Abstract
Background
Infection is still a frequent cause of morbidity and mortality in acute myelogenous leukemia (AML) patients receiving chemotherapy. Recently the main cause of infection has changed from gram-negative to gram-positive bacteria and the resistance to antibiotics has increased. This study aimed to access the effectiveness of antimicrobial prophylaxis (AP) with orally absorbable antibiotics.
Methods
Ninety-five AML patients receiving chemotherapy at Catholic Hemopoietic Stem Cell Transplantation Center from March 1999 to July 1999 were randomly divided into the AP group (250 mg ciprofloxacin twice a day, 150 mg roxithromycin twice a day, 50 mg fluconazole once a day) and the control group for a prospective analysis.
Results
The incidence of fever was 82.6% in the AP group and 91.6% in the control group (p = 0. 15). Though classification and sites of infections showed no difference between the two groups, the catheter associated infection occurred more frequently in the AP group in significance. The time interval between initiation of chemotherapy and onset of fever, white blood cell (WBC) count at the onset of fever, duration of leukopenia (WBC <1,000/mm3), duration of systemic antibiotic therapy, mortality due to infection and hospitalization period from the data starting chemotherapy showed no differences between the two groups. Infections due to gram negative bacteria decreased to 33.3% in the AP group (vs. 92% in the control group), but infections due to gram positive bacteria increased to 66.7% (vs. 8% in the control group). Gram negative bacteria showed 100% resistance to ciprofloxacin in the AP group and gram-positive bacteria showed 90–100% resistance to erythromycin, regardless of the presence of AP.
Conclusion
The AP could not reduce the occurrence of infection or infection associated death in AML patients receiving chemotherapy. On considering increased gram-positive infection and resistance to fluoroquinolone and macroiide, routine prescription of AP should be reconsidered. Further studies that assess the effectiveness of AP in other malignancies, aplastic anemia and bone marrow transplantation are required.
Keywords: Decontamination, Leukemia, Myelocytic, Acute
INTRODUCTION
Infections are still a frequent cause of morbidity and mortality in neutropenic patients receiving chemotherapy including remission induction therapy for hematologic malignancies. Some prophylactic methods have been studied for the last several decades and antimicrobial prophylaxis is widely being used1–3). Its rationale is based on “colonization resistance” that anaerobes in the bowel are preserved and predominance of gram-negative species as a potential pathogenic bacteria is suppressed. There are several reports that ciprofloxacin among fluoroquinolones is most frequently being used, by which the number of infections due to gram negative bacteria are decreased and the onset of fever is delayed4–13).
But the effects of antimicrobial prophylaxis (the incidence of bacterial infections and fever, use of systemic antibiotics, mortality due to infection etc.) vary according to the reporters. It is known to be related with an increasing trend of gram-positive infections, acquisition of resistance in gram-negative species like E. coli to fluoroquinolone and increased fungal infections, which requires reevaluation of the benefits and adverse effects of antimicrobial prophylaxis2, 3, 5, 14–18). According to Infectious Diseases Society of America (IDSA) guidelines in 1997, it was recommended that prophylactic antimicrobial agents should be considered for short periods of time in some special cases of profound and prolonged neutropenia instead of a routine usage of quinolones19).
We performed a prospective randomized trial to investigate the effectiveness of antimicrobial prophylaxis including ciprofloxacin, in acute myelogenous leukemia (AML) patients who were receiving chemotherapy.
PATIENTS AND METHODS
1. Patients
Adult patients with AML admitted at Catholic Hemopoietic Stem Cell Transplantation (CHSCT), the Catholic University of Korea for chemotherapy from March 1999 to July 1999 were included. Patients were not eligible if they were below 16 years old, pregnant or lactating state, epileptics and had a history of allergic reaction to quinolunes or macrolides, liver or renal diseases (aspartate aminotransferase and alanine aminotransferase over 3 times of the upper normal limit, total bilirubin over 2.5 mg/dL, serum creatinine over 2 mg/dL).
