Abstract
Background :
It has been suggested that chronic hepatitis C virus (HCV) infection is associated with diabetes. The aim of this study was to establish a potential relationship between chronic HCV infection and diabetes mellitus in Korean patients.
Methods :
We performed a prospective analysis of 404 patients with chronic viral hepatitis or liver cirrhosis who visited our hospital and analyzed whether age, sex, body mass index, alcohol consumption, hepatitis B virus (HBV) infection, HCV infection and cirrhosis were associated with diabetes. We also enrolled 627 diabetic patients and the seroprevalence of HBV surface antigen (HBsAg) and anti-HCV was determined.
Results :
Diabetes was observed more frequently in individuals with HCV infected chronic liver disease (24.0%) than in those with HBV infected (10.4%) (p<0.05). Univariate analyses revealed that age, alcohol consumption and HCV infection were significant independent predictors for diabetes. The mean age of the patients with HCV infected chronic liver disease was higher than that of HBV infected (56 ± 16 vs 44 ± 13, p<0.05). The prevalence of diabetes in HCV infected group was higher than that in HBV infected group in the age of 41–60 (p<0.05). In diabetic group, the seroprevalence of HBsAg positivity was 4.5% and that of anti-HCV was 2.1%.
Conclusion :
Our study demonstrates an association between diabetes and chronic HCV infection in Korean patients. The prevalence of diabetes in patients with HCV infected chronic liver disease is higher than that in those with HBV infected. Age and alcohol consumption are another risk factor for diabetes in patients with chronic viral liver disease.
Keywords: Hepatitis, Fibrosis, Diabetes mellitus, Hepatitis C virus, Prevalence
Introduction
It is known that patients with chronic liver disease have a high prevalence of diabetes mellitus and diabetics have an increased prevalence of liver cirrhosis1). Recent epidemiological data suggested that hepatitis C virus (HCV) infection may contribute to the development of diabetes. It has been reported that patients with chronic HCV infection were more likely to be diabetic compared to those with liver diseases of other etiologies2,3). In some reports from western countries, the prevalence of HCV infection in diabetics was higher than expected in the general population4,5). However, others reported negative association between HCV infection and diabetes6). HCV infection has been associated with a variety of extrahepatic disorders, in which either immunological or direct cytopathic mechanism is likely to play a role in its pathogenesis. It remains to be determined whether HCV infection predisposes to the development of diabetes. Unlike western countries where HCV infection is a major cause of chronic liver disease, hepatitis B virus (HBV) infection is a leading cause of chronic liver disease in Korea. Additionally, clinical characteristics of Korean diabetics are different from those of western diabetics7).
To establish a potential relationship between hepatitis viral infection and diabetes, we performed two studies in Koreans. First, a prospective cross-sectional study was done to determine the prevalence of diabetes in patients with chronic viral hepatitis or liver cirrhosis. Second, a seroprevalence study of anti-HCV and HBV surface antigen (HBsAg) was done to determine the prevalence of HBV and HCV infection in Korean diabetics.
PATIENTS AND METHODS
1. Chronic liver disease group
From September 1998 to February 2000, 404 patients with chronic viral hepatitis or liver cirrhosis who visited our hospital were prospectively enrolled and their serum aminotransferases, viral markers (HBsAg and anti-HCV), fasting blood glucose and liver ultrasonography were determined. Chronic viral hepatitis was defined as having elevated serum aminotransferases for more than six months, evidence of viral hepatitis serology and ultrasonographic features of chronic liver disease. HBV healthy carriers and patients with both viral markers were excluded. Alcoholic liver cirrhosis was defined when the history of alcohol intake more than 60 g/day for men and more than 20 g/day for women was present and either viral marker was not detected. The diagnosis of cirrhosis was made on the occurrence of signs of hepatic decompensation or ultrasonographic features of portal hypertension. The severity of liver cirrhosis was graded according to Child-Pugh’s classification.
