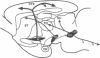
Abstract
Although certain neurophysiological functions of the amygdala complex in learning seem well established, the purpose of this review is to propose that an additional conceptualization of amygdala function is now needed. The research we review provides evidence that a subsystem within the amygdala provides a coordinated regulation of attentional processes. An important aspect of this additional neuropsychology of the amygdala is that it may aid in understanding the importance of connections between the amygdala and other neural systems in information processing.
Full text
PDF
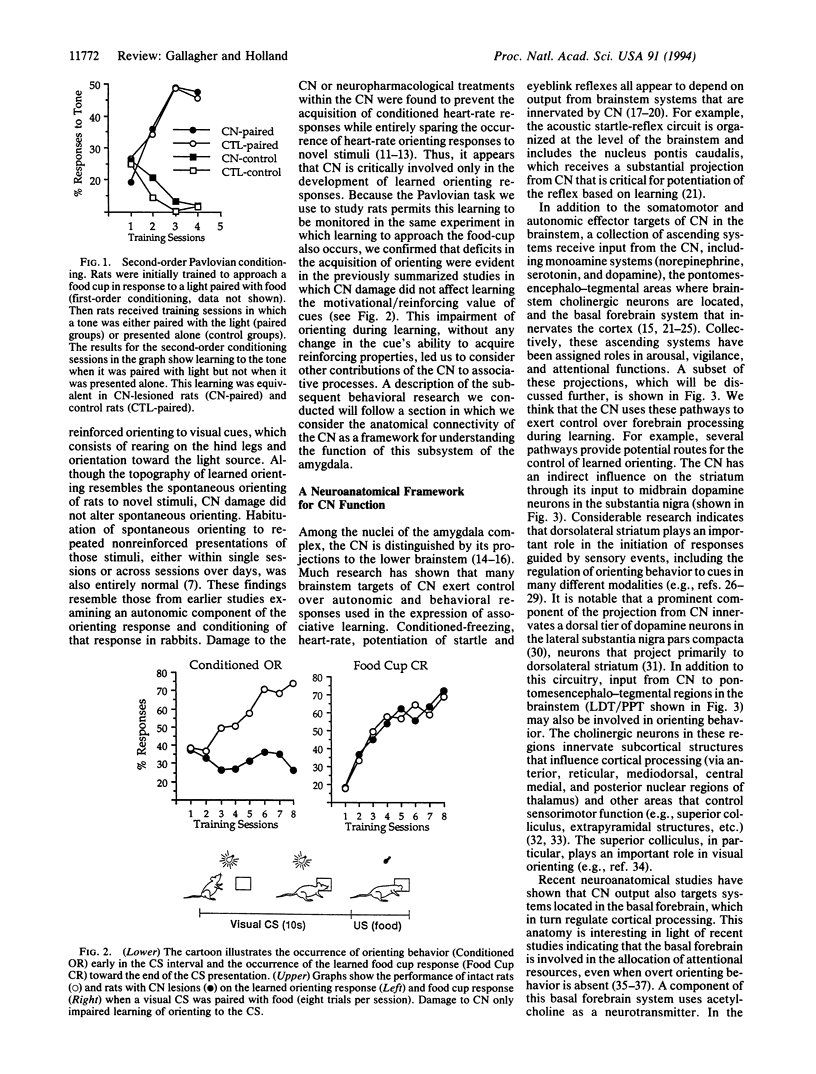

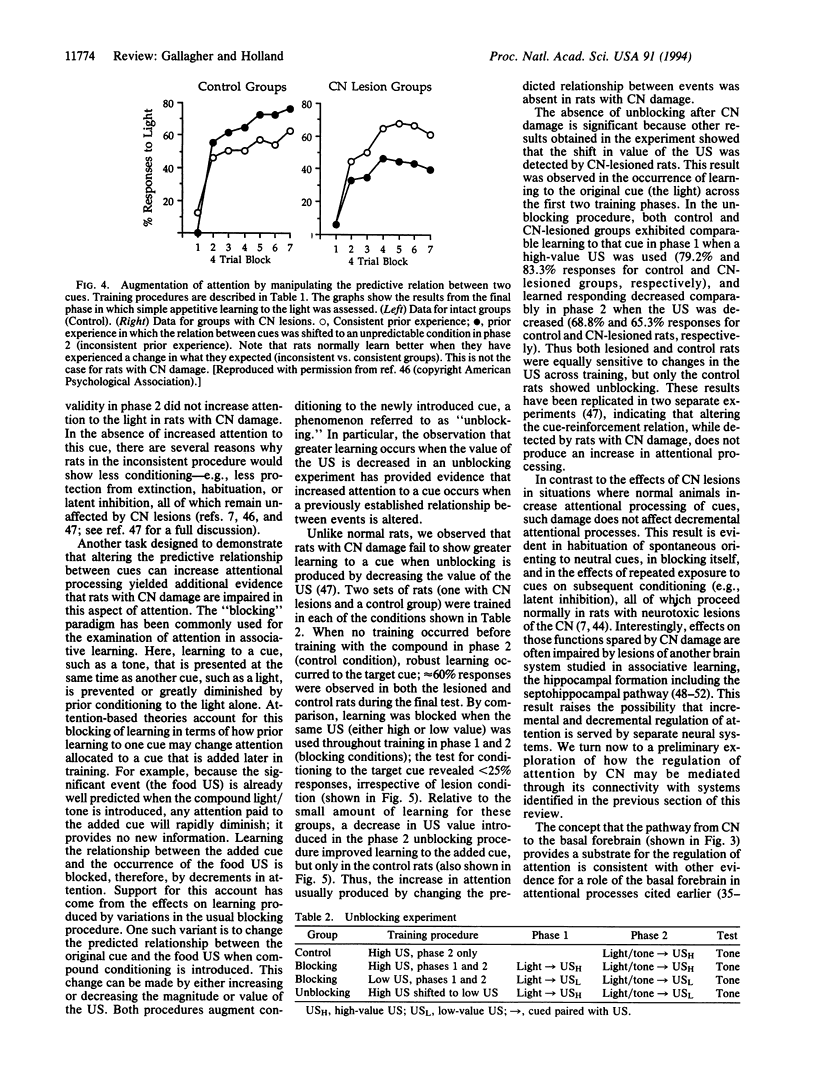
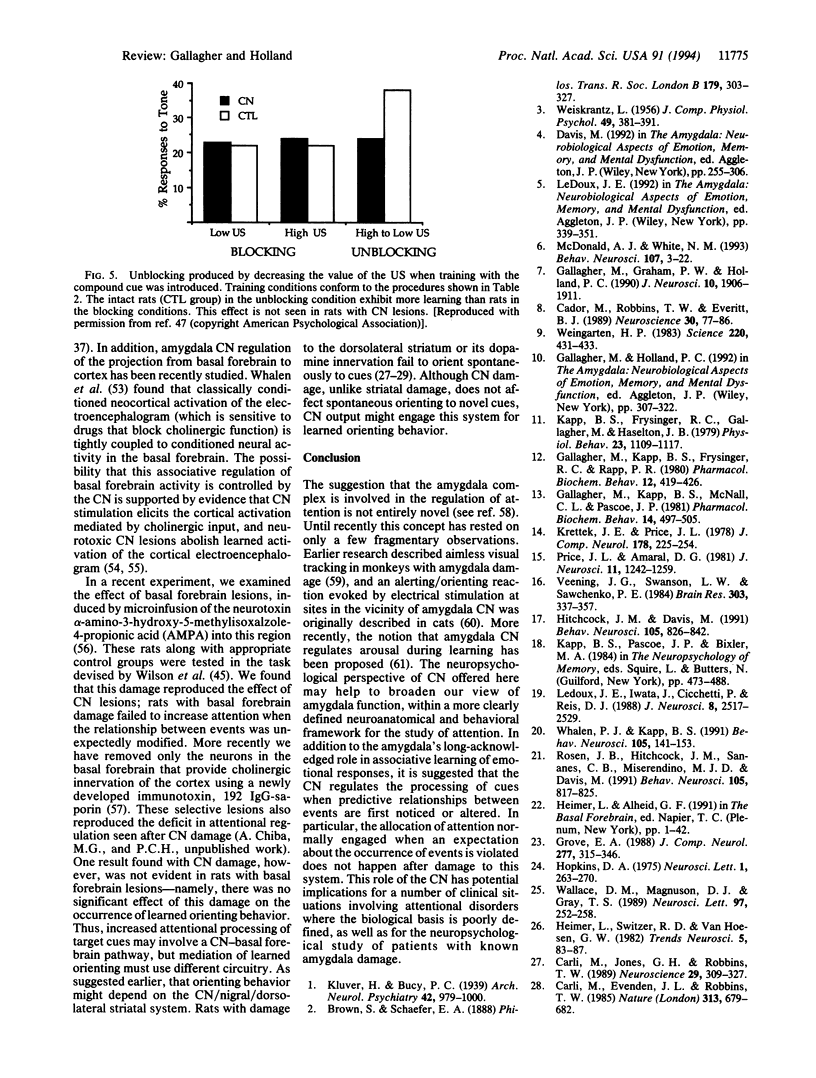

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw M. H., Mackworth N. H., Pribram K. H. The effect of resections of the inferotemporal cortex or the amygdala on visual orienting and habituation. Neuropsychologia. 1972 Jul;10(2):153–162. doi: 10.1016/0028-3932(72)90054-1. [DOI] [PubMed] [Google Scholar]
- Cador M., Robbins T. W., Everitt B. J. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30(1):77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Carli M., Evenden J. L., Robbins T. W. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985 Feb 21;313(6004):679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Carli M., Jones G. H., Robbins T. W. Effects of unilateral dorsal and ventral striatal dopamine depletion on visual neglect in the rat: a neural and behavioural analysis. Neuroscience. 1989;29(2):309–327. doi: 10.1016/0306-4522(89)90059-6. [DOI] [PubMed] [Google Scholar]
- Fairley P. C., Marshall J. F. Dopamine in the lateral caudate-putamen of the rat is essential for somatosensory orientation. Behav Neurosci. 1986 Oct;100(5):652–663. doi: 10.1037//0735-7044.100.5.652. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Moore R. Y. Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol. 1978 Aug 1;180(3):545–580. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Graham P. W., Holland P. C. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990 Jun;10(6):1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Kapp B. S., Frysinger R. C., Rapp P. R. beta-Adrenergic manipulation in amygdala central n. alters rabbit heart rate conditioning. Pharmacol Biochem Behav. 1980 Mar;12(3):419–426. doi: 10.1016/0091-3057(80)90047-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M., Kapp B. S., McNall C. L., Pascoe J. P. Opiate effects in the amygdala central nucleus on heart rate conditioning in rabbits. Pharmacol Biochem Behav. 1981 Apr;14(4):497–505. doi: 10.1016/0091-3057(81)90309-9. [DOI] [PubMed] [Google Scholar]
