Abstract
Background :
It has been debated which diagnostic test should be preferred for the diagnosis of Helicobacter pylori (HP) in patients with peptic ulcer diseases. Several limitations are reported in bleeding peptic ulcers because of intragastric blood and possibility of changed numbers of organisms by medication. This study was designed to find out the best method for diagnosis of HP infection, in aspect of deciding the times of detection and the specific tests in bleeding peptic ulcers.
Methods :
We prospectively examined histology, rapid urease test (CLO test), urea breath test (13C-UBT) and serology in HP diagnostics in 32 patients with bleeding peptic ulcers to detect HP infection. Each test was performed two times (four methods at first 24 hours and former three methods at 7th day after initial therapeutic endoscopy). We evaluated the sensitivity of each test, compared the two-times results and evaluated the effect of these tests to an outcome of endoscopic hemostasis.
Results :
Diagnostic sensitivities of histology, CLO test, 13C-UBT and serology are 75%, 67.8%, 100% and 100% at first endoscopy, and 71.4%, 78.5%, 89.3% at 7th day endoscopy, respectively. Histologic study and CLO test had diagnostic limitation at emergent first endoscopy contrary to UBT (p<0.01). Histologic study, CLO test and UBT have limitations at 7th day endoscopy. Only 3 patients (9.4%) rebled with subsequent complete endoscopic hemostasis and all diagnostic tests at initial endoscopy did not influence the outcome of hemostasis.
Conclusion :
First day histologic and CLO tests are inadequate methods in detecting HP infection in patients with bleeding peptic ulcers. 7-day histologic, CLO test and UBT have a low sensitivity. First-day UBT can be a standard test to diagnose HP infection in patients with bleeding peptic ulcers.
Keywords: Bleeding peptic ulcer, Helicobacter pylori, Urea breath test
INTRODUCTION
Helicobacter pylori (HP) has been strongly associated with peptic ulcers and it is thought to be a main etiologic factor in peptic ulcer disease1–6). The eradication of HP prevents recurrent bleeding in bleeding peptic ulcers7–15). Since several studies have reported that prevalence rate of HP infection may be underestimated in patients with bleeding peptic ulcers, it is debated that multiple tests or methods should be preferred for the diagnosis of HP in patients with bleeding peptic ulcer diseases9, 16–19). Although various diagnostic tests are introduced with their own efficient outcomes, several limitations as diagnostic tests are reported in bleeding peptic ulcers because of intragastric blood and possibility of changed numbers of organisms by anti-ulcer medication19–22). This study was designed to find out the best method for diagnosis of HP infection, in aspect of deciding the times of detection and the specific tests in bleeding peptic ulcers. We wanted to evaluate the validity that noninvasive test can execute a diagnostic role in bleeding peptic ulcers.
MATERIAL AND METHODS
Patients selection and characteristics
From September 1998 to July 1999, we prospectively tried tests two times (first 24 hrs and 7th day after initial therapeutic endoscopy) for 32 patients of bleeding peptic ulcers to detect HP infection by histologic study, rapid urease test (CLO test), carbon 13 labeled urea breath test (UBT) and serologic examination. We evaluated the sensitivity of each test, compared the two-times results and evaluated the effect to an outcome of endoscopic hemostasis.
We excluded the patients who has taken anti-ulcer medication or antibiotics or nonsteroidal antiinflammatory drugs (NSAIDs) before admission. Clinical features and endoscopic characteristics are recorded from each patient. Each patient or relative gave his or her written consent before the test and therapeutic endoscopy was performed. The test and treatment protocol had been approved by the Ethics Committee of the Soonchunhyang University Hospital (Chonan, Republic of Korea).
Diagnostic methods
During endoscopy, at first we performed endoscopic hemostasis by hemoclipping and/or hypertonic saline epinephrine (HSE) injection. After we confirmed initial hemostasis, we performed a multiple test for HP infection, including rapid urease test (CLO test, Ballard, Australia), which was observed for up to 24 hours, and histologic examination from endoscopic biopsy, 13C-labeled UBT and serologic examination for the presence of immunoglobin G (IgG) antibody to HP (Helicobacter pylori IgG, Radim, Italy).
After initial endoscopic hemostasis, a total of 4 biopsy specimens were obtained as two sets of antrum and body regardless of the presence of intragastric blood and one set of antrum and body was used for the CLO test and monitored for color change for up to 24 hours at room temperature. Another set was sent to an experienced pathologist for histologic examination with routine and special stains (modified Giemsa or Warthin-Starry silver stain).
