Abstract
Background :
Previously, the inhibition of coronary restenosis with Abciximab (ReoPro®)-coated stent in a porcine model was reported. ReoPro® inhibits platelet aggregation, the proliferation of vascular smooth muscle cells and the inflammatory reaction.
Methods :
A prospective randomized trial was performed to compare two types of stent for revascularization in the native coronary artery. The primary effective end points were major adverse coronary events (MACE): cardiac death, acute myocardial infarction, target vessel revascularization (TVR) and restenosis at the 6-month clinical and angiographic follow-ups.
Results :
One hundred and fifty-five patients were enrolled between August 2001 and June 2003. The mean ages (56.0±10.0 vs. 56.9±10.8 years), baseline diameter of stenosis and minimal luminal diameter were no different between the two groups. There was one myocardial infarction and revascularization during the hospital stay in control stent group. During the clinical follow-up there were two myocardial infarctions in control group. Follow-up coronary angiograms were performed in 62.3% (48/77) and 65.4% (51/78) of the coated and control groups, respectively. The diameter of stenosis and late loss were significantly less in the ReoPro®-coated stent group compared with the controls (16.4±5.8% vs. 34.3±6.1%, p=0.009; and 0.33±0.28 mm vs. 0.88±0.41 mm; p=0.002). The restenosis and TVR rates of the ReoPro®-coated stent were relatively lower compared with the control stent [14.6% (7/48) vs. 29.4% (15/51), p=0.062; and 9.2% (7/76) vs. 14.7% (11/75); p=0.327].
Conclusion :
A ReoPro®-coated stent is safe, and may be effective in the prevention of coronary restenosis.
Keywords: Stents, Coronary Artery Diseases, Platelets, Restenosis
INTRODUCTION
The incidence of coronary artery diseases, such as angina pectoris and myocardial infarction, has been increased rapidly over the past 10 years, and has become a major cause of death. Percutaneous coronary intervention (PCI) with stenting has been regarded as the most effective therapeutic modality for coronary artery disease, but the relatively high restenosis rate of 20–30% after PCI remains a major clinical problem1, 2).
Stent restenosis is due solely to neointimal hyperplasia (NIH) by stent-induced mechanical arterial injury and acute and chronic inflammation in the vessel wall, which activates smooth muscle cell (SMC) migration and proliferation3, 4). Local radiation therapy with beta or gamma radiation is known to control neointimal formation and has been performed in the treatment of in-stent restenosis, but notable results remain to be achieved5). The utilization of anti-proliferative agents delivered locally via drug-eluting stents has dramatically reduced the stent restenosis rate6).
Abciximab (ReoPro®) is a potent inhibitor that blocks the final pathway of platelet aggregation and decreases the short- and long-term event rates after PCI7–10). Besides its blocking effect for platelet aggregation, ReoPro® reacts with the CD11b/CD18 (macropharge-1 receptor) of vascular endothelial cells and macrophages, and inhibits the inflammatory reaction and proliferation of vascular SMC11–16). The possible mechanisms responsible for the inhibition of NIH by ReoPro® may be its antiplatelet, anti-inflammatory and anti-proliferative actions. In a previous animal study it was reported that ReoPro®-coated stents can effectively reduce neointima hyperplasia after coronary stenting17).
Here, a study was conducted to compare the long-term safety and efficacy of a ReoPro®-coated stents with those of a control bare metal stent in native human coronary artery lesions.
MATERIALS AND METHODS
Study Design and Population
This was a single-center prospective, randomized trial approved by the ethnics committee at institution, with all patients giving their written informed consent. The study was conducted between August 2001 and June 2003.
155 coronary artery disease patients were scheduled to undergo elective PCI for single, short (<15 mm in length), de novo lesions in native coronary arteries with diameters between 2.5 and 4.0 mm. The patients with left main diseases, bifurcation lesions, graft stenosis and left ventricular dysfunction were excluded. ReoPro®-coated and control stents were implanted in 77 and 78 patients, respectively.
Manufacturing Process of ReoPro®-coated Stent
A plasma polymerization reaction was performed to attach amine radicals to the stent surface. A stent was fixed in a tubular reactor made of a Pyrex glass tube, and then the pressure dropped to less than 5 mtorr using a vacuum pump. For attachment of the amine radicals to the stent surface, diaminocyclohexane (DACH) monomer was drifted into the tubular reactor at a constant dose rate (0.96 SCCM) and a plasma generated using a radiofrequency power generator. The power for polymerization of the plasma was 100 W for 5 minutes and then 60 W for 15 minutes.