2. Trial plan review and informed consents
The trial plan and the consent for this study were reviewed by a CHSCT Inquiry Committee for Clinical and Medical Studies and approved for every particular aspect, including the moral aspect. We explained the purpose, methods and expected adverse events of this study to each patient and then written informed consent was obtained voluntarily from each patient before enrollment in the study.
3. Antimicrobial prophylaxis
Patients were randomly divided into the antimicrobial prophylaxis group (250 mg ciprofloxacin twice a day, 150 mg roxithromycin twice a day, 50 mg fluconazole once a day) and the control group. Prophylactic antimicrobial agents were administered at least within 72 hours from the initiation of the chemotherapy and continued until the onset of fever (a single oral temperature of >38.3°C or >38.0°C over at least 1 hour)19), signs or symptoms of infection, serious adverse event related to the decontamination, recovery of the leukocyte count ≥1,000/mm3 or absolute neutrophil count ≥500/mm3. In neutropenic patients with fever, prophylactic antimicrobial agents were stopped immediately and the empirical broad-spectrum antimicrobial agents were administered according to CHSCT infection guidelines.
4. Patients’ evaluation and laboratory data
The subjects were daily checked for signs and symptoms of infection from when they were registered for the study and until they were discharged from the hospital. We daily recorded the highest and lowest body temperature of each patient. In patients with fever, culture of peripheral blood and specimens from the sites of infection in doubt were done. Complete blood cell count was checked daily and urinalysis, blood urea nitrogen, serum creatinine, serum electrolyte and liver function test were measured twice a week. Chest X-ray was taken at the day of registration and whenever it was needed clinically, thereafter. Time interval between the first day of chemotherapy and onset of fever, leukocyte count at the onset of fever, duration of leukocyte count <1000/mm3, duration of systemic antibiotics therapy, death associated with infection and hospitalization period were reviewed.
5. Classification of infections
Identification of significant bacteria from the trustful specimen like blood or urine was defined as “MDI (microbiologically defined infection)”, clear proof of infection without any causal bacteria as “CDI (clinically defined infection)” and fever without definite causes in clinical, X-ray, microbiologic ways as “UF (unexplained fever)”19). Bacteremia was defined as the same bacteria isolated from at least more than 2 sets of blood culture tests. In case of pneumonia, identification of bacteria on blood culture was only classified as MDI. Presentation of pulmonary infiltrates by X-ray and respiratory symptoms (cough, sputum, dyspnea) was classified as CDI.
6. Identification of microorganism and MIC measurement
The standard method for isolation and identification of pathogenic microorganism was used20). In the episode of fever, 3–5 mL peripheral blood was collected twice at an interval of one hour and put in BACTEC NR 6A, 7A blood culture containers (Becton Dickinson and Co., Sparks, MA, USA), containing soybean-casein digest broth, for culture. In addition, sites of infection in doubt were also checked for culture.
The antimicrobial susceptibility was tested by micro liquid broth dilution method using MicroScan (Dade Behring, West Sacramento, CA, USA). The degree of susceptibility was determined by National Committee for Clinical Laboratory Standards (NSSLS document M100-S9, 1999)21).
7. Data analysis and statistics
Statistical program SPSS 10.0 for windows was used with chi-square test or Fisher’s exact test and student’s unpaired t-test for comparison and analysis between the two groups, p value <0.05 was judged as significant.
RESULTS
1. Characteristics of patients
Total 95 patients were included in the study. 46 were assigned to the antimicrobial prophylaxis group and 49 to the control group. The mean ages were 33.5 and 39.9 (p=0.86). As to the chemotherapy, remission induction therapy was performed to 15 and 11 patients, consolidation to 22 and 27, reinduction to 9 and 11, respectively, which indicated no significant differences between the two groups (Table 1). There was no one who was discontinued prophylactic antimicrobials due to adverse events.
Table 1.
