV than during gas ventilation because the accumulation of heavy PFC in the dependent lung may increase the regional pleural pressures23), which can cause collapse of the lung units, and especially in the dependent lung. By turning the animals to the prone position, greater recruitment of the collapsed lung segments should have been possible. In the present study, the decrease in plateau pressure and the increase in compliance when the animals were turned to the prone position suggest that there was indeed recruitment of collapsed lung units.
The optimal duration of the prone position is uncertain. In a randomised trial of the prone position in ARDS patients, Gattinoni et al.24) found no survival benefit for the prone position compared to the supine position. However, in that study, the mean period of the prone position for their patients was only 7.0±1.8 hours per day. To maximize the benefits of the prone position, longer periods in the prone position may be needed. McAuley et al. have found progressive improvement for gas exchange, pulmonary shunt and extravascular lung water with the time for ARDS patients observed in prone position for up to 18 hours25). Relvas et al. have found consistent improvement for oxygenation in pediatric ARDS patients only if the prone position was maintained for over 12 hours daily26). To likewise maximize the benefit from a combined therapy of PLV and prone positioning, a longer period of prone positioning may be more beneficial, and this study was designed access the benefits and feasibility of prolonged prone positioning during PLV.
One of the primary concerns with using PLV is that heavy PFC could compromise the hemodynamics. A few studies have reported observing hemodynamic compromise during the total liquid ventilation; this could be corrected by insuring adequate hydration, which can prevent the decrease of cardiac output27). However, it has been reported that PLV can be performed forin animals and patients without any significant hemodynamic compromise8, 10–12, 28). Nevertheless, the hemodynamics of PLV in the prone position could be very different from the hemodynamics of PLV in the supine position because it is not known what the effects are for the redistribution of heavy PFC during the shift from the supine to the prone position. Another concern is the fact that in the supine position the heart lies on the PFC-filled lung, whereas in the prone position the heart is compressed between the sternum and the PFC-filled lungs, and this could decrease the cardiac output. That is why we were especially interested in the hemodynamic consequences of a prolonged prone position during PLV. Fortunately, there were no significant changes in the systemic hemodynamic variables during the 3 hours of prone position that were attributable to the change in position.
In this study, the mean PAP and PVRI showed tendencies to increase during the duration of this study, and there were significantly increased mean PAP values at P3 and S2 compared with the Base. Although physical shifts for the heavy PFC within the lung could have affected pulmonary vascular pressures, this is unlikely. Both the PAP and PVRI showed increasing tendencies, and this reached significance before the change in positioning from prone to supine. In addition, at least some of the models of acute lung injury using oleic acid have shown this tendency for an increased PAP and PVRI29). However, the significant increase for the mean PAP when the animals were turned back to a prone position warrants further study before a firm conclusion can be drawn.
Different animal models of acute lung injury have been used to investigate the pathogenesis, physiology and efficacy of new therapeutic strategies for acute lung injury, and none of the models has been shown to be superior30). The model used here incorporated increased permeability and surfactant dysfunction, both of which are an essential part of the pathophysiology of ALI and ARDS31). The PFC used in this study (PF-5080) is able to dissolve 56 mL O2/100 mL and 214 mL CO2/100 mL, which is similar to the most frequently used PFC, perflubron (49 mL O2/100 mL, 210 mL CO2/100 mL).
One limitation of this study was that we cannot completely rule out the possibility that the improved oxygenation and decreased shunt observed in this study may have been solely due to the effect of the prone positioning. This is unlikely because in our pilot study we did not observe any dramatic effects for the prone position without the PLV (data not shown). Because of the time constraints of our study, we did not examine only the effect of prone positioning since the primary objective of this study was to examine the effectiveness and feasibility of prolonged prone position during PLV.
In conclusion, using the prone position during PLV in an animal model of ALI appeared to improve the oxygenation without any hemodynamic compromise. Further research will be required to confirm our findings.
Acknowledgments
We want to thank Dr. Jae Min Lee and Dr. Jong Sung Kim of Animal Research Laboratory of Samsung Biomedical Research Institute for their assistance with the preparation of this study.
Footnotes
This study was supported by a Samsung grant, #SBRI C-A1-020-1.