ReoPro® (Centocor BV, Leiden, Netherlands) is a human-murine chimeric antibody Fab fragment (c7E3 Fab), which directly blocks glycoprotein IIb/IIIa receptors. A glass tube, 7 cm in length, with a 1 cm in diameter, was boiled in water for 5 minutes, removed and allowed to dry in an incubator. 2 mL of ReoPro® solution was also delivered to this glass tube, and the stent immersed in the solution. The carboxy radical of ReoPro® was introduced to the amine radicals attached to the stent to achieve covalent bonds and improved the attachment power between the stent and ReoPro®. This reaction was performed for 1 hour, after which the stent was removed and allowed to dry for more than 24 hours. The ReoPro®-coating on the surface of the stent was confirmed by scanning electron microscopy (Figure 1, 2). For the release kinetics of the ReoPro® from the stent, the stent was placed in a glass vial and immersed in 100 ml of phosphate-buffered saline. The amount of ReoPro® released into the buffer solution was measured using the UV absorbance at 278 nm (Figure 3).
Figure 1.
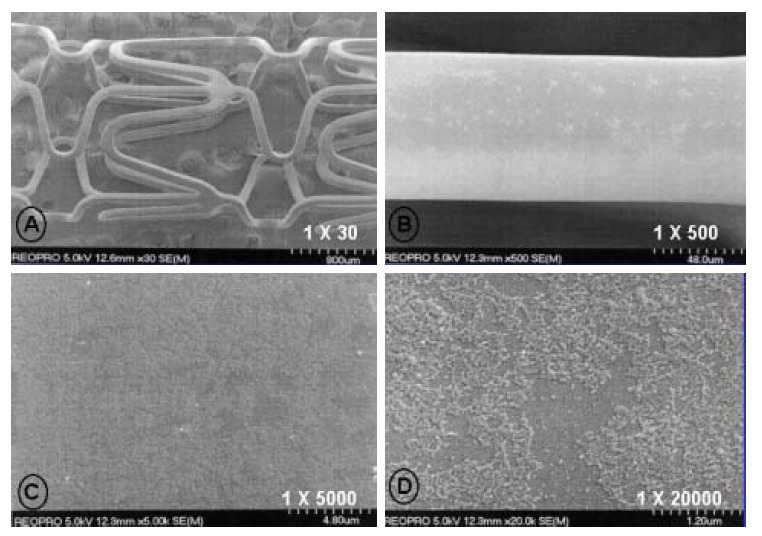
Scanning electron microscopic findings after ReoPro®-coating of the stent surface (Magnification ×30 in A, ×500 in B, ×5,000 in C and ×20,000 in D).
Figure 2.
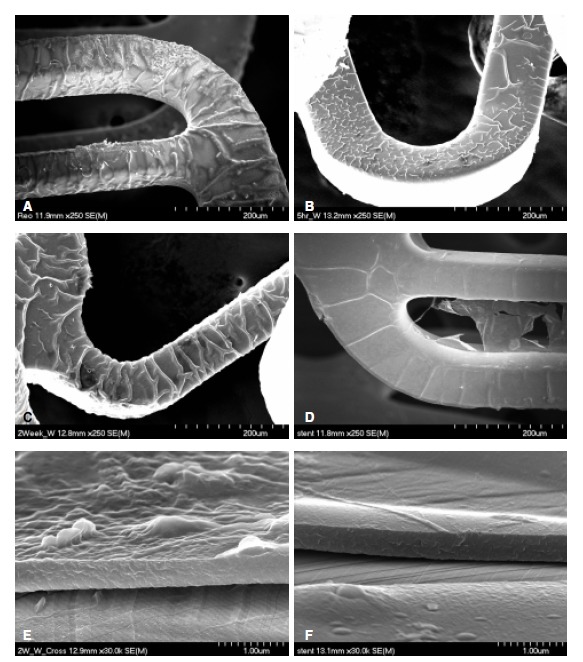
Scanning electron microscopic findings of ReoPro® grafting after the washing test. The stent surface of the ReoPro® grafting; immediately (A), 5 hours (B), 2 weeks (C) and 4 weeks (D) after the washing test. E–F: Cross sections of the stent of the ReoPro® grafting, immediately (E) and 1 months (F) after the washing tests. Magnification. 250 in A–D and 30,000 in E–F.
Figure 3.
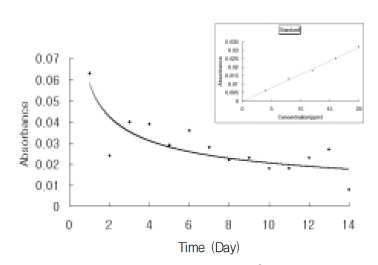
In vitro screening of the ReoPro® released from the stent surface. Right upper panel shows the linear relationship between the UV absorbance at 278 nm and the concentration of ReoPro® in water.
