Abstract
Background :
Airway hyperresponsiveness (AHR) to direct stimuli, such as methacholine (MCh), is observed not only in asthma but other diseases. AHR to indirect stimuli is suggested to be more specific for asthma. The purpose of this study was to determine whether asthmatic airway inflammation is more closely related to AHR to hypertonic saline (HS), an indirect stimulus, than to MCh.
Methods :
Sixty-four consecutive adult patients with suspected asthma (45 asthma and 19 non-asthma) performed a combined bronchial challenge and sputum induction with 4.5% saline, and MCh challenge on the next day.
Results :
Both HS-PD15 and MCh-PC20 were significantly lower in asthma patients than in non-asthma patients. However, the sensitivity / specificity for asthma was 48.9%/100%, respectively, in the HS test and 82.2% / 84.2%, respectively, in the MCh test. There was a significant relationship between HS-PD15 and MCh-PC20 and only 52.9% of patients with MCh-PC20≤4 mg/mL showed HS-AHR, but 4 patients with HS-AHR showed MCh-PC20>4 mg/mL. There were significant correlations between both HS-PD15 and MCh-PC20 and FEV1, or sputum eosinophils, but FEV1 was more closely related to MCh-PC20 (r=0.478, p<0.01) than to HS-PD15 (r=0.278, p<0.05), and sputum eosinophils were more closely related to HS-PD15 (r=−0.324, p<0.01) than to MCh-PC20 (r=−0.317, p<0.05). Moreover, the IL-5 level (r=−0.285, p<0.05) and IFN-γ/IL-5 ratio (r=0.293, p<0.05) in sputum were significantly related to HS-PD15, but not to MCh-PC20.
Conclusion :
HS-AHR may reflect allergic asthmatic airway inflammation more closely than MCh-AHR.
Keywords: Asthma, Inflammation, Methacholine, Saline Solution, Hypertonic
INTRODUCTION
Asthma is a chronic inflammatory disorder of the airways that is associated with airway hyperresponsiveness (AHR). With improvements in the method of examining sputum for inflammatory indices, direct noninvasive assessment of airway inflammation in asthma is now possible through the examination of hypertonic saline (HS)-induced sputum. HS provocation testing can also be used to measure AHR. A combined HS challenge and sputum induction procedure has been shown to be a useful means of comparing AHR and airway inflammation simultaneously1). However, inhalation of a direct stimulus, such as histamine or methacholine (MCh), is generally used to measure AHR. Although the severity of MCh-AHR is correlated with the number of inflammatory cells, such as mast cells, eosinophils and neutrophils in the airways of asthmatics, MCh-AHR is not very specific for a diagnosis of asthma because it is also seen in patients with other diseases, such as chronic obstructive pulmonary disease, cystic fibrosis and irritable bowel disease2). In contrast, naturally occurring bronchoconstriction in asthmatics is generally caused by indirect stimuli that act by releasing pharmacologically active substances from inflammatory cells or sensory neurons, and it has been suggested that AHR to these agents might be more specific for a diagnosis of asthma.
Indirect stimuli include exercise, isocapnic hyperventilation, hyper/hypotonic aerosols, metabisulphite/SO2, propranolol, adenosine, bradykinin and tachykinins3). Previous studies have shown that adenosine-AHR is more specific for a diagnosis of asthma4) and is better at predicting sputum eosinophilia5), corticosteroid sensitivity6) and response to hypoallergenic environmental therapy7) in asthma. However, few studies have investigated how the airway response to inhaled HS, as compared with MCh, reflects the underlying airway inflammatory processes. This study investigated whether HS-AHR is more closely associated with airway inflammation than is MCh-AHR.