After endoscopic hemostasis and aninvasive diagnostic test, we performed the 13C-UBT. The test consisted of a baseline breath sample and second breath sample collected 15 minutes after oral administration of 75 mg of 13C-labeled urea (Helikit™, Isotechnica Inc, Canada) dissolved in tap water. If the value of the carbon dioxide expired in both samples differed by more than 4 per mil, this was considered a positive result.
The blood sample for serologic evaluation was obtained before any medication was begun. An enzyme-linked immunosorbent assay for IgG antibody to HP was performed with a cutoff of 30 UR/mL. It was suggested by the manufacturer.
Criteria for second study
Through the first-day study, the patients who hade two or more positive results among multiple tests (CLO test, histologic examination, UBT and serologic examination) were included. We did not exclude patients with rebleeding to evaluate the safety of the diagnostic test. Patients were carefully observed and given follow-up treatment through histamine-2 receptor antagonists (ranitidine, 50 mg intravenously every 6 hours), antacid and antihemorrhagic drugs. However, we avoided other medicine or procedure which affect HP concentration of the stomach. At 7th day after initial study, we repeated the multiple test, except the serologic examination, for HP infection through the same methods an the first-day study.
Statistical analysis
The sensitivity of each test for HP infection was calculated. To assess whether each test was statistically different in sensitivity between the two day studies, Fisher’s exact or Chi-square test was used. Chi-square test was performed to evaluate the difference in sensitivity between the CLO test and the 13C-UBT.
RESULTS
Sensitivity of diagnostic tests
Diagnostic sensitivities of histologic study, CLO test, UBT and serologic examination were 75% (24/32), 68.8% (22/32), 100% (32/32), 100% (32/32) at first endoscopy, respectively, and those of the former three tests were 71.9% (23/32), 78.1% (25/32), 90.6% (29/32) at 7th day endoscopy.
Comparison of diagnostic test sensitivities
The sensitivities of the histologic study and CLO test were significantly lower than other tests (UBT or serologic examination) at first day study (p<0.01, Figure 1). Histologic, CLO, and UBT have limitations at 7th day endoscopy (Figure 2).
Figure 1.

This figure shows the comparison of diagnostic sensitivities at first day. The sensitivities of the histologic study and CLO test were significantly lower than other tests (UBT or serologic examination) (p<0.01).
Figure 2.
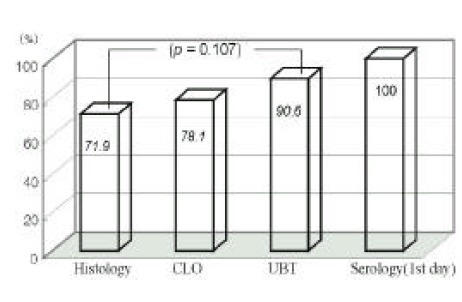
This figure shows the comparison of diagnostic sensitivities at 7th day. Histologic, CLO and UBT have limitations to detect HP infection. Sensitivity of UBT has a tendency of belnc higher than histology (p=0.107)
The sensitivities of the UBT had a tendency of decrease at examination of 7th day (p=0.238), and sensitivity of the CLO had a tendency of increment at examination of 7th day (p=0.199) (Figure 3).
Figure 3.
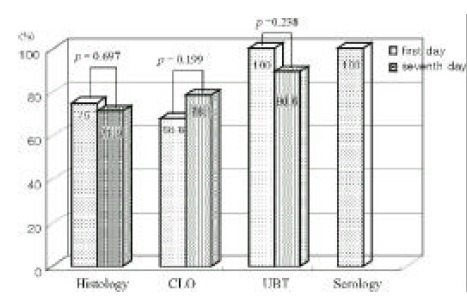
This figure shows the comparison of diagnostic sensitivities between 2-times study. The sensitivity of the UBT has a tendency of decrease at examination of 7th day, and sensitivity of the CLO has a tendency of increment at examination of 7th day.
The sensitivity of the diagnostic test according to the site of the lesion was not statistically different (Table 1).
Table 1.