In an animal study using a porcine coronary stent restenosis model, ReoPro®-coated stents were found to decreased the neointima area, percent diameter of stenosis and proliferating cell nuclear antigen positive rate more than the control stents17).
Study Procedure, Stent Implantation, and Angiography
Patients were randomly assigned to the groups in a 1:1 ratio. Lesions were treated with the use of standard interventional procedures. Each patient received one 17 mm or 18 mm stent. All lesions were pre-dilated. After successful predilation, the stents were mounted onto the delivery system and inflated to 10 to 16 atm.
All patients received aspirin (300 mg at least 12 hours prior to stent implantation and 100–200 mg daily, indefinitely) and clopidogrel (300 mg at least 6 h prior to stent implantation and 75 mg daily for 30 days). A bolus dose of 5,000 units of Heparin was given intravenously just before the PCI to maintain the activated clotting time to between 300 and 350 seconds over the duration of the procedure. When dalteparin, a low molecular weight heparin, was used, an initial bolus of 2,500 units was administered as a vascular sheath and advanced to an additional dose of 5,000 to 7,000 units during PCI. Successful PCI was defined as a residual stenosis of <30% with TIMI (Thrombolysis in Myocardial Infarction) flow ≥III, with no complications, such as Q wave acute myocardial infarction (AMI), emergent revascularization or cardiac death. Each angiogram or IVUS sequence was preceded by 200 to 300 μg of intracoronary nitroglycerin. The angiograms were analyzed with a validated QCA system (Phillips H5000 or Allular DCI program). Quantitative analyses of all angiographic data were performed by an independent skillful doctor. In-stent restenosis was defined as stenosis of ≥50% of the luminal diameter. Late lumen loss was defined as the difference between the minimal lumen diameters immediately after the stenting compared to that on the six month follow-up angiogram.
Bleeding events were classified as major, minor or insignificant according to the criteria of the TIMI Study group. Major bleeding events were defined as intracranial bleeding or a bleeding event that caused a decrease in hemoglobin of ≥4 g/dL or required a transfusion of ≥3 units of blood. Minor bleeding was defined as that resulting in a hemoglobin decrease of <4 g/dL or which required a transfusion of <3 units of blood. Thrombocytopenia was defined as a platelet count of 100×109/L.
Study end points
The primary end points were in-stent late lumen loss by quantitative angiography and a composite of major cardiac events, including cardiac death, any myocardial infarction and TVR at 12 months after the procedure. The secondary end points were percentage of the lumen diameter stenosis and the rate of restenosis.
Statistical Analysis
Statistical analyses were performed with the aid of the commercially available software (SPSS Version 11). The data are presented as rates or mean values±SD. Probability values are two-sided with the Student t- and Fisher’s exact tests for continuous and categorical variables, respectively. A value of p < 0.05 was considered significant.
RESULTS
ReoPro® coating and in vitro release pharmacokinetics The attachment of the ReoPro®-coating onto the surface of the stent was confirmed by scanning electron microscopy. The amount of ReoPro®-coating on the surface of the stent was 90 μg/stent, with a median thickness of 1 μm (Figure 1). The amount of ReoPro® released increased over time and that left on the stent surface 1 month after ReoPro® coating are shown in Figure 2. The in vitro screening of ReoPro® release from stent surface was evaluated, and is depicted in Figure 3.
Baseline clinical characteristics
In terms of gender and age, the ReoPro® stent group consisted of 64 males (83.1%), with a mean age of 56±10 years, and control stent group consisted of 53 males (67.9%), with a mean age of 57±11 years. There were no differences in the clinical diagnoses of the two groups, unstable angina pectoris was the most common: 39 patients (50.6%) and 39 patients (50.0%) in the ReoPro® and stent groups, respectively. The number of patients that had previously undergone PCI were 4 (5.2%) and 5 (5.4%) in the ReoPro® and control stent groups, respectively. There were no significant differences in risk factors for coronary artery disease, clinical diagnosis and left ventricular ejection fraction (Table 1).
Table 1.