MATERIALS AND METHODS
Subjects
Sixty-four consecutive patients with suspected asthma, who had all undergone both a combined HS challenge and sputum induction procedure, and a MCh challenge at Chonnam National University Hospital, participated in this study. The diagnosis of asthma was based on a clinical history of episodic wheezing, breathlessness, chest tightness or cough, and an improvement in forced expiratory volume in one second (FEV1) of ≥12% and at least 200 mL after anti-asthma treatment for two weeks. The remaining patients were considered as non-asthma, although some of them may have subclinical or mild asthma. The Institutional Review Board of Chonnam National University Hospital approved the study. All patients were informed of the experimental procedures and provided written informed consent.
Study design
On the first study day, baseline spirometry, allergy skin-prick tests and routine laboratory tests were performed after history taking and physical examination. Airway responsiveness to HS was measured, and then two puffs of salbutamol were inhaled and sputum induction using HS was continued. On the next day, at the same time of day as the HS-AHR, a MCh-AHR test was performed. The subjects were instructed in the use of a metered-dose inhaler through a valved holding chamber, and they were asked to complete a PEF diary. Anti-asthma medications, including fluticasone (1,000 μg/day) and prednisolone (30 mg/day for one week), were prescribed and a clinical evaluation was conducted at least two weeks later.
Allergy skin tests
Allergy skin-prick tests were carried out using 55 common allergen extracts with histamine and saline solution as positive and negative controls, respectively. After 15 minutes, a wheal more than 3 mm larger than that seen in the negative control was considered a positive prick test result, and subjects with at least one positive test result were considered atopic.
Airway responsiveness
Airway responsiveness to hypertonic saline solution was assessed using the method of Iredale et al.8) with 4.5% NaCl aerosol delivered from an ultrasonic nebulizer (Ultra-neb 2000, DeVilbiss, PA), through a Hans Rudolph 2700 two-way nonrebreathing valve box with a rubber mouthpiece and nose clips. The subjects inhaled 4.5% NaCl for doubling time periods (0.5, 1.0, 2.0, 4.0 and 8.0 minutes). FEV1 was measured in triplicate before the test (baseline), and in duplicate (30 and 90 sec) after each inhalation period with a spirometer (Spiro Analyzer ST-250, Fukuda Sangyo, Tokyo, Japan). The next challenge period followed within three minutes of the end of the previous one. If the FEV1 fell by less than 10%, the exposure time was doubled. If the fall in FEV1 was between 10 and 15%, the exposure time was kept the same. If the fall in FEV1 exceeded 15%, or after the maximal dose of solution had been delivered to the inspiratory port of the valve, the challenge was stopped. A dose-response curve was constructed by plotting the % change in FEV1 against the cumulative dose of aerosol delivered, expressed in mL on a log scale for each inhalation period. The provocative dose of HS causing a 15% fall in FEV1 (PD15) was calculated by linear interpolation of the log-dose-response curve. The degree of airway responsiveness to HS was categorized as severe AHR if PD15 was <2 mL, mild to moderate AHR if PD15 was ≥2 but ≤20 mL, and normal if PD15 was >20 mL. Patients not responding to the highest dose of HS were assigned a value twice the highest dose applied. Another index of airway responsiveness, the dose response ratio (DRR), was calculated by dividing the maximal % fall in FEV1 by the dose of saline required to produce it9).
The MCh challenge test followed a standardized tidal breathing method10). MCh in isotonic saline was aerosolized at room temperature in a DeVilbiss 646 nebulizer (DeVilbiss Co., Somerset, PA; output 0.13 mL/min). Dilution increments were 0.075, 0.15, 0.31, 0.62, 1.25, 2.5, 5.0, 10 and 25 mg/mL. The aerosols were inhaled by tidal breathing over 2 min at 5-min intervals, through the mouth with the nose clipped. The challenge test was discontinued if FEV1 dropped 20% or more from the baseline or if the maximal concentration of MCh was administered. The provocation concentration of MCh resulting in a 20% fall in FEV1 (PC20) was calculated by linear interpolation of the log-dose-response curve. The degree of airway responsiveness to MCh was categorized as moderate to severe AHR if PC20 was < 1 mg/mL, mild AHR if PC20 was ≥1 but ≤4 mg/mL, borderline AHR if PC20 was >4 but ≤16 mg/mL, and normal if PC20 was >16 mg/mL. Patients not responding to 25 mg/mL of MCh or HS were assigned a PC20 of 50 mg/mL. MCh-DRR was calculated by dividing the maximal % fall in FEV1 by the dose of MCh required to produce it.
