Abstract
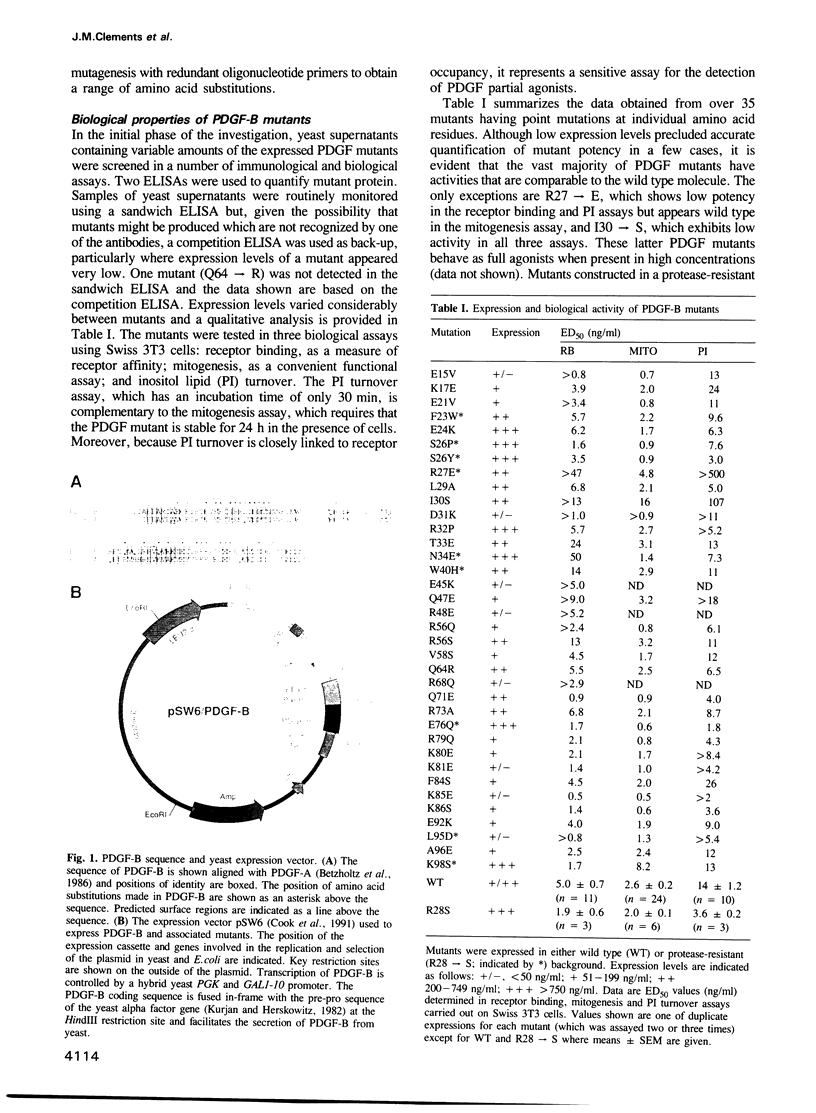
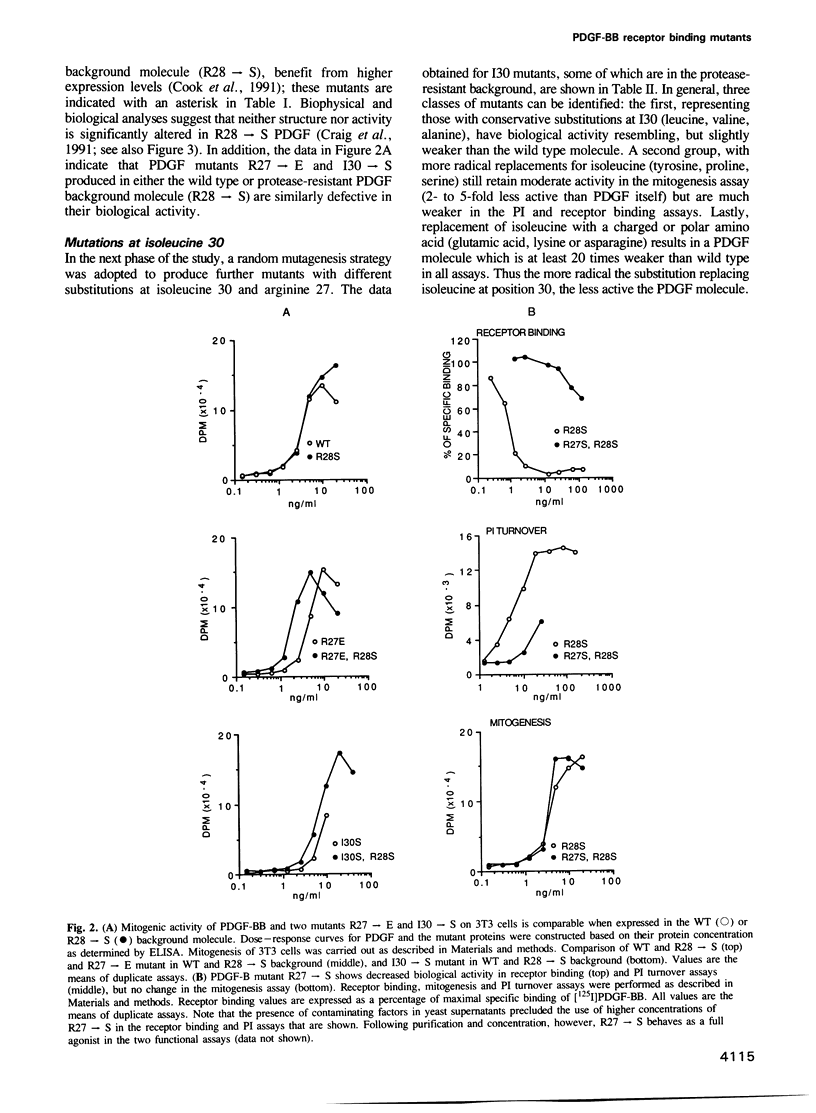
PDGF may be involved in the pathogenesis of a variety of disorders including atherosclerosis and certain types of cancer. There is currently little understanding of the molecular structure of PDGF and of the critical amino acid residues involved in receptor binding and cell activation. Two such PDGF-B chain residues, arginine 27 and isoleucine 30, have been identified by a site-directed mutagenesis programme. Substitutions in these positions can lead to PDGF mutants defective in both receptor affinity and cell activation as judged by displacement of [125I]PDGF-BB, mitogenic assay and inositol lipid turnover. Circular dichroism and fluorescence spectroscopy show that such mutations do not disrupt the structure of PDGF.
Full text
PDF
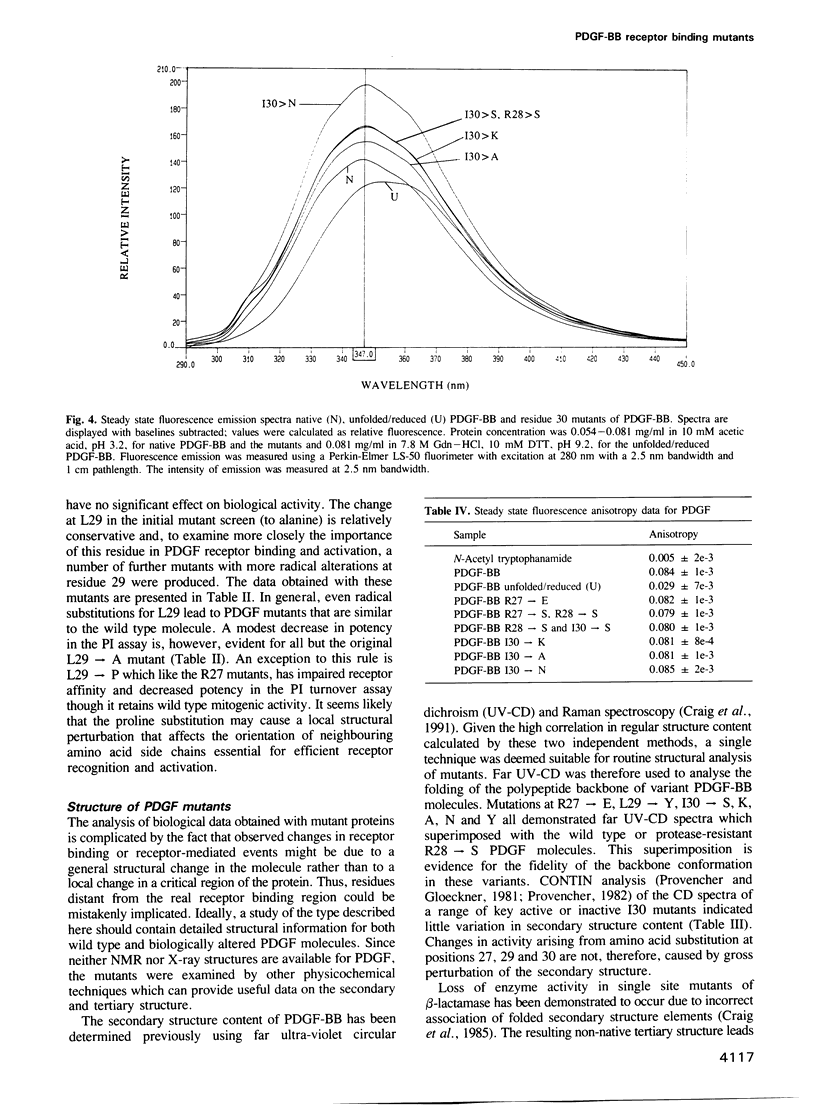



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bone E. A., Fretten P., Palmer S., Kirk C. J., Michell R. H. Rapid accumulation of inositol phosphates in isolated rat superior cervical sympathetic ganglia exposed to V1-vasopressin and muscarinic cholinergic stimuli. Biochem J. 1984 Aug 1;221(3):803–811. doi: 10.1042/bj2210803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Hollecker M., Creighton T. E., Pain R. H. Single amino acid mutations block a late step in the folding of beta-lactamase from Staphylococcus aureus. J Mol Biol. 1985 Oct 20;185(4):681–687. doi: 10.1016/0022-2836(85)90053-1. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler D. A., Montelione G. T., Niyogi S. K. Human epidermal growth factor. Distinct roles of tyrosine 37 and arginine 41 in receptor binding as determined by site-directed mutagenesis and nuclear magnetic resonance spectroscopy. FEBS Lett. 1990 Oct 1;271(1-2):47–50. doi: 10.1016/0014-5793(90)80368-s. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Gehrke L., Jobling S. A., Paik L. S., McDonald B., Rosenwasser L. J., Auron P. E. A point mutation uncouples human interleukin-1 beta biological activity and receptor binding. J Biol Chem. 1990 Apr 15;265(11):5922–5925. [PubMed] [Google Scholar]
