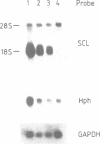
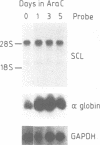
Abstract
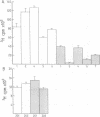
The SCL gene encodes a member of the helix-loop-helix family of transcription factors that have been implicated in regulation of differentiation and development. Although SCL mRNA is not detectable in normal thymocytes or peripheral T-lymphocytes, transcriptional activation occurs in T-cell tumours. A clue to the normal function of SCL has come from demonstration of high levels of SCL mRNA in erythroid cells. To illuminate the function of SCL in the erythroid lineage, an antisense SCL construct was introduced into the human erythroleukaemia cell line, K562. Cells electroporated with a vector containing antisense SCL grew more slowly than control cells which had received vector alone. Non-specific toxicity was excluded by showing that antisense SCL did not influence growth of Raji cells, a B-cell line that does not express endogenous SCL mRNA. Suppression of K562 growth was accompanied by increased spontaneous erythroid differentiation as measured by benzidine staining. K562 cells containing antisense SCL produced smaller colonies in agar and exhibited reduced clonogenicity compared with control cells. In addition, experiments in which K562 colonies were recloned showed that antisense SCL profoundly suppressed self-renewal of K562 cells. These data provide the first evidence that SCL promotes self-renewal in an erythroid cell line and raise the possibility that SCL may function to regulate proliferation of normal erythroid cells.
Full text
PDF
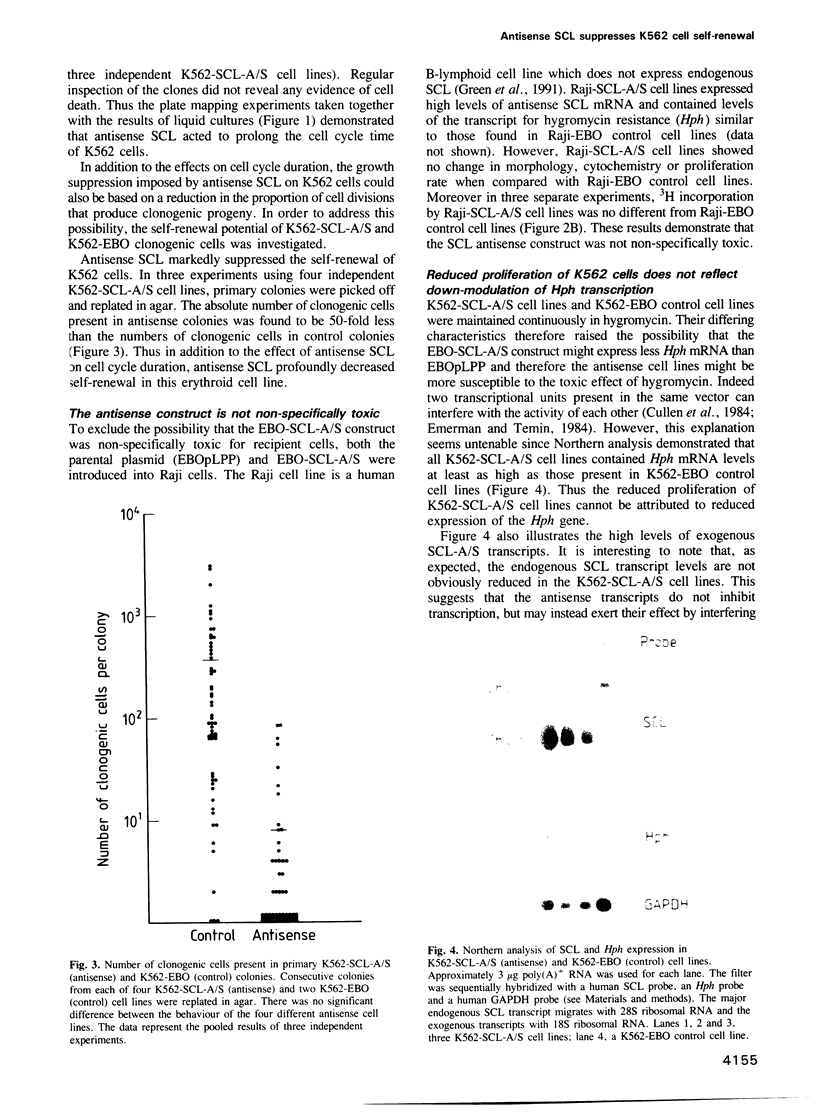
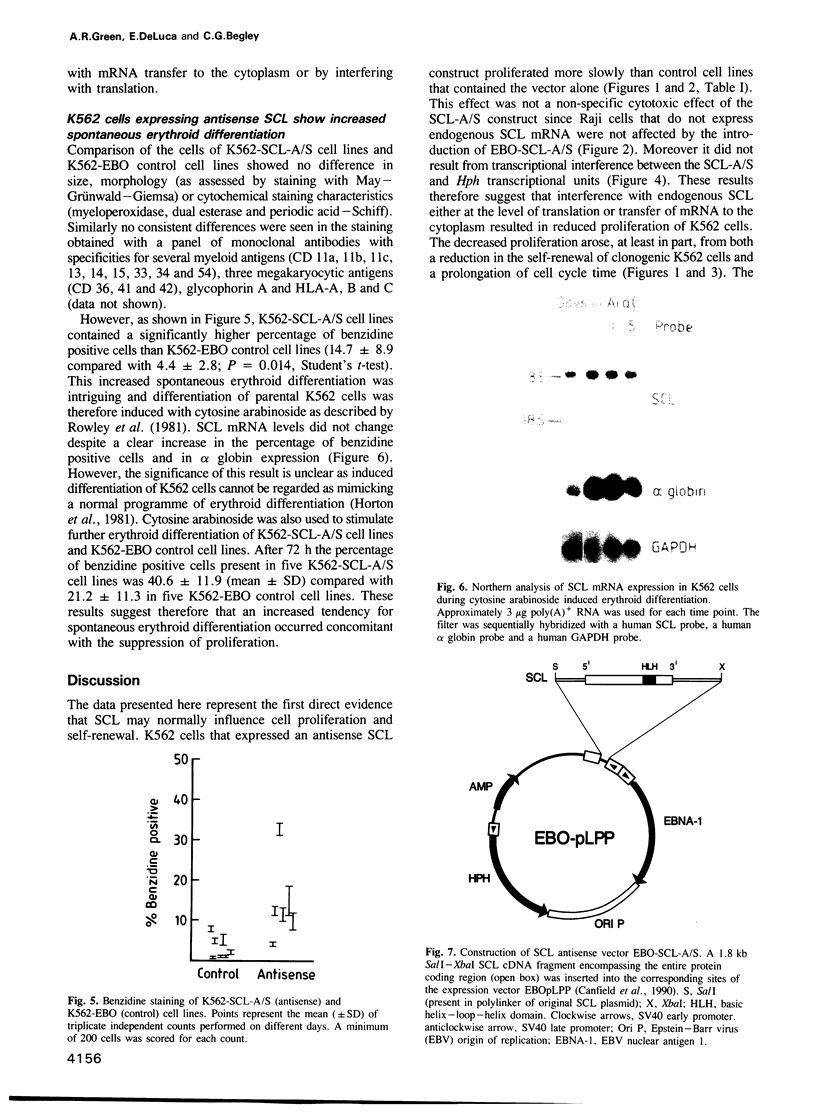


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. C., Jokinen M., Gahmberg C. G. Induction of erythroid differentiation in the human leukaemia cell line K562. Nature. 1979 Mar 22;278(5702):364–365. doi: 10.1038/278364a0. [DOI] [PubMed] [Google Scholar]
- Aplan P. D., Begley C. G., Bertness V., Nussmeier M., Ezquerra A., Coligan J., Kirsch I. R. The SCL gene is formed from a transcriptionally complex locus. Mol Cell Biol. 1990 Dec;10(12):6426–6435. doi: 10.1128/mcb.10.12.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Davey M. P., Nakahara K., Tchorz K., Kurtzberg J., Hershfield M. S., Haynes B. F., Cohen D. I., Waldmann T. A. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2031–2035. doi: 10.1073/pnas.86.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Benz E. J., Jr, Murnane M. J., Tonkonow B. L., Berman B. W., Mazur E. M., Cavallesco C., Jenko T., Snyder E. L., Forget B. G., Hoffman R. Embryonic-fetal erythroid characteristics of a human leukemic cell line. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3509–3513. doi: 10.1073/pnas.77.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard O., Guglielmi P., Jonveaux P., Cherif D., Gisselbrecht S., Mauchauffe M., Berger R., Larsen C. J., Mathieu-Mahul D. Two distinct mechanisms for the SCL gene activation in the t(1;14) translocation of T-cell leukemias. Genes Chromosomes Cancer. 1990 Jan;1(3):194–208. doi: 10.1002/gcc.2870010303. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Dunn S. M., Fecondo J. V., Culvenor J. G., Dührsen U., Burns G. F., Wawryk S. O. Regulation of expression of a human intercellular adhesion molecule (ICAM-1) during lymphohematopoietic differentiation. Blood. 1989 May 15;73(7):1896–1903. [PubMed] [Google Scholar]
- Brown L., Cheng J. T., Chen Q., Siciliano M. J., Crist W., Buchanan G., Baer R. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. EMBO J. 1990 Oct;9(10):3343–3351. doi: 10.1002/j.1460-2075.1990.tb07535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield V., Emanuel J. R., Spickofsky N., Levenson R., Margolskee R. F. Ouabain-resistant mutants of the rat Na,K-ATPase alpha 2 isoform identified by using an episomal expression vector. Mol Cell Biol. 1990 Apr;10(4):1367–1372. doi: 10.1128/mcb.10.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Chen Q., Cheng J. T., Tasi L. H., Schneider N., Buchanan G., Carroll A., Crist W., Ozanne B., Siciliano M. J., Baer R. The tal gene undergoes chromosome translocation in T cell leukemia and potentially encodes a helix-loop-helix protein. EMBO J. 1990 Feb;9(2):415–424. doi: 10.1002/j.1460-2075.1990.tb08126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordle S. R., Henderson E., Masuoka H., Weil P. A., Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991 Mar;11(3):1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. R., Salvaris E., Begley C. G. Erythroid expression of the 'helix-loop-helix' gene, SCL. Oncogene. 