Sputum induction and processing
The subjects were encouraged to expectorate into a sterile container after each dose of HS solution. Immediately after the HS challenge, the patients inhaled 200 μg of salbutamol and additional 4.5% saline until sputum was induced. Sputum was processed as previously described11). Briefly, opaque sputum portions were selected and digested with 0.1% dithiothreitol (Sigma, MO), and filtered through 53-μm nylon gauze (Spectrum Laboratories Inc., CA). Total cell counts were determined and cytospin (CF-120, Japan) slides were prepared. Differential cell counts were expressed as the percentage of non-squamous cells in a sample of 300 cells on each of two slides stained with Diff-Quik. The interferon (IFN)-γ, interleukin (IL)-4 and IL-5 levels in sputum were measured using commercially available ELISA kits (IFN-γ: Endogen, USA; IL-4 & IL-5: Quantikine HS, R&D Systems, MN).
Statistical analysis
Variables were summarized as the mean±SEM. All calculations of PD15 or PC20 were performed after log transformation. Data were compared using an unpaired Student’s t-test. Associations between variables were examined with the Spearman rank correlation coefficient. A value of p<0.05 was considered statistically significant.
RESULTS
Forty-five out of 64 patients with suspected asthma fulfilled the diagnostic criteria for asthma. Of the remaining non-asthma patients, 12 had rhinitis with or without sinusitis, 4 had gastroesophageal reflux disease, 1 had reaction to angiotensin converting enzyme inhibitor and 2 had an unknown etiology. The asthma group had more males and was younger than the non-asthma group. The baseline lung function was significantly lower, and the serum total IgE level and the proportion of atopy were significantly higher in the asthma group than in the non-asthma group (Table 1).
Table 1.
Comparison of Characteristics of Subjects between Asthma and Non-asthma
| Variables | Asthma | Non-asthma | p value |
|---|---|---|---|
| FEV1 (% predicted) | 86.2±1.90 | 102.3±3.36 | 0.000 |
| FEV1/FVC (%) | 78.1±1.48 | 82.0±2.04 | 0.149 |
| Blood eosinophils (/μL) | 394.3±41.84 | 280.5±102.75 | 0.223 |
| Log IgE | 2.4±0.08 | 2.0±0.12 | 0.002 |
| (Geometric mean, IU/mL) | (526.1) | (161.8) | |
| Atopy (%) | 76.3% | 26.3% | 0.000 |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
There was a significantly higher eosinophil differential count in the sputum of patients with asthma than in that of non-asthma patients (p<0.05). However, the IL-4, IL-5, IFN-γ levels and IFN-γ/IL-5 ratio in the sputum of patients with asthma were not significantly different from those in the non-asthma group (Table 2).
Table 2.