General characteristics of patients
| Prophylaxis | Control | p-value | |
|---|---|---|---|
| No. of patients | 46 | 49 | |
| Male : Female | 32:14 | 33:16 | 0.82 |
| Age (year)* | 33.5±11.3 | 39.9±10.8 | 0.86 |
| Chemotherapy (%) | 0.54 | ||
| Remission induction | 15 (33) | 11 (22) | |
| Consolidation | 22 (48) | 27 (55) | |
| Reinduction | 9 (19) | 11 (23) |
Mean±SD
2. Fever and infection
The incidence of fever was 82.6% (38 out of 46 patients) in the antimicrobial prophylaxis group and 91.6% (44 out of 49 patients) in the control group and there was no significant difference between the two groups (Figure 1, p=0.15). Infections were classified as 14 MDI, 19 CDI and 5 UF patients in the antimicrobial prophylaxis group, whereas the control group had 21, 15, 8, respectively. There was no difference between the two groups (Figure 2, p=0.34). Though there was no difference in the sites of infection between the two groups, the catheter infection was significantly more frequent in the antimicrobial prophylaxis group (Table 2).
Figure 1.
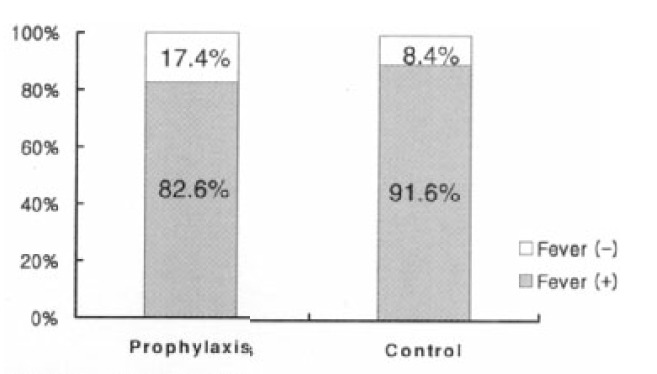
Incidence of fever
Figure 2.
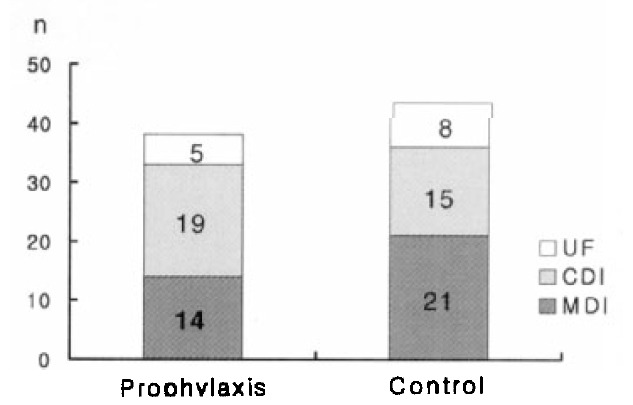
Classification of infection UF, unexplained fever; CDI, clinically defined infection; MDI, microbiologically defined infection
Table 2.
Sites of infection
| Prophylaxis (n=38) | Control (n=44) | p-value | |
|---|---|---|---|
| Oral cavity | 9 | 11 | 0.45 |
| Primary bacteremia | 2 | 6 | 0.10 |
| Pneumonia | 6 | 5 | 0.28 |
| Perianal infection | 5 | 5 | 0.40 |
| Neutropenic enterocolitis | 1 | 5 | 0.07 |
| Catheter infection | 5 | 0 | 0.006 |
| Other skin & soft tissue | 4 | 3 | 0.27 |
| Sinusitis | 1 | 1 | 0.46 |
| Unknown | 5 | 8 | 0.27 |
Mean time interval between initiation of chemotherapy and onset of fever was 13 days in the antimicrobial prophylaxis group and 11.9 days in the control group. Leukocyte count of peripheral blood at the onset of fever was 445/mm3 and 414/mm3, respectively. Duration of leukocyte count <1000/mm3 was 13.8 days and 11.7 days in each group. Duration of systemic antibiotics therapy was 12.2 days and 10.8 days, respectively. Mortality rate associated with infection was 4.3% and 4.1%. The duration of hospitalization based on the date when the chemotherapy started was 28.0 days and 26.5 days, respectively. There were no significant differences between the two groups (Table 3).