REFERENCES
- 1.Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA. 1995;273:306–309. [PubMed] [Google Scholar]
- 2.Abel SJ, Finney SJ, Brett SJ, Keogh BF, Morgan CJ, Evans TW. Reduced mortality in association with the acute respiratory distress syndrome (ARDS) Thorax. 1998;53:292–294. doi: 10.1136/thx.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuhrman BP, Paczan PR, DeFrancisis M. Perfluorocarbon-associated gas exchange. Crit Care Med. 1991;19:712–722. doi: 10.1097/00003246-199105000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Leonard RC. Liquid ventilation. Anaesth Intensive Care. 1998;26:11–21. doi: 10.1177/0310057X9802600102. [DOI] [PubMed] [Google Scholar]
- 5.Tutuncu AS, Faithful NS, Lachmann B. Comparison of ventilatory support with intratracheal perfluorocarbon administration and conventional mechanical ventilation in animals with acute respiratory failure. Am Rev Respir Dis. 1993;148:785–792. doi: 10.1164/ajrccm/148.3.785. [DOI] [PubMed] [Google Scholar]
- 6.Hirschl RB, Parent A, Tooley R, McCracken M, Johnson K, Shaffer TH, Wolfson MR, Bartlett RH. Liquid ventilation improves pulmonary function, gas exchange, and lung injury in a model of respiratory failure. Ann Surg. 1995;221:79–88. doi: 10.1097/00000658-199501000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernan LJ, Fuhrman BP, Kaiser RE, Penfil S, Foley C, Papo MC, Leach CL. Perfluorocarbon-associated gas exchange in normal and acid-injured large sheep. Crit Care Med. 1996;24:475–481. doi: 10.1097/00003246-199603000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Suh GY, Chung MP, Park SJ, Park JW, Kim HC, Kim H, Han J, Rhee CH, Kwon OJ. Partial liquid ventilation with perfluorocarbon improves gas exchange and decreases inflammatory response in oleic acid-induced lung injury in beagles. J Korean Med Sci. 1999;14:613–622. doi: 10.3346/jkms.1999.14.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley KP. Partial liquid ventilation-turning back a PAGE on evolution. Br J Anesth. 1997;78:1–2. doi: 10.1093/bja/78.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Hirschl RB, Pranikoff T, Wise C, Overbeck MC, Gauger P, Schreiner RJ, Dechert R, Bartlett RH. Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA. 1996;275:383–389. [PubMed] [Google Scholar]
- 11.Hirschl RB, Conrad S, Kaiser R, Zwischenberger JB, Bartlett RH, Booth F, Cardenas V. Partial liquid ventilation in adult patients with ARDS: a multicenter phase I–II trial. Ann Surg. 1998;228:692–700. doi: 10.1097/00000658-199811000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschl RB, Croce M, Gore D, Wiedemann H, Davis K, Zwischenberger J, Bartlett RH. Prospective, randomized, controlled pilot study of partial liquid ventilation in adult acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;165:781–787. doi: 10.1164/ajrccm.165.6.2003052. [DOI] [PubMed] [Google Scholar]
- 13.Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis. 1977;115:559–566. doi: 10.1164/arrd.1977.115.4.559. [DOI] [PubMed] [Google Scholar]
- 14.Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone positioning improves oxygenation in acute lung injury. Am J Respir Crit Care Med. 1994;150:184–193. doi: 10.1164/ajrccm.150.1.8025748. [DOI] [PubMed] [Google Scholar]
- 15.Pappert D, Rossaint R, Slama K, Gruning T, Falke KJ. Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest. 1994;106:1511–1516. doi: 10.1378/chest.106.5.1511. [DOI] [PubMed] [Google Scholar]
- 16.Lim CM, Koh Y, Chin JY, Lee JS, Lee SD, Kim WS, Kim DS, Kim WD. Respiratory and haemodynamic effects of the prone position at two different levels of PEEP in a canine acute lung injury model. Eur Respir J. 1999;13:163–168. doi: 10.1034/j.1399-3003.1999.13a30.x. [DOI] [PubMed] [Google Scholar]