Comparison of Measurements between Asthma and Non-asthma
| Variables | Asthma | Non-asthma | p value |
|---|---|---|---|
| SPUTUM | |||
| Eosinophils (%) | 9.7±2.40 | 2.7±1.30 | 0.013 |
| Neutrophils (%) | 14.9±3.22 | 13.9±5.66 | 0.879 |
| Lymphocytes (%) | 2.4±0.54 | 1.0±0.45 | 0.064 |
| Macrophages (%) | 72.2±3.98 | 80.1±4.01 | 0.166 |
| Epithelial cells (%) | 0.6±0.29 | 1.3±0.52 | 0.186 |
| IL-5 (pg/mL) | 6.4±0.20 | 5.9±0.55 | 0.311 |
| IL-4 (pg/mL) | 0.4±0.17 | 0.2±0.01 | 0.418 |
| IFN-γ (pg/mL) | 6.8±0.36 | 6.8±0.48 | 0.935 |
| IFN-γ/IL-5 | 1.1±0.04 | 1.2±0.08 | 0.083 |
| AHR | |||
| HS-Log PD15 | 1.2±0.08 | 1.7±0.02 | 0.000 |
| HS-DRR | 2.1±0.56 | 0.14±0.04 | 0.001 |
| HS-PD15 | 0.001 | ||
| 2–20 mL (%) | 20/45 (44.4%) | 0% | |
| ≤2 mL (%) | 2/45 (4.4%) | 0% | |
| MCh-Log PC20 | 0.95±0.14 | 1.63±0.10 | 0.000 |
| (Geometric mean MCh-PC20) | (8.9 mg/mL) | (42.3 mg/mL) | |
| MCh-DRR | 93.0±39.64 | 5.3±4.28 | 0.033 |
| MCh-PC20 | 0.000 | ||
| 4–16 mg/mL (%) | 5/45 (11.1%) | 1/19 (5.3%) | |
| 1–4 mg/mL (%) | 12/45 (26.7%) | 1/19 (5.3%) | |
| ≤1 mg/mL (%) | 20/45 (44.4%) | 1/19 (5.3%) | |
| Sputum Eosinophil | 14/45 (31.1%) | 1/19 (5.3%) | 0.026 |
| ≥4% + MCh-PC20 | |||
| ≤16 mg/mL |
IL, interleukin; IFN, interferon; AHR, airway hyperresponsiveness; HS, hypertonic (4.5%) saline; PD, provocative dose; DRR, dose-response ratio; MCh, methacholine; PC, provocative concentration.
HS-AHR was present only in patients with asthma, but only in 22 (48.9%). Compared to non-asthma patients, patients with asthma showed a significantly lower HS-PD15 (p< 0.001) and a significantly higher HS-DRR (p<0.005). Patients with asthma also had a significantly lower MCh-PC20 (p< 0.001) and a significantly higher MCh-DRR (p<0.05) (Table 2, Figure 1). Thirty-seven out of 45 (82.2%) patients with asthma, but only 3/19 (15.9%) non-asthma patients, also showed MCh-PC20 ≤ 16 mg/mL. The sensitivity, specificity and positive and negative predictive values of the tests for asthma were 48.9%, 100%, 100% and 45.2% in the HS test, and 82.2%, 84.2%, 92.5% and 66.7% in the MCh test, respectively. At the cut-off value of MCh-PC20 ≤ 4 mg/mL, the sensitivity, specificity and positive and negative predictive values of the test for asthma were 71.1%, 89.3%, 94.1% and 56.7%, respectively. Both sputum eosinophils ≥4% and MCh -PC20 ≤ 16 mg/mL were present in 14/45 (31.1%) patients with asthma and in 1/19 (5.3%) non-asthma patients (p<0.05). The specificity and positive and negative predictive values of the test for asthma were 94.7%, 93.3% and 36.7%, respectively.
Figure 1.
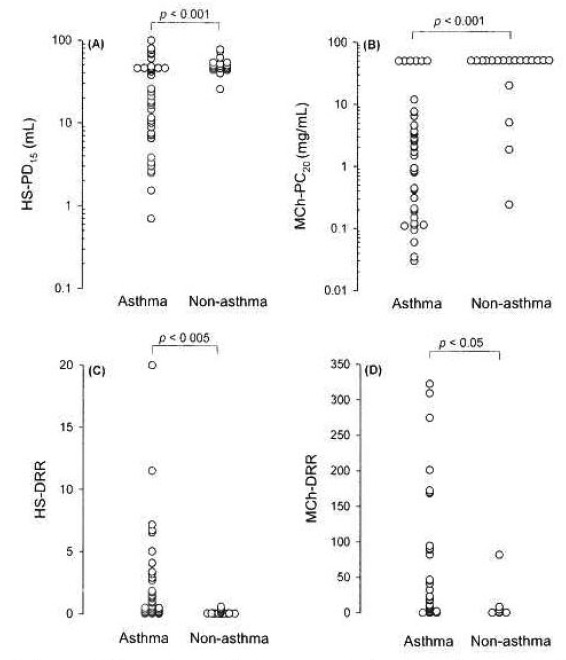
Comparisons of airway sensitivity (A and B) and of airway reactivity (C and D) between asthma and non-asthma in patients with suspected asthma. HS, hypertonic (4.5%) saline; MCh, methacholine; DRR, dose-response ratio.