Patients taking insulin or hypoglycemic drugs were considered as diabetics and fasting serum glucose levels of peripheral venous blood >140 mg/dL on more than one occasion were also used for the diagnosis of diabetes. Degree of alcohol consumption was graded as none, social drinking (less than 60 g/day for men, less than 20 g/day for women) or heavy drinking. Body mass index (BMI) was calculated using the standard formula of weight in kilograms divided by the square of height in meters and classified into four groups as underweight (20), normal (21–25), overweight (26–29) and obese (over 30).
2. Diabetes group
From July to December 1999, 672 patients with diabetes who visited our clinic were prospectively enrolled. Among them, 627 patients were tested for serum aminotransferases and viral hepatitis markers (HBsAg and anti-HCV). Some demographic features and laboratory assessment, including serum c-peptide level, were also determined.
3. Serological studies
Serum HBsAg was detected by using a commercial kit (AxSYM HBsAg V2 Abbott Laboratories, Abbott Park, IL). Serum anti-HCV was also assayed using a commercial kit (AxSYM HCV version 3.0 Abbott Laboratories, Abbott Park, IL), which employs recombinant antigens derived from different HCV regions (HCr43, c200, c100-3, NS5).
4. Statistical analysis
Results are expressed as mean (SD). Comparison between groups was done by using student’s t-test for continuous variables and chi-square test for categorical data. Analysis of variance was compared to determine the prevalence of diabetes in each group. Significance was accepted at the level of p<0.05. Data entry and analysis was done using SPSS Windows release 7.5 computer packages.
RESULTS
1. Clinical characteristics of patients with chronic liver disease
Of the 404 patients with chronic liver disease, 225 patients were chronic viral hepatitis and 179 patients were liver cirrhosis (Table 1). The mean age was 44 years in chronic hepatitis group and 56 years in cirrhosis group. Among the patients with chronic hepatitis, 157 patients (69.8%) had HBsAg and 68 patients (30.2%) had HCV marker. The HCV infected group had a higher proportion of patients over the age of 61. The mean age of HCV infected group (52.4 ± 16.0 yrs) was also higher than that of HBV infected group (39.8 ± 12.0 yrs) (p=0.002). However, there was no significant difference in sex, BMI and alcohol consumption between HBV and HCV infected groups. In cirrhotics, 102 patients (57.0%) had HBsAg and 28 patients (15.6%) had HCV marker. Forty-three patients (24.0%) were diagnosed as alcoholic liver cirrhosis and 6 patients were cryptogenic. The HCV infected cirrhotics (66.6 ± 9.2 yrs) were older than the HBV infected (51.5 ± 10.9 yrs) (p<0.05). There were no significant differences in BMI, alcohol consumption and Child- Pugh score between HBV and HCV infected groups.
Table 1.
Clinical characteristic of patients with chronic hepatitis and liver cirrhosis according to the etiology
| Chronic hepatitis (n=225) | Liver cirrhosis (n=179) | |||||
|---|---|---|---|---|---|---|
| HBV (n=157) | HCV (n=68) | HBV (n=102) | HCV (n=28) | Alcohol (n=43) | Cryptogenic (n=6) | |
| Age (years) | ||||||
| <40 | 86 | 20 | 14 | 0 | 3 | 1 |
| 41–60 | 60 | 22 | 63 | 8 | 19 | 0 |
| 61< | 11 | 26 | 25 | 20 | 21 | 5 |
| mean (SD) | 40 ± 12 | 52 ± 16 | 52 ± 11 | 67 ± 9 | 58 ± 12 | 65 ± 14 |
| Sex | ||||||
| Male | 105(67%) | 45(66%) | 67(66%) | 14(50%) | 42(98%) | 1(17%) |
| Female | 52(33%) | 23(34%) | 35(34%) | 14(50%) | 1(2%) | 5(83%) |
| BMI | 23.4 ± 3.7 | 23.7 ± 3.5 | 23.8 ± 3.7 | 23.2 ± 3.0 | 22.2 ± 3.6 | 23.0 ± 6.4 |
| Alcohol | ||||||
| No | 84(54%) | 37(54%) | 66(65%) | 21(75%) | 0 | 6(100%) |
| Social | 53(34%) | 21(31%) | 26(25%) | 4(14%) | 0 | 0 |
| Heavy | 20(13%) | 10(15%) | 10(10%) | 3(11%) | 43(100%) | 0 |
| Child-Pugh | ||||||
| A | 49(48%) | 13(46%) | 19(44%) | 3(50%) | ||
| B | 36(35%) | 10(36%) | 20(47%) | 2(33%) | ||
| C | 17(17%) | 5(18%) | 4(9%) | 1(17%) | ||
BMI; body mass index
2. Prevalence of diabetes and factors associated with diabetes in patients with chronic liver disease
Diabetes was observed in 29 patients (12.9%) among chronic viral hepatitis group and in 34 patients (19.0%) among cirrhotics (Table 2). The prevalence of diabetes was higher in patients with HCV infection (24.0%) than that in patients with HBV infection (10.4%) (p<0.05). In patients with chronic viral hepatitis, the prevalence of diabetes (23.5%) in HCV infected group was also higher than in HBV infected (8.2%) (p=0.002). In cirrhotics, the prevalence of diabetes in HCV infected group (25%) tended to be higher than in HBV infected (13.7%) (p=0.07). However, the mean age of HCV infected group (56 ± 16 yrs) was significantly higher than that of HBV infected (44 ± 13 yrs) (p<0.05).