Baseline clinical characteristics
| Group I (n=77) ReoPro®-coated stent | Group II (n=78) Control stent | p | |
|---|---|---|---|
| Age (years) | 56.0±10.0 | 56.9±10.5 | 0.584 |
| Male (%) | 64 (83.1) | 53 (67.9) | 0.039 |
| Risk factor (%) | |||
| Current smoking | 45 (58.4) | 42 (53.8) | 0.628 |
| Hypertension | 37 (48.1) | 40 (51.3) | 0.749 |
| Hypercholesterolemia | 28 (36.4) | 26 (33.3) | 0.738 |
| Diabetes mellitus | 18 (23.4) | 12 (15.4) | 0.228 |
| Clinical Diagnosis (%) | 0.746 | ||
| Stable angina | 7 (9.1) | 7 (9.0) | |
| Unstable angina | 39 (50.6) | 39 (50.0) | |
| Non-ST elevation MI | 9 (11.7) | 7 (9.0) | |
| ST elevation MI | 20 (26.0) | 22 (28.2) | |
| Old myocardial infarction | 2 (2.6) | 3 (3.8) | |
| Combined vascular disease (%) | |||
| Peripheral | 1 (1.3) | 1 (1.3) | 1.00 |
| Hemorrhagic stroke | 0 (0.0) | 0 (0.0) | 1.00 |
| Transient ischemic attack | 2 (2.6) | 5 (6.4) | 0.189 |
| Family history | 3 (3.9) | 3 (3.8) | 1.00 |
| Previous coronary procedure (%) | |||
| Percutaneous coronary intervention | 4 (5.2) | 5 (6.4) | 1.00 |
| Bypass surgery | 0 (0.0) | 0 (0.0) | 1.00 |
| Left ventricular function (%) | |||
| Mean ejection fraction | 62.8±10.1 | 62.8±12.0 | 0.994 |
MI, myocardial infarction
Quantitative angiographic analysis
Stenting was successfully performed in all patients, without the need for additional stenting and with no complications. The target artery, lesion classification by the ACC/AHA classification, TIMI flow and mean reference diameter and lesion length were similar between the two groups. The stent sizes and lengths were 3.32±0.35 and 17.1±1.1 mm, and 3.28±0.43 and 17.5±4.4 mm in the ReoPro® and control stent groups, respectively, which were not significant (Table 2).
Table 2.
Coronary angiographic characteristics
| Group I (n=77) ReoPro®-coated stent | Group II (n=78) Control stent | p | |
|---|---|---|---|
| Diseased vessels (%) | 0.704 | ||
| Right coronary artery | 20 (26.0) | 16 (20.5) | |
| Left anterior descending artery | 45 (58.4) | 50 (64.1) | |
| Left circumflex artery | 12 (15.6) | 12 (15.4) | |
| Diseased vessel number (%) | 0.117 | ||
| One vessel | 68 (88.3) | 59 (75.6) | |
| Two vesel | 8 (10.4) | 16 (20.5) | |
| Three vessel | 1 (1.3) | 3 (3.8) | |
| ACC/AHA classification (%) | 0.085 | ||
| Type B1 | 68 (88.3) | 58 (74.4) | |
| Type B2 | 8 (10.4) | 17 (21.8) | |
| Type C | 1 (1.3) | 2 (2.6) | |
| TIMI flow (%) | 0.855 | ||
| TIMI flow 0 | 2 (2.6) | 4 (5.1) | |
| TIMI flow 1 | 1 (1.3) | 1 (1.3) | |
| TIMI flow 2 | 21 (27.3) | 17 (21.8) | |
| TIMI flow 3 | 53 (68.8) | 56 (71.8) | |
| Pre-dilation balloon (mm) | |||
| Balloon length | 20.0±0.0 | 20.2±1.1 | 0.159 |
| Balloon size | 3.31±0.34 | 3.26±0.43 | 0.436 |
| Stent size (mm) | 3.32±0.35 | 3.28±0.43 | 0.567 |
| Stent indication (%) | 0.574 | ||
| elective and suboptimal results | 67 (87.0) | 70 (89.7) | |
| Acute closure and dissection | 10 (13.0) | 8 (10.3) | |
| Stent length (mm) | 17.1±1.1 | 17.5±4.4 | 0.432 |
ACC/AHA, American College of Cardiology/American Heart Association; TIMI, Thrombolysis In Myocardial Infarction.
In-hospital MACE and bleeding events
The PCI success rates were 100% in both groups. During hospitalization, an emergent percutaneous revascularization for myocardial infarction due to acute stent thrombosis occurred in 1 patient of the control stent group (Table 3). No events of major and minor bleeding occurred in either group. The incidences of bleeding complications were not significantly different (Table 4).
Table 3.
In-hospital primary outcomes after stenting
| Group I (n=77) ReoPro®-coated stent | Group II (n=78) Control stent | p | |
|---|---|---|---|
| Success rate (%) | 77 (100) | 78 (100) | 1.00 |
| Primary end points (%) | |||
| Cardiac death | 0 (0.0) | 0 (0.0) | 1.00 |
| Acute myocardial infarction | 0 (0.0) | 1 (1.3) | 0.503 |
| Emergent revascularization | 0 (0.0) | 1 (1.3) | 0.503 |
| Emergent bypass surgery | 0 (0.0) | 0 (0.0) | 1.00 |
Table 4.