Table 3.
Quantifiable parameters to compare the efficacy of decontamination
| Prophylaxis | Control | p-value | |
|---|---|---|---|
| Time interval between initiation of chemotherapy and onset of fever (days) | 13.0±3.8 | 11.9±3.9 | 0.19 |
| Mean WBC count at the onset of fever (/mm3) | 445±424 | 416±380 | 0.75 |
| Duration of leukopenia (WBC <1,000/mm3) | 13.8±82 | 11.7±7.1 | 0.18 |
| Duration of systemic antibiotics use (days) | 12.2±9.0 | 10.8±5.5 | 0.40 |
| Death due to infection (%) | 2 (4.3) | 2 (4.1) | 0.47 |
| Hospitalization day (days) | 28.0±9.1 | 26.5 ±6.5 | 0.36 |
Fifteen microorganisms were identified as causal agents in 14 MDI patients of the antimicrobial prophylaxis group. They were 10 gram-positive bacteria (66.7%) and 5 gram-negative bacteria (33.3%). On the other hand, 25 microorganisms isolated in the MDI patients of the control group were 23 gram-negative (92.0%) and only 2 gram-positive bacteria (8.0%) (Table 4). Relative risk of infection due to gram-positive bacteria in the antimicrobial prophylaxis group was 4.67 (p=0.002, 2.03 <95% confidence interval <10.74), which was statistically significant.
Table 4.
Microorganisms causing infection
| Prophylaxis (n=15/14 persons) | Control (n=25/21 persons) | |
|---|---|---|
| G (+) Staphylococcus aureus | 1 | 1 |
| Staphylococcus haemolyticus | 1 | 0 |
| Staphylococcus epidermidis | 4 | 0 |
| Streptococcus mitis | 2 | 0 |
| Streptococcus pneumoniae | 0 | 1 |
| Enterococcus faecalis | 2 | 0 |
| Total | 10 | 2 |
|
| ||
| G (−) Escherichia coii | 3 | 9 |
| Klebsiella pneumoniae | 1 | 6 |
| Klebsiella oxytoca | 0 | 1 |
| Pseudomonas aeruginosa | 1 | 0 |
| Enterobacter cloacae | 0 | 3 |
| Aeromonas hydrophila | 0 | 3 |
| ESurkhoideria cepacia | 0 | 1 |
| Total | 5 | 23 |
G (+), Gram positive bacteria; G (−), Gram negative bacteria
The antimicrobial resistance rate was as follow; gram-negative bacteria isolated in the antimicrobial prophylaxis group had 100% resistance to ciprofloxacin, whereas only 3 out of 23 gram-negative organisms (13%) in the control group had resistance to ciprofloxacin. The resistance rate to erythromycin of gram-positive bacteria isolated in the antimicrobial prophylaxis and the control group was 90% and 100%, respectively (Table 5).
Table 5.
Incidence and resistance patterns of infection by Gram positive or negative organisms
| Prophylaxis | Control | |
|---|---|---|
| EM resistant gram positive organisms (%) | 9/10 (90%) | 2/2 (100%) |
| CPFX resistant gram negative organisms (%) | 5/5 (100%) | 3/23 (13%) |
EM, erythromycin; CPFX, ciprofloxacin
DISCUSSION
Infection is still the most frequent cause of morbidity and mortality among complications occurred during neutropenic state after chemotherapy. Recently more cytotoxic chemotherapy for acute leukemia is being intoduced. As the result, more profound and prolonged neutropenia is developed and the risks of infections tend to increase. Several things have been reported to reduce infectious complications in the neutropenic patients; That is, improvement in the hospital hygiene, withdrawal of every raw food, installation of laminar air flow and high-efficacy particulate air system, administration of G-CSF/GM-CSF and prophylactic antimicrobial agents2–17). Among these, the antimicrobial prophylaxis introduced by van der Waaij, et al. adopts the theory of “colonization resistance” that keeping bowel anaerobes can prevent colonization and overgrowth of aerobic gram-negative bacteria as a potential pathogenic organism1, 8, 22, 23). Ideal agents to meet these theory are reported to have characteristics as follow; good responsiveness to the most pathogenic bacteria frequently isolated in the infections of patients with supressed marrow, relatively little effect to anaerobes in the bowel, long half-life and high therapeutic index as well as minimal toxicity1, 9, 11, l6). Ciprofloxacin is being widely used as an absorbable oral agent, which has merits to effectively reduce gram-negative bacteremia to 1–2% and delay the onset of fever in neutropenia4, 6, 17, 18).