Table 2.
Prevalence of diabetes mellitus in patients with chronic hepatitis and liver cirrhosis
| Groups | No. of patients | Age (mean SD, yrs) | No. of diabetics | p |
|---|---|---|---|---|
| Chronic hepatitis | 225 | 44 ± 14 | 29(12.9%) | 0.002 |
| HBV | 157 | 40 ± 12 | 13(8.2%) | |
| HCV | 68 | 52 ± 16 | 16(23.5%) | |
| Liver cirrhosis | 179 | 56 ± 12 | 34(19.0%) | 0.07 |
| HBV | 102 | 52 ± 11 | 14(13.7%) | |
| HCV | 28 | 67 ± 9 | 7(25%) | |
| Alcohol | 43 | 58 ± 12 | 13(30.2%) | |
| Total | 404 | 49 ± 15 | 63(15.6%) | 0.001 |
| HBV | 259 | 44 ± 13 | 27(10.4%) | |
| HCV | 96 | 56 ± 16 | 23(24.0%) |
Significant differences for the comparison of the prevalence of diabetes between HBV and HCV are in bold type
The univariate analyses revealed that age, degree of alcohol consumption and HCV infection were significant independent predictors for diabetes (Table 3). Sex and BMI had little impact on the prevalence of diabetes. The impact of age factor on the frequency of diabetes was assessed after stratifying the patients by age. The prevalence of diabetes was 3.2% in patients under the age of 40, 17.4% in those with the age of 41–60 and 26.9% in those over the age of 61 (Figure 1). In the age of 41–60 years, the prevalence of diabetes in HCV infected group was higher than that in HBV infected (p<0.05).
Table 3.
Analysis of chronic liver disease cohort for variables associated with diabetes
| Variables | Diabetes (%)
|
||
|---|---|---|---|
| Diabetes (N=63) | No diabetes (N=341) | p | |
| Age | 0.001 | ||
| <40 | 4(3.2%) | 120(96.8%) | |
| 41–63 | 30(17.4%) | 142(82.6%) | |
| > 61 | 29(26.9%) | 79(73.1%) | |
| Sex | 0.066 | ||
| Male | 49(17.9%) | 225(82.1%) | |
| Female | 14(10.8%) | 116(89.2%) | |
| Alcohol | 0.012 | ||
| No | 27(12.4%) | 190(87.6%) | |
| Social | 18(17.1%) | 87(83.9%) | |
| Heavy | 18(21.4%) | 64(78.6%) | |
| BMI | 0.246 | ||
| < 20 | 8(9.4%) | 77(90.6%) | |
| 21–25 | 34(15.8%) | 181(84.2%) | |
| 26–29 | 16(18.0%) | 73(78.0%) | |
| 30 < | 3(20%) | 12(80%) | |
| Diagnosis | 0.093 | ||
| Cirrhosis | 34(19.0%) | 145(81%) | |
| Noncirrhosis | 29(12.9%) | 196(76.9%) | |
| Etiology | Etiology | ||
| HBV | 27(10.4%) | 233(89.6%) | |
| HCV | 23(24.0%) | 72(76%) | |
Significant differences are in bold type
Figure 1.