Bleeding and hematologic complications after stenting
| Group I ReoPro®-coated stent | Group II Control stent | p | |
|---|---|---|---|
| Major bleeding (%) | 0 (0.0) | 0 (0.0) | 1.00 |
|
| |||
| Minor bleeding (%) | |||
| Vascular access site (hematoma<5 cm) | 1 (1.4) | 1 (1.4) | 1.00 |
| Vascular access site (>5 cm) | 0 (0.0) | 1 (1.4) | 1.00 |
| Gingival bleeding | 0 (0.0) | 0 (0.0) | 1.00 |
| Gastrointestinal bleeding | 0 (0.0) | 0 (0.0) | 1.00 |
| Urinary bleeding | 0 (0.0) | 1 (1.4) | 1.00 |
|
| |||
| Stroke or Intracranial bleeding | 0 (0.0) | 0 (0.0) | 1.00 |
| Thrombocytopenia (<100,000/mm3) | 0 (0.0) | 0 (0.0) | 1.00 |
Long-term angiographic and clinical follow-up results
A 6-month follow-up coronary angiography was performed in 48 (62.3%) and 51 (65.4%) of the ReoPro® and control stent groups, respectively. At the six-month follow-up angiogram, the minimal luminal diameter of the stent segment was significantly greater in the ReoPro® stent group. The late losses were 0.33±0.28 and 0.88±0.41 mm in ReoPro® and control stent groups (p=0.002), and the in-stent restenosis (ISR) developed in 7 (14.6%) and 15 (29.4%) patients in the ReoPro® and 15 control stent groups, respectively (p=0.062) (Table 5, Figure 4).
Table 5.
Quantitative coronary angiographic results
| Group I (n=48) ReoPro®-coated stent | Group II (n=51) Control stent | p | |
|---|---|---|---|
| Follow-up number | 48 (62.3) | 51 (65.4) | 0.740 |
| Follow-up duration (day) | 202.1±61.1 | 218.0±59.9 | 0.194 |
| Restenosis rate (%) | 7/48 (14.6) | 15/51 (29.4) | 0.062 |
| Follow-up diameter stenosis (%) | 16.4±5.8 | 34.3±6.1 | 0.009 |
| Reference diameter (mm) | 2.94±0.38 | 2.98±0.29 | 0.693 |
| Lesion length (mm) | 14.8±1.8 | 13.7±2.0 | 0.671 |
| Late loss (mm) | 0.33±0.28 | 0.88±0.41 | 0.002 |
Figure 4.
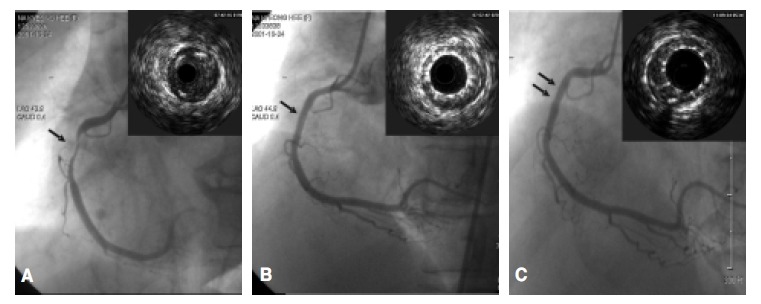
Coronary angiogram (CAG) and intracoronary ultrasonography (IVUS) show critical stenosis in the proximal right coronary artery (RCA), with abundant atheromatous plaques in the lesion before stenting (A). After successful ReoPro®-coated stent placement, the coronary artery revealed good patency, without residual stenosis (B). At the 6-month follow-up CAG and IVUS, no in-stent restenosis in the previously stented RCA was observed (C).
A total of 97% patients were followed up for a period of 14.1±6.9 months. During the follow-up period, two patients in the control stent group suffered a myocardial infarction, but there were no cardiac deaths in either group. TVR was performed in 11 (14.7%) and 7 (9.2%) patients of the control and ReoPro® stent groups, respectively (Table 6).
Table 6.