Sensitivity of Diagnostic Tests According to Site of Lesion
| Gastric ulcer (n=21) | Duodenal ulcer (n=11) | |||
|---|---|---|---|---|
|
| ||||
| First | Seventh | First | Seventh | |
| Histologic study | 70 | 65 | 81.8 | 90 |
| CLO test | 60 | 70 | 90.9 | 87 |
| UBT | 100 | 75 | 100 | 80 |
CLO; Campylobacter-like organism,
UBT; Urea breath test
As outcomes of endoscopic hemostatic treatment, initial hemostasis rate was 93.8% (30/32), rebleeding rate was 9.4% (3/32) and operation rate was 6.2% (2/32). 3 rebleeding patients were completely controlled by subsequent complete endoscopic hemostasis. All diagnostic tests at initial endoscopy did not influence the outcome of hemostasis compared to our previous result for hemostatic efficacy in bleeding peptic ulcers20).
DISCUSSION
There is still no established gold standard for the diagnosis of HP infection. The choice of diagnostic modality to determine HP infection status depends on test availability, ease of use, HP treatment history and whether esophagogastroduodenoscopy is planned. It has been debated which diagnostic test should be preferred for the diagnosis of HP in patients with peptic ulcer diseases. There are many presumable limitations of various diagnostic tests, which include changes of urease activity, changes of pH in sample or test medium, changes of number of HP organism, cost, time consumption, technical personal effort, clinical state of patients and patient’s preference. Intragastric blood and possibility of changed numbers of organisms by medication have been known as representative problem to diagnose HP infection correctly in bleeding peptic ulcer. In our designed two-times test (first day and seventh day), intragastric blood, clinically emergent state and invasiveness of the test itself are suspected as limitary factors at first day; unavoidable medicine, including acid suppressive agent, antacid and antihemorrhagic drug, are suspected as limitary factors at seventh day.
There are few published studies that compare the sensitivity of the invasive and noninvasive diagnostic test. It is important to determine the best diagnostic method of HP infection, because it is possible that invasive test aggravates the ulcer bleeding in the emergent clinical state of peptic ulcer bleeding. Noninvasive tests have disputed that these are more useful than the invasive tests and more accurately reflect HP infection status24, 25). High frequency of positive results in noninvasive tests (13C-UBT and IgG serologic studies) was detected in our bleeding peptic ulcer patient in the first day, and UBT showed relatively high sensitivity at the seventh day. Sensitivity of the histologic test and CLO test was significantly low in the first day. In bleeding patients, we could not take more than four biopsy samples. Although more numbers of biopsies in extended site are expected to increase the result of sensitivity, we could not expand the design to take more biopsy samples in bleeding patients who were clinically unstable, in fact.
Since almost results of diagnostic tests exceed 97% of positive predictive value in peptic ulcer disease, repetitive tests are considered unnecessary in a patient who has one of the positive parameters for HP22). However, the prevalence rate of HP infection in patients with bleeding peptic ulcers has been reported to be lower than the rate in patients with nonbleeding ulcers in several studies, and our positive results of 68.8% CLO test and 75% of histologic test are low like the results of those studies19–21). If we have to select one diagnostic test to detect HP infection in patients with bleeding peptic ulcers, we make important decision about which test is preferred to represent an the correct state of HP infection in bleeding peptic ulcers. There have been known factors that indicate false negative result of each diagnostic test and these include the bleeding and medication of antibiotics, H2-receptor antagonist or proton pump inhibitors, as well as limitation of each test itself.
Although UBT is a good diagnostic noninvasive test, the factor related to false negative results is not discussed in patients with bleeding peptic ulcers. Cut-off value and gastric emptying time are main factors that decide correct diagnostic yield26–30). Intragastric blood and 7 days medication and gastric motility are suspected of false negative factors in the clinical setting of bleeding peptic ulcers. We may consider intragastric blood and changed gastric emptying time related to an unstable clinical state, and these can affect hydrolysis of labeled urea by urease producing HP, in contrast to unavoidable medication of H2 receptor blocker which can decrease the amount of urease itself. In our study, 100% positive result of UBT in the first day represents that intragastric blood and gastric motility of unstable clinical state are not a factor to change results, and 90.6% of 7 days can make us suspect the effect of medication.
CLO and histologic studies could not completely detect HP infection in two-times tests also. This means that these are not good diagnostic tests in patients with bleeding peptic ulcers.
Serological testing has been well-known for easy performance and high positive result, but it also has limitations because interpretation of its results is difficult to differentiate past infection in countries with a high HP prevalence rate. Therefore, it is usually used in a limited setting of follow-up studies after HP treatment.