There were significant relationships between HS-PD15 and MCh-PC20 (r=0.600, p<0.01), and between HS-DRR and MCh-DRR (r=0.576, p<0.01). Only 18 out of 34 (52.9%) patients with MCh-PC20≤4 mg/mL showed HS-AHR, but also only 18 out of 22 (81.8%) patients with HS-AHR showed MCh-PC20≤4 mg/mL.
Significant correlations between both HS-PD15 and MCh-PC20 and FEV1 or sputum eosinophils were found, but FEV1 was more closely related to MCh-PC20 (r=0.478, p<0.01) than to HS-PD15 (r=0.278, p<0.05), and sputum eosinophils were more closely related to HS-PD15 (r=−0.324, p<0.01) than to MCh-PC20 (r=−0.317, p<0.05) (Figure 2). Moreover, the IL-5 level (r=−0.285, p<0.05) and IFN-γ/IL-5 ratio (r=0.293, p<0.05) in sputum were significantly related to the HS-PD15, but not to the MCh-PC20 (Table 3).
Figure 2.
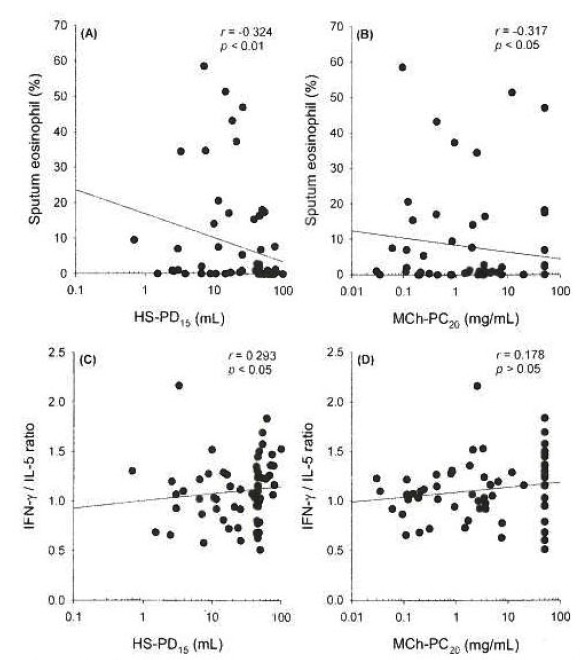
Relationship between hypertonic saline-PD15 or methacholine-PC20 and sputum eosinophils (%) (A and B) or IFN-γ/IL-5 ratio (C and D).
Table 3.
Correlations (rs) of lung function measurements with inflammatory markers in sputum
| FEV1 | HS-PD15 | HS-DRR | MCh-PC20 | MCh-DRR | |
|---|---|---|---|---|---|
| Eosinophil | −0.815 | −0.324** | 0.218 | −0.317* | 0.323* |
| Neutrophil | −0.278* | 0.016 | 0.011 | −0.093 | 0.117 |
| Lymphocyte | −0.339** | 0.015 | 0.061 | −0.350** | 0.334** |
| Macrophage | 0.232 | 0.218 | −0.191 | 0.241 | −0.275* |
| IL-5 | −0.077 | −0.285* | 0.345* | −0.223 | 0.222 |
| IL-4 | 0.003 | −0.097 | 0.037 | −0.062 | 0.129 |
| IFN-γ | 0.209 | 0.123 | 0.018 | 0.015 | −0.073 |
| FN-γ/IL-5 | 0.252* | 0.293* | −0.215 | 0.178 | −0.232 |
rs, Spearman rank correlation coefficient.