- Gonzales C., Chesselet M. F. Amygdalonigral pathway: an anterograde study in the rat with Phaseolus vulgaris leucoagglutinin (PHA-L). J Comp Neurol. 1990 Jul 8;297(2):182–200. doi: 10.1002/cne.902970203. [DOI] [PubMed] [Google Scholar]
- Grove E. A. Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol. 1988 Nov 15;277(3):315–346. doi: 10.1002/cne.902770302. [DOI] [PubMed] [Google Scholar]
- Heckers S., Ohtake T., Wiley R. G., Lappi D. A., Geula C., Mesulam M. M. Complete and selective cholinergic denervation of rat neocortex and hippocampus but not amygdala by an immunotoxin against the p75 NGF receptor. J Neurosci. 1994 Mar;14(3 Pt 1):1271–1289. doi: 10.1523/JNEUROSCI.14-03-01271.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L., Alheid G. F. Piecing together the puzzle of basal forebrain anatomy. Adv Exp Med Biol. 1991;295:1–42. doi: 10.1007/978-1-4757-0145-6_1. [DOI] [PubMed] [Google Scholar]
- Hitchcock J. M., Davis M. Efferent pathway of the amygdala involved in conditioned fear as measured with the fear-potentiated startle paradigm. Behav Neurosci. 1991 Dec;105(6):826–842. doi: 10.1037//0735-7044.105.6.826. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Gallagher M. Amygdala central nucleus lesions disrupt increments, but not decrements, in conditioned stimulus processing. Behav Neurosci. 1993 Apr;107(2):246–253. doi: 10.1037//0735-7044.107.2.246. [DOI] [PubMed] [Google Scholar]
- Holland P. C., Gallagher M. Effects of amygdala central nucleus lesions on blocking and unblocking. Behav Neurosci. 1993 Apr;107(2):235–245. doi: 10.1037//0735-7044.107.2.235. [DOI] [PubMed] [Google Scholar]
- Kapp B. S., Frysinger R. C., Gallagher M., Haselton J. R. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979 Dec;23(6):1109–1117. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kapp B. S., Supple W. F., Jr, Whalen P. J. Effects of electrical stimulation of the amygdaloid central nucleus on neocortical arousal in the rabbit. Behav Neurosci. 1994 Feb;108(1):81–93. doi: 10.1037//0735-7044.108.1.81. [DOI] [PubMed] [Google Scholar]
- Kaye H., Pearce J. M. Hippocampal lesions attenuate latent inhibition and the decline of the orienting response in rats. Q J Exp Psychol B. 1987 May;39(2):107–125. [PubMed] [Google Scholar]