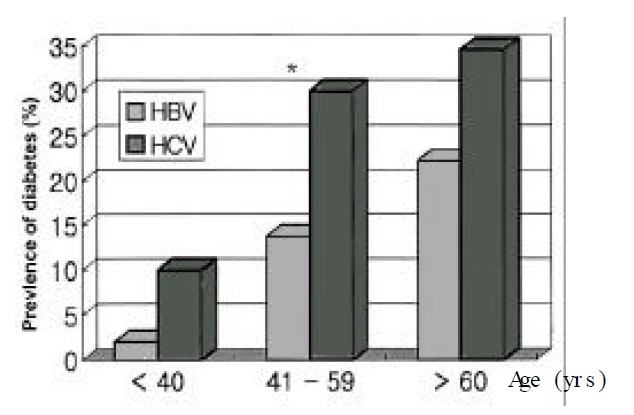
Prevalence of diabetes according to the etiology of chronic liver disease in three age (groups HBV vs HCV from youngest to oldest group, 2% vs. 10%, p=0.39; 13.8% vs. 30%, p=0.03; 22.3% vs. 34.7%, p=0.55.)
*p<0.05 significant difference
3. Prevalence of hepatitis viral infection in diabetics
All patients with diabetes who visited our clinic were asked for serum hepatitis viral markers. Among those 672 patients, 627 patients (93.3%) were finally tested (Table 4). Among the diabetics tested for viral markers, 28 patients (4.5%) showed positive for serum HBsAg and 13 patients (2.1%) had serum anti-HCV. One patient (0.2%) showed positivity to both viral markers. Several clinical and laboratory characteristics between HBsAg and anti-HCV antibody positive groups were similar (Table 5).
Table 4.
Distribution of age and sex in diabetic groups
| Group | Sex | Age (mean SD, yrs) | |
|---|---|---|---|
|
| |||
| Male (n) | Female (n) | ||
| HBsAg (−), anti-HCV (−) | 290 | 295 | 57.2 ± 12.1 |
| HBsAg (+), anti-HCV (−) | 14 | 14 | 54.4 ± 11.1 |
| HBsAg (−), anti-HCV (+) | 9 | 4 | 57.2 ± 8.7 |
| HBsAg (+), anti-HCV (+) | 0 | 1 | 58 |
| Non-tested | 12 | 33 | 59.1 ± 12.6 |
|
| |||
| Total | 325 | 347 | 57.2 ± 12.1 |
Table 5.
Physical and laboratory data in viral marker positive diabetic groups
| HBsAg (+) (n=28) | HBsAg (+) (n=28) | p | |
|---|---|---|---|
| BMI (kg/m2) | 24.0 ± 3.8 | 24.7 ± 3.3 | |
| Waist/hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | |
| Abnormal LFT (%) | 9(32.1) | 5(38.5) | |
| Random c-peptide (ng/mL) | 1.8 ± 1.2 | 2.6 ± 1.4 | 0.1 |
DISCUSSION
Disorders in carbohydrate metabolism and impaired glucose tolerance were observed in patients with cirrhosis. Hyperinsulinemia and insulin resistance has been observed in cirrhotic patients who developed diabetes. In recent epidemiological studies, the association between diabetes and HCV infection has been suggested. HCV infection is related to a variety of extrahepatic manifestations such as mixed cryoglobulinemia and membranous proliferative glomeruone-phritis. Recently, hyperglycemia is suggested to be one of the extrahepatic manifestations attributed to HCV infection.
The prevalence of HCV infection in the Korean population is known as 1.3–1.6%8,9) and the etiologic proportion of HCV in chronic liver disease is only 20–30%8). Korea is one of the endemic area of HBV infection and HBV is a common cause of chronic liver disease. The prevalence of HBV infection is 5–7% in the general population10,11). In a study, the prevalence of diabetes and impaired glucose tolerance in the adult Korean population was 7.2% and 8.9%, respectively12). The prevalence is less than 1.0% in subjects less than 40 years of age and increases thereafter12).