- 17.Cakar N, der Kloot TV, Youngblood M, Adams A, Nahum A. Oxygenation response to a recruitment maneuver during supine and prone positions in an oleic acid-induced lung injury model. Am J Respir Crit Care Med. 2000;161:1949–1956. doi: 10.1164/ajrccm.161.6.9907113. [DOI] [PubMed] [Google Scholar]
- 18.Max M, Kuhlen R, Lopez F, Reyle-Hahn SM, Baumet JH, Rossaint R. Combining partial liquid ventilation and prone position in experimental acute lung injury. Anesthesiology. 1999;91:796–803. doi: 10.1097/00000542-199909000-00032. [DOI] [PubMed] [Google Scholar]
- 19.Gauger PG, Overbeck MC, Chambers SD, Cailipan CI, Hirschl RB. Partial liquid ventilation improves gas exchange and increases EELV in acute lung injury. J Appl Physiol. 1998;84:1566–1572. doi: 10.1152/jappl.1998.84.5.1566. [DOI] [PubMed] [Google Scholar]
- 20.Yang QH, Kaplowitz MR, Lai-Fook SJ. Regional variations in lung expansion in rabbits:prone vs supine position. J Appl Physiol. 1989;67:1371–1376. doi: 10.1152/jappl.1989.67.4.1371. [DOI] [PubMed] [Google Scholar]
- 21.Wiener-Kronish JP, Gropper MA, Lai-Fook SJ. Pleural pressure in dogs measured using a rib capsule. J Appl Physiol. 1985;59:597–602. doi: 10.1152/jappl.1985.59.2.597. [DOI] [PubMed] [Google Scholar]
- 22.Mutoh T, Lamm WJ, Embree LJ, Hildebrandt J, Albert RK. Abdominal distension alters regional pleural pressures and chest wall mechanics in pigs in vivo. J Appl Physiol. 1991;70:2611–2618. doi: 10.1152/jappl.1991.70.6.2611. [DOI] [PubMed] [Google Scholar]
- 23.Barbas CS, Amato MB, Goldner M. Effect of PEEP on regional pleural pressures (PPL) during partial liquid ventilation (PLV) in dogs with oleic acid induced lung injury (OAI) Am J Respir Crit Care Med. 1997;155:A86. [Google Scholar]
- 24.Gattinoni L, Tognoni G, Pesenti A, Taccone P, Mascheroni D, Labarta V, Malacrida R, di Giulio P, Fumagalli R, Pelosi P, Brazzi L, Latini R. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 25.McAuley DF, Giles S, Fichter H, Perkins GD, Gao F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002;28:414–418. doi: 10.1007/s00134-002-1248-z. [DOI] [PubMed] [Google Scholar]
- 26.Relvas MS, Silver PC, Sagy M. Prone positioning of pediatric patients with ARDS results in improvement in oxygenation if maintained > 12 h daily. Chest. 2003;124:269–274. doi: 10.1378/chest.124.1.269. [DOI] [PubMed] [Google Scholar]
- 27.Curtis SE, Fuhrman BP, Howland DF, DeFrancisis M, Motoyama EK. Cardiac output during liquid (perfluorocarbon) breathing in newborn piglets. Crit Care Med. 1991;19:225–230. doi: 10.1097/00003246-199102000-00019. [DOI] [PubMed] [Google Scholar]
- 28.Houmes RJ, Verbrugge SJ, Hendrik ER, Lachmann B. Hemodynamic effects of partial liquid ventilation with perfluorocarbon in acute lung injury. Intensive Care Med. 1995;21:966–972. doi: 10.1007/BF01700657. [DOI] [PubMed] [Google Scholar]
- 29.Hofman WF, Ehrhart IC, Granger WM, Miller DA. Sequential cardiopulmonary changes after oleic-acid injury in dogs. Crit Care Med. 1985;13:22–27. doi: 10.1097/00003246-198501000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal C, Caronia C, Quinn C, Lugo N, Sagy M. A comparison among animal models of acute lung injury. Crit Care Med. 1998;26:912–916. doi: 10.1097/00003246-199805000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Hirschl RB, Tooley R, Parent A, Johnson K, Bartlett RH. Evaluation of gas exchange, pulmonary compliance, and lung injury during total and partial liquid ventilation in the acute respiratory distress syndrome. Crit Care Med. 1996;24:1001–1008. doi: 10.1097/00003246-199606000-00021. [DOI] [PubMed] [Google Scholar]