Long-term clinical results
| Group I (n=76) ReoPro®-coated stent | Group II (n=75) Control stent | p | |
|---|---|---|---|
| Clinical follow-up number (14.1±6.9 months) | 76 (98.7) | 75 (96.2) | 0.620 |
| Total follow-up MACE | |||
| Cardiac death | 0 (0.0) | 0(0.0) | 1.00 |
| Acute myocardial infarction | 0 (0.0) | 2 (2.6) | 0.497 |
| TVR | 7 (9.2) | 11 (14.7) | 0.327 |
| Non-TVR | 2 (2.6) | 5 (6.7) | 0.276 |
| CABG | 0 (0.0) | 0 (0.0) | 1.00 |
MACE, major adverse cardiac events; TLR, Target lesion revascularization; CABG, coronary artery bypass graft
DISCUSSION
This is the first human study on ReoPro®-coated stents for the prevention of ISR. The clinical outcomes with up to 6-months follow-up suggest that the use of ReoPro®-coated stents is safe and feasible. The angiographic follow-up results at six months demonstrated a low amount of NIH and late loss. Nevertheless, neointimal proliferation was not completely abolished by ReoPro®-coated stents. It is possible that drug dosing, absorption and elution kinetics may have influenced our results and limited the anti-proliferative effects of ReoPro®.
A glycoprotein IIb/IIIa receptor blocker is a most potent inhibitor of platelet aggregation, and is activated on platelets by collagen, thrombin and adenosine diphosphate, which act as a receptor for fibrinogen and von Willebrand factor, causing thrombus formation18, 19). In a previous large-scale trial, blockade of this receptor by abciximab, a human-murine chimeric antibody Fab fragment, was shown to reduce the incidence of acute ischemic events by 35% among patients undergoing “high-risk” PCI7). ISR is due to thrombus formation caused by vascular injury after stenting, inflammatory reaction, NIH caused by SMC proliferation and the accumulation of extracellular matrix20, 21). ReoPro® administration decreases inflammatory markers, such as C-reactive protein, interleukin-6 and tumor necrosis factor- after stent implantation22). It may be caused by the interaction between CD11b/CD18 (macrophage-1 receptor), a receptor located on the surface of neutrophils and monocytes, and ReoPro®. The interaction between CD11b/18 and ReoPro® results in the inhibition of cell-to-cell adhesion and the interaction between a cell and the extracellular matrix14–16, 23). The anti-inflammation effects, were not confirmed by the former study using a porcine coronary stent restenosis model24).
Also, ReoPro® has an affinity for vitronectin receptors on SMC, endothelial cells and platelets, with the blockade of this vitronectin receptor by ReoPro® inhibiting the migration and proliferation of SMC after stenting, which may prevent ISR11–13, 18, 19, 25).
ReoPro® was expected to have a clinical effect on the prevention of restenosis after PCI26). There are only a few results of the effects of ReoPro® on the prevention of restenosis post-PCI, but the EPISTENT study27) found that ReoPro® was effective on diabetics. In the ERASER study28), there was a small amount of evidence suggesting an anti-restenosis effect. The effect of a single injection of ReoPro® is considered insufficient for preventing the SMC proliferation and anti-inflammatory actions of neointima formation within the stent.
There have been studies, such as that by Aggarwal et. al29), where a drug-coated stent was made to release the drug to the target lesion. They verified that the monoclonal platelet glycoprotein IIb/IIIa antibody of rabbits could be coated onto a stent using a polymer, and also reported platelet accumulation, increased blood flow and vessel patency. Baron et. al30), using a monoclonal antibody Fab fragment (c7E3 Fab), reported that c7E3 Fab could be coated onto a stent using a polymer, where 53% of the drug still remained on the stent after 12 hours of washing, which was then released. Furthermore, they affirmed that c7E3 Fab was located in both the intima and media, and had an additional effect on SMC.
A drug-eluting stent is a device that releases a single or multiple bioactive agents into the bloodstream, which can subsequently be deposited in or have an affect on the tissues adjacent to the stent. Avoiding systemic toxicity, stent-based local drug-release at the site of vascular injury, via a polymer-coated stent, is an attractive method for achieving an effective local concentration of a drug. The safety and efficacy of such an approach critically depends on the delicate combination of drug, polymer and kinetics of release. In the present study, the effective coating of ReoPro® onto a stent surface was observed by scanning electron microscopy, with the constant release of ReoPro® confirmed by an in vitro release curve. This study has shown that a ReoPro® stent is feasible, produces significant inhibition of NIH and has potential therapeutic benefits in the prevention of stent restenosis.
This is the first study in humans to demonstrate that coated stents are feasible and safe. There were no complications or side effects related to the ReoPro® coated stent procedure compared with the control group. Particularly, no bleeding event was caused by the ReoPro®. Our clinical study has demonstrated that a ReoPro®-coated stent is effective in the prevention of in-stent neointimal hyperplasia, with no acute or subacute stent thromboses, even in patients with acute myocardial infarction and unstable angina with a short course of anti-platelet therapy. These observations suggest that vasculoprotective agents, such as ReoPro®, may provide an alternative approach to anti-proliferative agents in the prevention of ISR and warrant further investigations with a large, randomized multi-center trial.