In conclusion, first-day histologic and CLO test are an inadequate method in detecting HP infection in patients with bleeding peptic ulcers. 7-day histologic, CLO test and UBT have a low sensivity. Intragastric blood at first day and anti-ulcer medications during 7-days are suspected as risk factors of decreased sensitivity. First-day UBT can be a standard test to diagnose HP infection in patients with bleeding peptic ulcers.
REFERENCES
- 1.Kim IH, Kim PS, Kwon S, Lee DH, Choi W, Cho HG, Kim HG, Shin YW, Kim YS, Kim YB. Analysis of Risk Factors of the Peptic Ulcer Bleeding. Kor J Gastroenterol. 2000;35:178–185. [Google Scholar]
- 2.Jang BK, Ahn SH, Hur JW, Hwang JS, Kang YW, Park SK. The Effect of Helicobacter pylori infection on Peptic Ulcer Bleeding. Kor J Gastroenterol. 1999;34:295–300. [Google Scholar]
- 3.You JY, Kim HY, Kim YB, Park CK, Jang MK, Lee JY, Lee JH, Kim SG, Rho BY, Choi JH, Seo JY, Kim JS, Kim JH. The Effect of Helicobacter pylori Infection on Peptic Ulcer Bleeding. Kor J Gastroenterol. 1998;31:451–465. [Google Scholar]
- 4.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1314. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 5.Price AB, Levi J, Dolby JM, Dunscombe PL, Smith A, Clark J, Stephenson ML. Campylobacter pyloridis in peptic ulcer disease: microbiology, pathology, and scanning electron microscopy. Gut. 1985;26:1183–1188. doi: 10.1136/gut.26.11.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui WM, Ho J, Lam SK. Pathogenetic role of Helicobacter pylori in duodenal ulcer disease. Dig Dis Sci. 1991;36:424–430. doi: 10.1007/BF01298869. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Saeed ZA, Malaty HM. Effect of treatment of Helicobacter pylori infection on the long-term recurrence of gastric or duodenal ulcer. Ann Intern Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 8.Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow D, Blackbourn SJ. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;2:1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- 9.McColl KE. The role of Helicobacter pylori eradication in the management of acute bleeding peptic ulcer. Eur J Gastroenterol Hepatol. 1995;7:753–755. [PubMed] [Google Scholar]
- 10.Jasperson D, Koerner T, Schorr W, Brennenstuhl M, Raschka C, Hammer C-H. Helicobacter pylori eradication reduces the rate of rebleeding in ulcer hemorrhage. Gastrointest Endosc. 1995;41:5–7. doi: 10.1016/s0016-5107(95)70267-9. [DOI] [PubMed] [Google Scholar]
- 11.Rokkas T, Karameris A, Mavrogeorgis A, Rallis E, Giannikos N. Eradication of Helicobacter pylori reduces the possibility of rebleeding in peptic ulcer disease. Gastrointest Endosc. 1995;41:1–4. doi: 10.1016/s0016-5107(95)70266-0. [DOI] [PubMed] [Google Scholar]
- 12.Graham DY, Hepps KS, Ramirez FC, Lew GM, Saeed ZA. Treatment of Helicobacter pylori reduced the rate of rebleeding in peptic ulcer disease. Scand J Gastroenterol. 1993;28:939–942. doi: 10.3109/00365529309098288. [DOI] [PubMed] [Google Scholar]
- 13.Labenz J, Borsch G. Highly significant change of the clinical course of relapsing and complicated peptic ulcer disease after cure of Helicobacter pylori infection. Am J Gastroenterol. 1994;89:1785–1788. [PubMed] [Google Scholar]
- 14.Labenz J, Borsch G. Role of Helicobacter pylori eradication in the prevention of peptic ulcer bleeding relapse. Digestion. 1994;55:19–23. doi: 10.1159/000201117. [DOI] [PubMed] [Google Scholar]
- 15.Jaspersen D. Helicobacter pylori eradication: the best longterm prophylaxis for ulcer bleeding recurrence ? Endoscopy. 1995;27:622–625. doi: 10.1055/s-2007-1005771. [DOI] [PubMed] [Google Scholar]
- 16.Henrksson AE, Edman AC, Held M, Wadstrom T. Helicobacter pylori and acute bleeding peptic ulcer. Eur J Gastroenterol Hepatol. 1995;7:769–771. [PubMed] [Google Scholar]