1991 Mar;6(3):475–479. [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Cedar S. H., Edwards P. A. Expression of red cell specific determinants during differentiation in the K562 erythroleukaemia cell line. Scand J Haematol. 1981 Oct;27(4):231–240. doi: 10.1111/j.1600-0609.1981.tb00478.x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Packham G., Cook A., Farrell P. J. The BZLF1 protein of EBV has a coiled coil dimerisation domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991 Feb;6(2):195–204. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Ludwig S. R., Wessler S. R. Maize R gene family: tissue-specific helix-loop-helix proteins. Cell. 1990 Sep 7;62(5):849–851. doi: 10.1016/0092-8674(90)90259-h. [DOI] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Atchison M. L. Functional characterization of the developmentally controlled immunoglobulin kappa 3' enhancer: regulation by Id, a repressor of helix-loop-helix transcription factors. Mol Cell Biol. 1991 Feb;11(2):1040–1047. doi: 10.1128/mcb.11.2.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley P. T., Ohlsson-Wilhelm B. M., Farley B. A., LaBella S. Inducers of erythroid differentiation in K562 human leukemia cells. Exp Hematol. 1981 Jan;9(1):32–37. [PubMed] [Google Scholar]
- Ruezinsky D., Beckmann H., Kadesch T. Modulation of the IgH enhancer's cell type specificity through a genetic switch. Genes Dev. 1991 Jan;5(1):29–37. doi: 10.1101/gad.5.1.29. [DOI] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Spencer C. A., Groudine M. Control of c-myc regulation in normal and neoplastic cells. Adv Cancer Res. 1991;56:1–48. doi: 10.1016/s0065-230x(08)60476-5. [DOI] [PubMed] [Google Scholar]
- Thisse B., Stoetzel C., Gorostiza-Thisse C., Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988 Jul;7(7):2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Visvader J., Begley C. G. Helix-loop-helix genes translocated in lymphoid leukemia. Trends Biochem Sci. 1991 Sep;16(9):330–333. doi: 10.1016/0968-0004(91)90137-k. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Lyman G. H., Wickstrom E. Anti-c-myc DNA increases differentiation and decreases colony formation by HL-60 cells. In Vitro Cell Dev Biol. 1989 Mar;25(3 Pt 1):297–302. doi: 10.1007/BF02628470. [DOI] [PubMed] [Google Scholar]