But, the main cause of infection is being changed from gram-negative bacteria to gram-positive bacteria by antimicrobial prophylaxis itself, use of central venous catheter, mucositis related to the chemotherapy and increases in using antacid and antihistamine agent (H2-blocker). In case of the CHSCT using the high-efficacy particulate air system, after ciprofloxacin was prescribed for prophylaxis, gram-positive infection remarkably increased, by which gram-positive bacteria of MDI increased from 32.5% during 1981–84 to 64.4% during 1995–9724–27). Furthermore, emergence of resistance to these prophylactic antimicrobial agents is also important matter, especially, most of the E. coli isolated from neutropenic patients receiving antimicrobial prophylaxis show fluoroquinolone resistance. We had reported that the resistance to fluoroquinolone of E. coli isolated at the CHSCT is upto 92.9%27). Recently there is a report that the combined use of penicillin, roxithromycin, rifampin, vancomycin28–30) and fluconazole as an antifungal agent31–33) is effective to prevent gram-positive bacterial infection such as streptococcal infection; emerging organism in a high-risk group. Combination therapy of ciprofloxacin, roxithromycin, fluconazole is currently used at the CHSCT.
We tried to investigate the effectiveness of antimicrobial prophylaxis in AML patients under the condition unlike the past, that is, more frequent gram-positive bacterial infection than gram-negative one and increased resistance to antimicrobial agents used for prophylaxis.
The frequency of fever after the chemotherapy is upto 82.6% in the antimicrobial prophylaxis group and 91.6% in the control group in this study, which showed no difference between the two groups and also no difference between the classifications of infection. Sites of infection didn’t show any difference between the two groups, but the catheter infection occurred more frequently in the antimicrobial prophylaxis group in significance (Table 2). Because most of the catheter associated infections are caused by methicillin-resistant Staphylococcus epidermidis in CHSCT34), it can not be considered for the assessment of effectiveness of antimicrobial prophylaxis. And time interval between initiation of chemotherapy and onset of fever, WBC count at the onset of fever, duration of leukopenia (WBC <1,000/mm3), duration of systemic antibiotic therapy, mortality due to infection, hospitalization period from the date starting the chemotherapy showed no differences between the two groups, which indicated antimicrobial prophylaxis was not effective to prevent infections.
Comparing the causative organisms, gram-positive bacteria was 66.7% in the antimicrobial prophylaxis group, but in the control group gram-negative bacteria was 92%. It indicated that the total number of causative bacteria showed no differences between the two groups but the number of gram-negative bacteria decreased by antimicrobial prophylaxis and the number of gram-positive bacteria increased as a result. And the relative risk of infection due to gram-positive bacteria significantly increased by the antimicrobial prophylaxis. In addition, gram-negative bacteria with resistance to ciprofloxacin was 100% in the antimicrobial prophylaxis group, whereas it was 13% in the control group, which implied that the use of ciprofloxacin induced the resistance. Therefore, it should be carefully considered that the routine preventive use of ciprofloxacin is discontinued based on the above result. In case of gram-positive bacteria, resistance to erythromycin was very high by 90–100% without regard to the antimicrobial prophylaxis so that the relation between resistance and therapeutic efficacy of antimicrobial prophylaxis should be studied later. But it also implies that the preventive use of macrolide is useless.