- Giese N., LaRochelle W. J., May-Siroff M., Robbins K. C., Aaronson S. A. A small v-sis/platelet-derived growth factor (PDGF) B-protein domain in which subtle conformational changes abrogate PDGF receptor interaction and transforming activity. Mol Cell Biol. 1990 Oct;10(10):5496–5501. doi: 10.1128/mcb.10.10.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammacher A., Mellström K., Heldin C. H., Westermark B. Isoform-specific induction of actin reorganization by platelet-derived growth factor suggests that the functionally active receptor is a dimer. EMBO J. 1989 Sep;8(9):2489–2495. doi: 10.1002/j.1460-2075.1989.tb08385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factors: a family of isoforms that bind to two distinct receptors. Br Med Bull. 1989 Apr;45(2):453–464. doi: 10.1093/oxfordjournals.bmb.a072334. [DOI] [PubMed] [Google Scholar]
- Hosang M., Rouge M., Wipf B., Eggimann B., Kaufmann F., Hunziker W. Both homodimeric isoforms of PDGF (AA and BB) have mitogenic and chemotactic activity and stimulate phosphoinositol turnover. J Cell Physiol. 1989 Sep;140(3):558–564. doi: 10.1002/jcp.1041400322. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khym J. X. An analytical system for rapid separation of tissue nucleotides at low pressures on conventional anion exchangers. Clin Chem. 1975 Aug;21(9):1245–1252. [PubMed] [Google Scholar]
- King C. R., Giese N. A., Robbins K. C., Aaronson S. A. In vitro mutagenesis of the v-sis transforming gene defines functional domains of its growth factor-related product. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5295–5299. doi: 10.1073/pnas.82.16.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- LaRochelle W. J., Giese N., May-Siroff M., Robbins K. C., Aaronson S. A. Molecular localization of the transforming and secretory properties of PDGF A and PDGF B. Science. 1990 Jun 22;248(4962):1541–1544. doi: 10.1126/science.2163109. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Parker J. M., Guo D., Hodges R. S. New hydrophilicity scale derived from high-performance liquid chromatography peptide retention data: correlation of predicted surface residues with antigenicity and X-ray-derived accessible sites. Biochemistry. 