- Krettek J. E., Price J. L. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978 Mar 15;178(2):225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- LeDoux J. E., Iwata J., Cicchetti P., Reis D. J. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988 Jul;8(7):2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R. J., White N. M. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993 Feb;107(1):3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Muir J. L., Dunnett S. B., Robbins T. W., Everitt B. J. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 1992;89(3):611–622. doi: 10.1007/BF00229886. [DOI] [PubMed] [Google Scholar]
- Pearce J. M., Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980 Nov;87(6):532–552. [PubMed] [Google Scholar]
- Posner M. I., Dehaene S. Attentional networks. Trends Neurosci. 1994 Feb;17(2):75–79. doi: 10.1016/0166-2236(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Price J. L., Amaral D. G. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J Neurosci. 1981 Nov;1(11):1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert E. J., Bennett T. L., Lane P., French J. Hippocampectomy and the attenuation of blocking. Behav Biol. 1978 Feb;22(2):147–160. doi: 10.1016/s0091-6773(78)92170-3. [DOI] [PubMed] [Google Scholar]
- Robbins T. W., Everitt B. J., Marston H. M., Wilkinson J., Jones G. H., Page K. J. Comparative effects of ibotenic acid- and quisqualic acid-induced lesions of the substantia innominata on attentional function in the rat: further implications for the role of the cholinergic neurons of the nucleus basalis in cognitive processes. Behav Brain Res. 1989 Dec 1;35(3):221–240. doi: 10.1016/s0166-4328(89)80143-3. [DOI] [PubMed] [Google Scholar]
- Rosen J. B., Hitchcock J. M., Sananes C. B., Miserendino M. J., Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci. 1991 Dec;105(6):817–825. doi: 10.1037/0735-7044.105.6.817. [DOI] [PubMed] [Google Scholar]
- Russchen F. T., Amaral D. G., Price J. L. The afferent connections of the substantia innominata in the monkey, Macaca fascicularis. J Comp Neurol. 1985 Dec 1;242(1):1–27. doi: 10.1002/cne.902420102. [DOI] [PubMed] [Google Scholar]
- Satoh K., Fibiger H. C. Cholinergic neurons of the laterodorsal tegmental nucleus: efferent and afferent connections. J Comp Neurol. 1986 Nov 15;253(3):277–302. doi: 10.1002/cne.902530302. [DOI] [PubMed] [Google Scholar]