The antimicrobial prophylaxis therapy was introduced when most of infections in the neutropenic patients were due to gram-negative bacteria, especially Pseudomonas aeruginosa. After that, anti-pseudomonal antimicrobials were developed to reduce gram-negative infection. As gram-positive infection is being increased by increased invasive procedure and increased frequency of mucositis induced from the toxic chemotherapy, the appropriateness of antimicrobial prophylaxis should be reevaluated. Especially, the prophylactic use of ciprofloxacin could reduce gram-negative infection, but the resistance increased remarkably and it was not effective to the gram-positive bacteria, which indicated that the effectiveness of ciprofloxacin should be reviewed in the state of bowel colonization of gram-positive bacteria.
In case of fluoroquinolones, it was reported that it could reduce gram-negative infection and delayed the onset of fever, but there was no report that it could decrease infection-related morbidity or prevent the gram-positive infection. There is another report about the additional use of rifampin, penicillin, roxithromycin, vancomycin to prevent increasing gram-positive bacterial infection, but their effectiveness are not clear2, 8, 18, 28–30). Cruciani et al. presented that prophylactic use of fluroquinolone macrolide, penicillin, vancomycin could reduce bacteremia by gram-positive bacteria, whereas they also reported that it could not reduce the episodes of fever and death due to infection5). In general, it is a predominant opinion if there was no evidence of gram-positive infection, prophylactic or empirical use of vancomycin should be restricted to prevent unnecessary use and emergence of resistant organisms19, 30, 35)
The limitation of this study is that the effectiveness of prophylactic fluconazole to prevent fungal infection was not investigated. Though there is a report that prolonged neutropenia may induced more frequent fungal infections, definitive fungal infection is hard to be diagnosed because; i) isolation of fungus from blood culture is very difficult and infrequent, ii) its pathogenic role is not clear if it is isolated from the feces in the patients with gastrointestinal symptoms and iii) invasive procedure to confirm pathogenic organisms in neutropenic patients with pneumonia is hard to be done. In this study, there was no fungus as causative agents of MDI and it was thought to be related to the early administration of amphotericin B according to infection guidelines of CHSCT. Fungal infection in neutropenic patients is being still studied.
Finally, in AML patients receiving chemotherapy, antimicrobial prophylaxis could not reduce the episodes of fever, infection associated death, hospitalization period. Although it made gram-negative infection decrease, gram-positive infection increased on the contrary. All gram-negative bacteria was resistant to ciprofloxacin with prophylaxis, whereas gram-positive bacteria was highly resistant to erythromycin by 90–100% regardless of the presence of antimicrobial prophylaxis. In the future, the effectiveness of antimicrobial prophylaxis in other malignancies, aplastic anemia and bone marrow transplantation should be studied comprehensively. And prophylactic antibiotics should be reevaluated as the result.