1986 Sep 23;25(19):5425–5432. doi: 10.1021/bi00367a013. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Raines E. W., Ross R. Purification of human platelet-derived growth factor. Methods Enzymol. 1985;109:749–773. doi: 10.1016/0076-6879(85)09128-5. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Hart C. E., Phillips P. E., Forstrom J. W., Ross R., Murray M. J., Bowen-Pope D. F. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989 May 25;264(15):8771–8778. [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severinsson L., Ek B., Mellström K., Claesson-Welsh L., Heldin C. H. Deletion of the kinase insert sequence of the platelet-derived growth factor beta-receptor affects receptor kinase activity and signal transduction. Mol Cell Biol. 1990 Feb;10(2):801–809. doi: 10.1128/mcb.10.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund M., Hedin U., Sejersen T., Heldin C. H., Thyberg J. Arterial smooth muscle cells express platelet-derived growth factor (PDGF) A chain mRNA, secrete a PDGF-like mitogen, and bind exogenous PDGF in a phenotype- and growth state-dependent manner. J Cell Biol. 1988 Feb;106(2):403–413. doi: 10.1083/jcb.106.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. W. Protein secondary structure analysis using Raman amide I and amide III spectra. Methods Enzymol. 1986;130:311–331. doi: 10.1016/0076-6879(86)30016-8. [DOI] [PubMed] [Google Scholar]
- Zurawski S. M., Imler J. L., Zurawski G. Partial agonist/antagonist mouse interleukin-2 proteins indicate that a third component of the receptor complex functions in signal transduction. EMBO J. 1990 Dec;9(12):3899–3905. doi: 10.1002/j.1460-2075.1990.tb07610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Asua L. J., Rozengurt E., Dulbecco R. Kinetics of early changes in phosphate and uridine transport and cyclic AMP levels stimulated by serum in density-inhibited 3T3 cells. Proc Natl Acad Sci U S A. 1974 Jan;71(1):96–98. doi: 10.1073/pnas.71.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]