Footnotes
This study was supported by grants from the Ministry of Health Welfare (01-PJ1-PG3-20500-0016) and Chonnam National University Hospital (CUHRI-U-200125), and was presented at the Scientific Sessions of the 2003 American Heart Association, Orlando, FL.
REFERENCES
- 1.Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, Emanuelsson H, Marco J, Legrand V, Materne P. A comparison of balloon-expandable stent implantation with balloon angioplasty in patients with coronary artery disease. N Eng J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 2.Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, Detre K, Veltri L, Ricci D, Nobuyoshi M. A randomized comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 3.Herdeg C, Oberhoff M, Baumbach A, Blattner A, Axel DI, Schroder S, Heinle H, Karsch KR. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000;35:1969–1976. doi: 10.1016/s0735-1097(00)00614-8. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Teirstein PS, Moses JW, Tripuraneni P, Lansky AJ, Jani S, Wong SC, Fish D, Ellis S, Holmes DR, Kerieakes D, Kuntz RE. Localized intracoronary gamma-radiation therapy to inhibit the recurrence of restenosis after stenting. N Engl J Med. 2001;344:250–256. doi: 10.1056/NEJM200101253440402. [DOI] [PubMed] [Google Scholar]
- 5.Waksman R, Bhargava B, White L, Chan RC, Mehran R, Lansky AJ, Mintz GS, Satler LF, Pichard AD, Leon MB, Kent KK. Intracoronary beta-radiation therapy inhibits recurrence of in-stent restenosis. Circulation. 2000;101:1895–1898. doi: 10.1161/01.cir.101.16.1895. [DOI] [PubMed] [Google Scholar]
- 6.Morice MC, Serruy PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, Molnar F, Falotico R. A randomized comparison of a sirolimuseluting stent with standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 7.EPIC (Evaluation of 7E3 in Preventing Ischemic Complications) Investigators Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high-risk coronary angioplasty. N Engl J Med. 1994;330:956–961. doi: 10.1056/NEJM199404073301402. [DOI] [PubMed] [Google Scholar]
- 8.EPILOG (Evaluation in PTCA to improve Long-Term outcome GP IIb/IIIa Blockade Study Group) Investigators Platelet glycoprotein IIb/IIIa receptor blockade and low-dose heparin during percutaneous coronary revascularization. N Engl J Med. 1997;336:1689–1696. doi: 10.1056/NEJM199706123362401. [DOI] [PubMed] [Google Scholar]
- 9.CAPTURE (C7E3 Fab AntiPlatelet Therapy in Unstable Refractory angina) Investigators Randomized placebo-controlled trial of abciximab before and during coronary intervention in refractory unstable angina. Lancet. 1997;349:1429–1435. [PubMed] [Google Scholar]
- 10.Platelet Receptor Inhibition in ischemic Syndrome Management in Patients Limited by Unstable Sings and Symptoms (PRISM-PLUS) Study Investigators Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non-Q-wave myocardial infarction. N Engl J Med. 1998;338:1488–1497. doi: 10.1056/NEJM199805213382102. [DOI] [PubMed] [Google Scholar]
- 11.Nakada MT, Jordan RE, Knight DM. Abciximab (ReoPro, chimeric 7E3 Fab) cross-specificity with [alpha]V [beta]3 integrin receptors: a potential mechanism for the prevention of restenosis. J Am Coll Cardiol. 1997;29:A243. [Google Scholar]
- 12.Reverter JC, Beguin S, Kessels H, Kuman R, Hemker HC, Coller BS. Inhibition of platelet-mediated, tissue factor-induced thrombin generation by the mouse/human chimeric 7E3 antibody potential implications for the effect of c7E3 Fab treatment of an acute thrombosis and “clinical restenosis.”. J Clin Invest. 1996;98:863–874. doi: 10.1172/JCI118859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shappel SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD8) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 14.Simon DI, Xu H, Ortelpp S, Rogers C, Rao NK. 7E3 monoclonal antibody directed against the platelet glycoprotein IIb/IIIa cross-reacts with the leukocyte integrin Mac-1 and blocks adhesion to fibrinogen and ICAM-1. Arterioscler Thromb Vasc Biol. 1997;17:528–535. doi: 10.1161/01.atv.17.3.528. [DOI] [PubMed] [Google Scholar]