REFERENCES
- 1.Arnow PM. Prevention of bacterial infection in the transplant recipient: the role of selective bowel decontamination. Infect Dis Clin din North Am. 1995;9:849–862. [PubMed] [Google Scholar]
- 2.Kerr KG. The prophylaxis of bacterial infections in neutropenic patients. J Antimicrob Chemother. 1999;44:587–591. doi: 10.1093/jac/44.5.587. [DOI] [PubMed] [Google Scholar]
- 3.Verhoef J. Prevention of infections in the neutropenic patient. Clin infect Dis. 1993;17(Suppl 2):S359–367. doi: 10.1093/clinids/17.supplement_2.s359. [DOI] [PubMed] [Google Scholar]
- 4.Bow EJ, Loewen R, Vaughan D. Reduced requirement for antibiotic therapy targeting Gram-negative organisms in febrile, neutropenic patients with cancer who are receiving antibacterial chemoprophylaxis with oral quinolones. Clin infect Dis. 1995;20:907–912. doi: 10.1093/clinids/20.4.907. [DOI] [PubMed] [Google Scholar]
- 5.Cruciani M, Rampazzo R, Malena M, Lazzarini L, Todeschini G, Messori A, Concia E. Prophylaxis with fluroquinolones for bacterial infections in neutropenic patients: a meta-analysis. Clin Infect Dis. 1996;23:795–805. doi: 10.1093/clinids/23.4.795. [DOI] [PubMed] [Google Scholar]
- 6.De Pauw BE, Donnelly JP, Novakova DW, Schattenberg A. Options and limitations of long-term oral ciprofloxacin as antibacterial prophylaxis in allogeneic bone marrow transplant recipients. Bone Marrow Transplant. 1990;5:179–182. [PubMed] [Google Scholar]
- 7.Donnelly JP, Maschmeyer G, Daenen S. Selective oral antimicrobial prophylaxis for the prevention of infection in acute leukemia - ciprofloxacin versus co-trimoxazole plus colistin. Eur J Cancer. 1992;28A:873–878. doi: 10.1016/0959-8049(92)90138-r. [DOI] [PubMed] [Google Scholar]
- 8.Engels EA, Lau J, Barza M. Efficacy of quinolone prophylaxis in neutropenic cancer patients: a meta-analysis. J Clin Oncol. 1998;16:1179–1187. doi: 10.1200/JCO.1998.16.3.1179. [DOI] [PubMed] [Google Scholar]
- 9.Klastersky J. Chemoprophylaxis of Gram-negative infections in neutropenic patients. Eur Urol. 1990;17(Suppl 1):40–45. doi: 10.1159/000464090. [DOI] [PubMed] [Google Scholar]
- 10.Kurrle E, Schmeriser T, Kern W. Selective decontamination in neutropenic patients. Epidemiol infect. 1992;109:327–335. doi: 10.1017/s0950268800050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Kaliish LA, Finberg R. Ciprofloxacin versus trimethoprim/sulfamethoxazole for prophylaxis of bacterial infections in bone marrow transplant recipients: a randomized, controlled trial. J Clin Oncol. 1995;13:239–250. doi: 10.1200/JCO.1995.13.1.239. [DOI] [PubMed] [Google Scholar]
- 12.Lew MA, Kehoe K, Ritz J, Antman KH, Nadler L, Takvorian T, Mayer R, Kalish L, Finberg R. Prophylaxis of bacterial infections with ciprofloxacin in patients undergoing bone marrow transplantation. Transplantation. 1991;51:630–636. doi: 10.1097/00007890-199103000-00017. [DOI] [PubMed] [Google Scholar]
- 13.de Marie S, van den Broek PJ, Willemze R, van Furth R. Strategy for antibiotic therapy in febrile neutropenic patients on selective antibiotic decontamination. Eur J Clin Microbiol Infect Dis. 1993;12:897–906. doi: 10.1007/BF01992162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martino R, Subira M, Altes A, Lopez R, Sureda A, Domingo-Albos A, Pericas R, Brunet S. Effect of discontinuing prophylaxis with norfloxacin in patients with hematologic malignancies and severe neutropenia. Acta Haematol. 1998;99:206–211. doi: 10.1159/000040840. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly JP. Is there a rationale for the use of antimicrobial prophylaxis in neutropenic patients? J intern Med. 1997;242(Suppl 740):79–88. [PubMed] [Google Scholar]
- 16.Patrick CC. Use of fluoroquinolones as prophylatic agents in patients with neutropenia. Padiatr Infect Dis J. 1997;16:135–139. doi: 10.1097/00006454-199701000-00038. [DOI] [PubMed] [Google Scholar]
- 17.Klastersky J. Science and pragmatism in the treatment and prevention of neutropenic infection. J Antimicrob Chemother. 1998;41(Suppl D):13–24. doi: 10.1093/jac/41.suppl_4.13. [DOI] [PubMed] [Google Scholar]