In our study, the prevalence of diabetes in patients with chronic viral hepatitis was 12.9%, which seems to be higher than that in the general population12). The prevalence of diabetes in cirrhotics was 19.4%. However, the prevalence of diabetes was not significantly different between chronic hepatitis and cirrhosis groups. Additionally, univariate analysis revealed that cirrhosis was not an independent risk factor for diabetes in our study. Cirrhosis is known to cause glucose intolerance and may have higher postprandial serum glucose concentration even though the fasting level is in the normal range. In our study, diabetes was diagnosed by the fasting serum glucose concentration, so the prevalence of diabetes in cirrhotics might be underestimated.
Diabetes was observed more frequently in HCV infected patients than in HBV infected patients (p<0.05). However, the distribution of age between the two groups was different. HCV infected group had a higher proportion of patients over the age 60. So, we stratified the age into three groups. In our analysis, significant difference of diabetes prevalence between HBV and HCV infected patients was present only in the age of 41–60 years. Under the age of 40, similarity of diabetic prevalence may be partially explained by the relatively low overall prevalence of diabetes in this age and the small number of enrolled patients. Over the age of 61, higher prevalence of diabetes with aging and chronicity of liver disease may confound our analysis. In support of our findings, Mason et al.5) also reported the similar prevalence of diabetes between HCV and HBV infected chronic liver disease over the age of 61. Several cohort studies of chronic liver disease also showed that diabetes was observed in 24% to 26% of patients with HCV infection while it was detected in 9% to 13% of patients with HBV infection3,5,13–15). However, a few reports disproved the association of HCV infection and diabetes6,16).
In our chronic liver disease group, we excluded healthy HBV carriers, in whom HBV was not considered to affect the development of diabetes. Other variables associated with diabetes were systematically analyzed. Alcohol consumption was a risk factor for diabetes, while the degree of alcohol consumption was similar, irrespective of viral etiology. So, alcohol consumption might not contribute to the high prevalence of diabetes in patients with HCV infection. BMI was not a risk factor for diabetes in our study. That could be explained by the fact that obese patients (BMI over 30) were relatively rare. In Korea, obese people, whose BMI is over 30, is relatively rare, in general, and even in diabetics7).
In our diabetes group, the seroprevalence of HBsAg was 4.5%, which is similar to that in the Korean population10,11). However, the seroprevalence of anti-HCV was 2.1%, which seems to be higher than that in the Korean population (1.3–1.6%)8,9). Our data rather suggest that the diabetic population is more likely to have HCV infection.
The mechanism by which HCV infection may have predisposed to diabetes was not established yet. Although there is no evidence, HCV may injure pancreatic islet cells directly, like coxsackievirus, cytomegalovirus and adenovirus. El-Zayadi et al.17) reported that HCV seropositives were more prone to require insulin therapy, reflecting pancreatic beta cell destruction and insulin deficiency. However, in our study, random serum c peptide level was not different between HBsAg and anti-HCV positive diabetic group, which seems to be contradictory to the idea that HCV infection may involve pancreatic beta cells as an extrahepatic target. Cirrhotics with HCV infection and alcoholic cirrhotics have common histological features of increased hepatic fat and iron deposition. It has been suggested that hepatic fat deposition may contribute to the development of insulin resistance18). Fasting plasma insulin levels from patients with HCV-related cirrhosis and diabetes were elevated, which was consistent with insulin resistance, but acute insulin response to glucose loading was blunted, suggesting the concomitant pancreatic beta cell dysfunction and insulin deficiency15).
In conclusion, our study demonstrates an association between diabetes and chronic HCV infection. Age and alcohol consumption are additional risk factors for diabetes in patients with chronic liver disease.