p<0.05,
p<0.01
DISCUSSION
In this study, the well-known characteristic features of asthma - i.e., sputum eosinophilia, HS-AHR and MCh-AHR -clearly differentiated asthma from non-asthma. Moreover, the specificity and positive predictive value of the HS-AHR test for asthma were higher than those of the MCh-AHR test. This is in accordance with the hypothesis that AHR to indirect stimuli, which acts by releasing pharmacologically active substances from inflammatory cells or sensory neurons, might be more specific for a diagnosis of asthma.
Human lung mast cells release histamine via a non-IgE-mediated pathway following a hyperosmolar stimulus in vitro12), and sensory nerves in dogs are stimulated by injection of HS into a lobar bronchus13). Rather than acting directly on effector cells (airway smooth muscle cells, bronchial endothelial cells, mucus producing cells) to limit airflow, a change in osmolarity in the airways might cause the endogenous release of substances that cause airflow limitation, and the presence of these substances might be associated with inflammation. Therefore, the airway responses to osmotic challenge are reported to be specific for asthma14, 15)
There was a significant relationship between HS-AHR and MCh-AHR. At the cut-off value of MCh-PC20≤4 mg/mL, the specificity and positive predictive value for asthma were increased at the expense of a reduced sensitivity for asthma. Therefore, the high specificity of HS-AHR for asthma might simply result from the low sensitivity of HS-AHR for asthma. Of course, the patients responsive to MCh were not necessarily responsive to HS. However, the patients responsive to HS also did not always demonstrate MCh-AHR, which is consistent with the results of Anderson et al.16). Moreover, even at the low cut-off value, the specificity of the MCh test did not reach that of the HS test.
In this study, sputum eosinophil counts were more closely related to HS-AHR than to MCh-AHR, although the relationship between eosinophils and MCh-AHR was significant. It has been shown that HS-AHR is strongly associated with higher levels of sputum eosinophils and sputum mast cells17). In addition, acute treatment with sodium cromoglycate18) or nedocromil sodium19) and long-term treatment with aerosol steroids17) reduce the airway responses to osmotic stimuli. However, the severity of MCh-AHR is also correlated with the number of inflammatory cells in the airways of asthmatics and is reduced with nedocromil sodium or inhaled glucocorticoids2). Nonetheless, there is a dichotomy between HS-AHR and MCh-AHR with respect to airway inflammation, since adenosine-AHR is better at predicting sputum eosinophilia5), corticosteroid sensitivity6) and the response to hypoallergenic environmental therapy7) in asthma. Actually, Gibson et al.20) demonstrated that sputum eosinophils are correlated with airway responsiveness to HS, but not to MCh, in corticosteroid-treated asthma.
It has been shown that the airway caliber is an important determinant of MCh-AHR5, 21, 22), so MCh is a more sensitive index of airway responsiveness in patients with severe asthma, in whom airway wall remodeling in addition to airway inflammation plays an important role23). Ichinose et al.21) showed that the baseline MCh-AHR can be predicted from the airway caliber, while the reversible component of MCh-AHR appeared to be associated with inflammatory parameters in sputum. We also showed that FEV1 was more closely related to MCh-PC20 than to HS-PD15, although the relationship between FEV1 and HS-PD15 was significant.
In addition, the IL-5 level and IFN-γ/IL-5 ratio in sputum were significantly related to HS-PD15, but not to MCh-PC20, in this study. Since IL-5 is the main cytokine causing eosinophilia and asthma is a Th2 cytokine-associated allergic disease (i.e., the IFN-γ/IL-5 ratio is decreased), these results suggest that allergic airway inflammation is related to HS-AHR. De Meer et al.22) also showed that airway responsiveness to adenosine was related to airway allergy.