- 18.Klastersky J. Prevention of infection in neutropenic cancer patients. Curr Opin Oncol. 1996;8:270–277. doi: 10.1097/00001622-199607000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Huges WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, Feld R, Pizzo P, Rolston KVI, Shenep JL, Young LS. guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Clin Infect Dis. 1997;1997;25:551–573. doi: 10.1086/513764. [DOI] [PubMed] [Google Scholar]
- 20.Murray PR, Baron EJ, Ptaller MA, Tenover FC, Yolken RH. Manual of clinical microbiology. 7th ed. Washington; ASM press: 1999. pp. 33–104. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards (NCCLS) NCCLS document M100-S9. 1. Vol. 19. NCCLS; 940 West Valley Road, Suite 1400, Wayne, PA 19087-1898, USA: 1999. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. [Google Scholar]
- 22.van der Waaij D, Berghius-de Vries JM, Lekkerkerk-van der Wees JE. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg. 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–414. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on Gram-positive and resistant bacteria. Clin Infect Dis. 1999;29:490–494. doi: 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 25.Jones RN. Contemporary antimicrobial susceptibility patterns of bacterial pathogens commonly associated with febrile patients with neutropenia. Clin Infect Dis. 1999;29:495–502. doi: 10.1086/598621. [DOI] [PubMed] [Google Scholar]
- 26.Choi JH, Kim YJ, Lee DG, Shin WS, Kim SW, Bae SS, Kim SH, Yoo JH, Kim KM, Han KJ, Lee JW, Min WS, Kim CC. Infectious features in patients with acute leukemia. Korean J Infect Dis. 1999;31:217–224. [Google Scholar]
- 27.Yoo JH, Huh DH, Choi JH, Shin WS, Kang MW, Kim CC, Kim DJ. Molecular epidemiological analysis of quinolone -resistant Escherichia coll causing bacteremia in neutropenic patients with leukemia in Korea. Clin Infect Dis. 1997;25:1385–1391. doi: 10.1086/516132. [DOI] [PubMed] [Google Scholar]
- 28.Kern WV, Hay B, Kern P, Marre R, Arnold R. A randomized trial of roxithromycin in patients with acute leukemia and bone marrow transplant recipients receiving fluoroquinolone prophylaxis. Antimicrob Agents Chemother. 1994;38:465–472. doi: 10.1128/aac.38.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidalgo M, Hornedo J, Lumbreras C, Trigo JM, Gomez C, Perea S, Ruiz A, Hitt R, Cortes-funes H. Lack of ability of ciprofloxacin-rifampin prophylaxis to decrease infection-related morbidity in neutropenic patients given cytotoxic therapy and peripheral blood stem cell transplants. Antimicrob Agents Chemother. 1997;41:1175–1177. doi: 10.1128/aac.41.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ford CD, Reilly W, Wood J, Classen DC, Burke JP. Oral antimicrobial prophylaxis in bone marrow transplnat recipients: randomized trial of ciprofloxacin versus ciprofloxacin-vancomycin. Antimicrob Agents Chemother. 1998;42:1402–1405. doi: 10.1128/aac.42.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamac K, Senol E, Haznedar R. Prophylactic use of fluconazole in neutropenic cancer patients. Postgrad Med J. 1995;71:284–286. doi: 10.1136/pgmj.71.835.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertens JA, Boogaerts MA. Antifungal prophylaxis in neutropenia. Curr Opin Infect Dis. 1999;12:549–555. doi: 10.1097/00001432-199912000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Bohme A, Karthaus M, Hoelzer Antifungal prophylaxis in neutropenic patients with hematologic malignancies: is there a real benefit? Chemotherapy. 1999;45:224–232. doi: 10.1159/000007187. [DOI] [PubMed] [Google Scholar]
- 34.Infection control Newsletter of the St. Mary’s hospital. 1999;4:1–2. [Google Scholar]
- 35.Young SD, Feld R. Fever associated with chemotherapy-induced neutropenia: a review of current therapeutic approaches. Curr Opin Infect Dis. 1998;11:401–409. doi: 10.1097/00001432-199808000-00002. [DOI] [PubMed] [Google Scholar]