- 17.Laine LA. Helicobacter pylori and complicated ulcer disease. Am J Med. 1996;100:52–59. doi: 10.1016/s0002-9343(96)80229-4. [DOI] [PubMed] [Google Scholar]
- 18.Hosking SW, Yung MY, Chung SC, Li AKC. Differing prevalence of Helicobacter in bleeding and nonbleeding ulcers[Abst] Gastroenterology. 1992;102:A85. [Google Scholar]
- 19.Tu IK, Lee CL, Wu CH, Chen TK, Chan CC, Huang SH, Lee SC. Comparison of invasive and noninvasive tests for detecting Helicobacter pylori infection in bleeding peptic ulcer. Gastrointest Endosc. 1999;49:302–306. doi: 10.1016/s0016-5107(99)70005-2. [DOI] [PubMed] [Google Scholar]
- 20.Archimandritis A, Tzivras M, Souyioultzis S, Papaparaskevas I, Apostolopoulos P, Delladetsima I, Avlami A, Davaris PS. High rate of false negative rapid urease test (CLO) in patients with upper gastrointestinal bleeding (UGB)[Abst] Gut. 1997;41(1 suppl):A76. [Google Scholar]
- 21.Lai KC, Hui WM, Lam SK. Bleeding ulcers have high false negative rate for antral Helicobacter pylori when tested with urease test[Abst] Gastroenterology. 1996;110:A167. [Google Scholar]
- 22.Lerang F, Moum B, Mowinckel P, Haug JB, Ragnhildstveit E. Accuracy of seven different tests for the diagnosis of Helicobacter pylori infection and the impact of H2-receptor antagonist on test results. Scand J Gastroenterol. 1998;33:364–9. doi: 10.1080/00365529850170982. [DOI] [PubMed] [Google Scholar]
- 23.Chung IK, Hahm JS, Kim HS, Park SH, Lee MH, Kim SJ. Comparison of the hemostatic efficacy of the endoscopic hemoclip method with hypertonic saline-epinephrine injection and a combination of the two for the management of bleeding peptic ulcers. Gastrointest Endosc. 1999;49:13–18. doi: 10.1016/s0016-5107(99)70439-6. [DOI] [PubMed] [Google Scholar]
- 24.Cutler AF, Havstad S, Chen KM, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and noninvasive tests to diagnose Helicobacter pylori infection. Gastroenterology. 1995;109:136–141. doi: 10.1016/0016-5085(95)90278-3. [DOI] [PubMed] [Google Scholar]
- 25.Andersen LP, Killerick S, Pedersen G, Thoreson AC, Jorgensen F, Rath J, Larson NA, Borup O, Krogfelt K, Scheibel J, Rune S. An analysis of seven different methods to diagnose Helicobacter pylori infections. Scand J Gastroenterol. 1998;33:24–30. doi: 10.1080/00365529850166167. [DOI] [PubMed] [Google Scholar]
- 26.Lin SK, Lambert JR, Schembri M, Nicholson L, Finlay M, Wong C, Coulepis A. A comparison of diagnostic tests to determine Helicobacter pylori infection. J Gastroenterol Hepatol. 1992;7:203–209. doi: 10.1111/j.1440-1746.1992.tb00963.x. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Munoz JE, Leodolter A, Sauerbruch T, Malfertheiner P. A citric acid solution is an optimal test drink in the 13C-urea breath test for the diagnosis of Helicobacter pylori infection. Gut. 1997;40:459–462. doi: 10.1136/gut.40.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leodolter A, Dominguez-Munoz JE, von Amim U, Manes G, Malfertheiner P. 13C-urea breath test for the diagnosis of Helicobacter pylori infection. A further simplification for clinical practice. Scand J Gastroenterol. 1998;33:267–270. doi: 10.1080/00365529850170847. [DOI] [PubMed] [Google Scholar]
- 29.Mion F, Deleclus H, Rousseau M, Berger F, Brazier J, Minaire Y. 13C-urea breath test for the diagnosis of Helicobacter pylori infection: comparison with histology. Gastroenterol Clin Biol. 1994;18:1106–1111. [PubMed] [Google Scholar]
- 30.Ohara S, Kato M, Asaka M, Toyota T. Studies of 13C-urea breath test for the diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol. 1998;33:6–13. doi: 10.1007/pl00009968. [DOI] [PubMed] [Google Scholar]