Although the MCh challenge was very specific for asthma when it was interpreted together with sputum inflammatory markers, combining the HS challenge with sputum induction seems to be a useful means of comparing AHR and airway inflammation simultaneously. Unfortunately, the combined study has a reduced success rate in obtaining an adequate sputum sample, as well as increased side effects1). Taken together, examination of HS-AHR may be preferred as a way of confirming a diagnosis of asthma, because of high specificity, and for monitoring patients with asthma, primarily because it provides a way of inferring parallel changes in airway inflammation. Further studies are needed to determine whether reduction of airway wall inflammation in asthma is also more closely associated with a reduction in HS-AHR than with a reduction in MCh-AHR.
Acknowledgments
The authors would like to thank Miss Young-Ah Koh for her excellent laboratory assistance.
Footnotes
This study was financially supported by the Chonnam National University in the program, 2001
REFERENCES
- 1.Jones PD, Hankin R, Simpson J, Gibson PG, Henry RL. The tolerability, safety and success of sputum induction and combined hypertonic saline challenge in children. Am J Respir Crit Care Med. 2001;164:1146–1149. doi: 10.1164/ajrccm.164.7.2103015. [DOI] [PubMed] [Google Scholar]
- 2.O’Byrne PM. Airway hyperreponsiveness. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, editors. Allergy Principles & Practice. 5th ed. Toronto: Mosby; 1998. pp. 859–866. [Google Scholar]
- 3.Van Schoor J, Joos GF, Pauwels RA. Indirect bronchial hyperresponsiveness in asthma: mechanisms, pharmacology and implications for clinical research. Eur Respir J. 2000;16:514–533. doi: 10.1034/j.1399-3003.2000.016003514.x. [DOI] [PubMed] [Google Scholar]
- 4.Avital A, Springer C, Bar-Yishay E, Godfrey S. Adenosine, methacholine and exercise challenges in children with asthma or paediatric chronic obstructive pulmonary disease. Thorax. 1995;50:511–516. doi: 10.1136/thx.50.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van den Berge M, Meijer RJ, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, Postma DS. PC20 adenosine 5′-mono-phosphate is more closely associated with airway inflammation than PC20 methacholine. Am J Respir Crit Care Med. 2001;163:1546–1550. doi: 10.1164/ajrccm.163.7.2010145. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berge M, Kerstjens HA, Meijer RJ, de Reus DM, Koeter GH, Kauffman HF, Postma DS. Corticosteroid-induced improvement in the PC20 of adenosine monophosphate is more closely associated with reduction in airway inflammation than improvement in the PC20 of methacholine. Am J Respir Crit Care Med. 2001;164:1127–1132. doi: 10.1164/ajrccm.164.7.2102135. [DOI] [PubMed] [Google Scholar]
- 7.Benckhuijsen J, van den Bos JW, van Velzen E, de Bruijn R, Aalbers R. Differences in the effect of allergen avoidance on bronchial hyperresponsiveness as measured by methacholine, adenosine 5′-monophosphate and exercise in asthmatic children. Pediatr Pulmonol. 1996;22:147–153. doi: 10.1002/(SICI)1099-0496(199609)22:3<147::AID-PPUL2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Iredale MJ, Wanklyn SA, Phillips IP, Krausz T, Ind PW. Non-invasive assessment of bronchial inflammation in asthma: no correlation between eosinophilia of induced sputum and bronchial responsiveness to inhaled hypertonic saline. Clin Exp Allergy. 1994;24:940–945. doi: 10.1111/j.1365-2222.1994.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor G, Sparrow D, Talor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis. 1987;136:1412–1417. doi: 10.1164/ajrccm/136.6.1412. [DOI] [PubMed] [Google Scholar]