REFERENCES
- 1.Petrides AS. Liver disease and diabetes mellitus. Diabetes Rev. 1994;2:2–18. [Google Scholar]
- 2.Allison MED, Wreghitt T, Palmer CR, Alexander GJM. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 3.Ozyilkan E, Arslan M. Increased prevalence of diabetes mellitus in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1996;91:1480–1481. [PubMed] [Google Scholar]
- 4.Simo R, Hernandez C, Genesca J, Jardi R, Mesa J. High prevalence of hepatitis C virus infection in diabetic patients. Diabetes Care. 1996;19:998–1000. doi: 10.2337/diacare.19.9.998. [DOI] [PubMed] [Google Scholar]
- 5.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJM, Xu L, Guo L, Jacob S, Regenstein F, Zimmerman R, Everhart J, Wasserfall C, Maclaren N, Perrillo R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 6.Mangia A, Schiavone G, Lezzi G, Marmo R, Gruno F, Villani MR, Cascavilla I, Fantasia L, Andriulli A. HCV and diabetes mellitus: evidence for a negative association. Am J Gastroenterol. 1998;93:2363–2367. doi: 10.1111/j.1572-0241.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 7.Min HK. Non-insulin-dependent diabetes mellitus (NIDDM) in Korea. Diabet Med. 1996;13(9 Suppl 6):S13–15. [PubMed] [Google Scholar]
- 8.Kim BS, Park YM. Prevalence of hepatitis C virus related to liver disease in Korea. Gastroenterol Jpn. 1993;28(Suppl 5):17–22. doi: 10.1007/BF02989198. [DOI] [PubMed] [Google Scholar]
- 9.Park BC, Han BH, Ahn SY, Lee SW, Lee DH, Lee YN, Seo JH, Kim KW. Prevalence of hepatitis C antibody in patients with chronic liver disease and hepatocellular carcinoma in Korea. J Viral Hepat. 1995;2:195–202. doi: 10.1111/j.1365-2893.1995.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee MS, Kim DH, Kim H, Lee SH, Kim CY, Park TS, Yoo KY, Park BJ, Ahn YO. Hepatitis B vaccination and reduced risk of primary liver cancer among male adults: a cohort study in Korea. Int J Epidemiol. 1998;27:316–319. doi: 10.1093/ije/27.2.316. [DOI] [PubMed] [Google Scholar]
- 11.Ahn YO. Strategy for vaccination against hepatitis B in areas with high epidemicity: focus on Korea. Gut. 1996;38(Suppl 2):S3–66. doi: 10.1136/gut.38.suppl_2.s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YS, Lee HK, Koh CS, Min HK, Yoo KY, Kim Y, Shin YS. Prevalence of diabetes and IGT in Yonchon County, South Korea. Diabetes Care. 1995;18:545–548. doi: 10.2337/diacare.18.4.545. [DOI] [PubMed] [Google Scholar]
- 13.Fraser GM, Harman I, Meller N, Niv Y, Porath A. Diabetes mellitus is associated with chronic hepatitis C but not chronic hepatitis B virus infection. Isr J Med Sci. 1996;32:526–530. [PubMed] [Google Scholar]
- 14.Grimbert S, Valensi P, Levi-Marchal C, Perret G, Richardet JP, Raffoux C, Trinchet JC, Beaugrand M. High prevalence of diabetes mellitus in patients with chronic hepatitis C. A case control study. Gastroenterol Clin Biol. 1996;20:544–548. [PubMed] [Google Scholar]
- 15.Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O’Rahilly S, Shore S, Tom B, Alexander GJM. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 16.Sortiropoulos A, Peppas TA, Skliros O, Apostolou O, Kotsini V, Pappas SI. Low prevalence of hepatitis C infection in Greek diabetic patients. Diabet Med. 1999;16:250–252. doi: 10.1046/j.1464-5491.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 17.el-Zayadi AR, Selim OE, Hamdy H, Dabbous H, Andy A, Moniem SA. Association of chronic hepatitis C infection and diabetes mellitus. Tropical Gastroenterol. 1998;19:141–144. [PubMed] [Google Scholar]
- 18.Cavallo-Perin P, Cassder M, Bozzo C, Bruno A, Nuccio P, DallOmo AM, Marucci M, Pagano G. Mechanism of insulin resistance in liver cirrhosis. Evidence of a combined receptor and postreceptor defect. J Clin Invest. 1985;75:1659–1665. doi: 10.1172/JCI111873. [DOI] [PMC free article] [PubMed] [Google Scholar]