- 15.Mickelson JK, Ali MN, Kleiman NS, Lakkis NM, Chow TW, Hughes BJ, Smith CW. Chimeric 7E3 Fab (ReoPro) decreases detectable CD IIb on neutrophils from patients undergoing coronary angioplasty. J Am Coll Cardiol. 1999;33:97–106. doi: 10.1016/s0735-1097(98)00532-4. [DOI] [PubMed] [Google Scholar]
- 16.Lefkovits J, Topol EJ. Platelet glycoprotein IIb/IIIa receptor antagonists in coronary artery disease. Eur Heart J. 1996;17:9–18. doi: 10.1093/oxfordjournals.eurheartj.a014698. [DOI] [PubMed] [Google Scholar]
- 17.Kang KT, Jeong MH, Kim NH, Lee SH, Rhew JY, Park JC, Lee SU, Kim KH, Ahn YK, Cho JG, Park JC, Cho DL, Kang JC. The inhibitory effect of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on porcine coronary stent restenosis. Korean Med J. 2001;60:314–323. [Google Scholar]
- 18.Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, 3rd, Loop FD, Peterson KL, Reeves TJ, Williams DO, Winters WL., Jr Guidelines for percutaneous transluminal coronary angioplasty: a report of the American College of Cardiology/American Heart Association Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Percutaneous Transluminal Coronary Angioplasty) Circulation. 1988;78:486–502. doi: 10.1161/01.cir.78.2.486. [DOI] [PubMed] [Google Scholar]
- 19.Weitz JI, Bates SM. Beyond heparin and aspirin: new treatments for unstable angina and non-Q wave myocardial infarction. Arch Intern Med. 2000;160:749–758. doi: 10.1001/archinte.160.6.749. [DOI] [PubMed] [Google Scholar]
- 20.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 21.Virmani R, Farb A. Pathology of in-stent restenosis. Curr Opin Lipidol. 1999;10:499–506. doi: 10.1097/00041433-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Lincoff AM, Kereiakes DJ, Mascelli MA, Deckelbaum LI, Barnathan ES, Patel KK, Frederick B, Nakada MT, Topol EJ. Abciximab suppresses the rise in levels of circulating inflammatory markers after percutaneous coronary revascularization. Circulation. 2001;104:163–167. doi: 10.1161/01.cir.104.2.163. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz M, Nordt T, Bode C, Peter K. The GP IIb/IIIa inhibitor abciximab (c7E3) inhibits the binding of various ligands to the leukocyte integrin Mac-1 (CD11b/CD18, alpha(M)beta(2)) Thromb Res. 2002;107:121–128. doi: 10.1016/s0049-3848(02)00207-4. [DOI] [PubMed] [Google Scholar]
- 24.Park OY, Jeong MH, Kim JH, Kim W, Lim SY, Sim DS, Yang BR, Lee SH, Lee SH, Hong YJ, Kim JH, Park WS, Ahn YG, Park JT, Cho JG, Cho DR, Park JC, Kang JC. The inhibitory effect of platelet glycoprotein IIb/IIIa receptor blocker-coated stent on porcine coronary stent restenosis. Korean Circ J. 2003;33:439–445. [Google Scholar]
- 25.Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions. Circulation. 1989;80:198–205. doi: 10.1161/01.cir.80.1.198. [DOI] [PubMed] [Google Scholar]
- 26.Blindt R, Bosserhoff AK, Zeiffer U, Krott N, Hanrath P, vom Dahl J. Abciximab inhibits the migration and invasion potential of human coronary artery smooth muscle cells. J Mol Cell Cardiol. 2000;32:2195–2206. doi: 10.1006/jmcc.2000.1245. [DOI] [PubMed] [Google Scholar]
- 27.EPISTENT Investigators Randomized placebo-controlled and balloon angioplasty-controlled trial to assess safety of coronary stenting with use of platelet glycoprotein IIb/IIIa blockade. Lancet. 1998;352:87–92. doi: 10.1016/s0140-6736(98)06113-3. [DOI] [PubMed] [Google Scholar]
- 28.ERASER (Evaluation of Reo-Pro And Stenting to Eliminate Restenosis) Investigators Acute platelet inhibition with abciximab does not reduce in-stent restenosis. Circulation. 1999;100:799–806. doi: 10.1161/01.cir.100.8.799. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal RK, Ireland DC, Azrin MA, Ezekowitz MD, de Bono DP, Gershlick AH. Antithrombotic potential of polymer-coated stents eluting platelet glycoprotein IIb/IIIa receptor antibody. Circulation. 1996;94:3311–3317. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- 30.Baron JH, Gershlick AH, Hogrefe K, Armstrong J, Holt CM, Aggarwal RK, Azrin M, Ezekowitz M, de Bono DP. In vitro evaluation of c7E3-Fab (ReoPro) eluting polymer-coated coronary stents. Cardiovasc Res. 2000;46:585–594. doi: 10.1016/s0008-6363(00)00042-0. [DOI] [PubMed] [Google Scholar]