- 10.Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O’Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness: standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Eur Respir J. 1993;6(Suppl. 16):53–83. doi: 10.1183/09041950.053s1693. [DOI] [PubMed] [Google Scholar]
- 11.Fahy JV, Liu J, Wong H, Boushley HA. Cellular and biochemical analysis of induced sputum from asthmatics and from healthy subjects. Am Rev Respir Dis. 1993;147:1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- 12.Eggleston PA, Kagey-Sobotka A, Lichtenstein LM. A comparison of the osmotic activation of basophils and human lung mast cells. Am Rev Respir Dis. 1987;135:1043–1048. doi: 10.1164/arrd.1987.135.5.1043. [DOI] [PubMed] [Google Scholar]
- 13.Pisarri TE, Jonzon A, Coleridge HM, Coleridge JC. Vagal afferent and reflex responses to changes in surface osmolarity in lower airways of dogs. J Appl Physiol. 1992;73:2305–2313. doi: 10.1152/jappl.1992.73.6.2305. [DOI] [PubMed] [Google Scholar]
- 14.Galdes-Sebaldt M, McLaughlin FJ, Levison H. Comparison of cold air, ultrasonic mist and methacholine inhalations as tests of bronchial reactivity in normal and asthmatic children. J Pediatr. 1985;107:526–530. doi: 10.1016/s0022-3476(85)80009-3. [DOI] [PubMed] [Google Scholar]
- 15.Makker HK, Holgate ST. Relation of the hypertonic saline responsiveness of the airways to exercise induced asthma symptom severity and to histamine or methacholine reactivity. Thorax. 1993;48:142–147. doi: 10.1136/thx.48.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson SD, Smith CM, Rodwell LT, du Toit JI, Riedler J, Robertson CF. The use of nonisotonic aerosols for evaluating bronchial hyperresponsiveness. In: Spector S, editor. Provocation Challenge Procedures. New York: Marcel Dekker; 1995. pp. 249–278. [Google Scholar]
- 17.Gibson PG, Wlodarczyk JW, Hensley MJ, Gleeson M, Henry RL, Cripps AW, Clancy RL. Epidemiological association of airway inflammation with asthma symptoms and airway hyperresponsiveness in childhood. Am J Respir Crit Care Med. 1939;158:36–41. doi: 10.1164/ajrccm.158.1.9705031. [DOI] [PubMed] [Google Scholar]
- 18.Anderson SD, du Toit JI, Rodwell LT, Jenkins CR. Acute effect of sodium cromoglycate on airway narrowing induced by 4.5 percent saline aerosol. Outcome before and during treatment with aerosol corticosteroids in patients with asthma. Chest. 1994;105:673–680. doi: 10.1378/chest.105.3.673. [DOI] [PubMed] [Google Scholar]
- 19.Rodwell LT, Anderson SD, du Toit JI, Seale JP. Nedocromil sodium inhibits the airway response to hyperosmolar challenge in patients with asthma. Am Rev Respir Dis. 1992;146:1149–1155. doi: 10.1164/ajrccm/146.5_Pt_1.1149. [DOI] [PubMed] [Google Scholar]
- 20.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000;105:752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 21.Ichinose M, Takahashi T, Sugiura H, Endoh N, Miura M, Mashito Y, Shirato K. Baseline airway hyperresponsiveness and its reversible component: role of airway inflammation and airway calibre. Eur Respir J. 2000;15:248–253. doi: 10.1034/j.1399-3003.2000.15b05.x. [DOI] [PubMed] [Google Scholar]
- 22.De Meer G, Heederik D, Postma D. Bronchial responsiveness to adenosine-5′-monophosphate (AMP) and methacholine differ in their relationship with airway allergy and baseline FEV1. Am J Respir Crit Care Med. 2002;165:327–331. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- 23.Holgate ST. Adenosine provocation: a new test for allergic type airway inflammation. Am J Respir Crit Care Med. 2002;165:317–319. doi: 10.1164/ajrccm.165.3.2112045a. [DOI] [PubMed] [Google Scholar]