- Schmajuk N. A., DiCarlo J. J. Stimulus configuration, classical conditioning, and hippocampal function. Psychol Rev. 1992 Apr;99(2):268–305. doi: 10.1037/0033-295x.99.2.268. [DOI] [PubMed] [Google Scholar]
- Solomon P. R., Moore J. W. Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol. 1975 Dec;89(10):1192–1203. doi: 10.1037/h0077183. [DOI] [PubMed] [Google Scholar]
- Solomon P. R. Role of the hippocampus in blocking and conditioned inhibition of the rabbit's nictitating membrane response. J Comp Physiol Psychol. 1977 Apr;91(2):407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- Veening J. G., Swanson L. W., Sawchenko P. E. The organization of projections from the central nucleus of the amygdala to brainstem sites involved in central autonomic regulation: a combined retrograde transport-immunohistochemical study. Brain Res. 1984 Jun 15;303(2):337–357. doi: 10.1016/0006-8993(84)91220-4. [DOI] [PubMed] [Google Scholar]
- Voytko M. L., Olton D. S., Richardson R. T., Gorman L. K., Tobin J. R., Price D. L. Basal forebrain lesions in monkeys disrupt attention but not learning and memory. J Neurosci. 1994 Jan;14(1):167–186. doi: 10.1523/JNEUROSCI.14-01-00167.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISKRANTZ L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956 Aug;49(4):381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- Wallace D. M., Magnuson D. J., Gray T. S. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989 Feb 27;97(3):252–258. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- Weingarten H. P. Conditioned cues elicit feeding in sated rats: a role for learning in meal initiation. Science. 1983 Apr 22;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Whalen P. J., Kapp B. S. Contributions of the amygdaloid central nucleus to the modulation of the nictitating membrane reflex in the rabbit. Behav Neurosci. 1991 Feb;105(1):141–153. doi: 10.1037//0735-7044.105.1.141. [DOI] [PubMed] [Google Scholar]
- Whalen P. J., Kapp B. S., Pascoe J. P. Neuronal activity within the nucleus basalis and conditioned neocortical electroencephalographic activation. J Neurosci. 1994 Mar;14(3 Pt 2):1623–1633. doi: 10.1523/JNEUROSCI.14-03-01623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf N. J., Butcher L. L. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986 May;16(5):603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. The primate superior colliculus and the shift of visual attention. Invest Ophthalmol. 1972 Jun;11(6):441–450. [PubMed] [Google Scholar]